ABSTRACT
Adenosine deaminase-1 (ADA1) regulates the concentration of adenosine as the main modulator of oocyte maturation. There is compelling evidence for the association of ADA1 gene polymorphisms with many diseases but the importance of ADA1 polymorphisms in polycystic ovary syndrome (PCOS) has not been studied before. This study investigates serum total ADA activity (tADA), ADA1 and ADA2 isoenzyme activities, and genotype and allele frequencies of G22A and A4223C polymorphisms in healthy and PCOS women. In this case-control study 200 PCOS patients and 200 healthy women were enrolled. Genomic DNA was extracted from whole blood and the PCR-RFLP technique was used to determine the G22A and A4223C variants. The genotype frequencies were calculated and the association between polymorphic genotypes and enzyme activities were determined. tADA activity was significantly lower in the PCOS group compared with the control group (27.76±6.0 vs. 39.63±7.48, respectively). PCOS patients also showed reduced activity of ADA1 and ADA2. PCOS was not associated with G22A polymorphism whereas AA, AC, and CC genotypes of A4223C polymorphism were found distributed differently between the control and the PCOS women where the C allele showed a strong protective role for PCOS (odds ratio=1.876, p=0.033). The present study for the first time showed that lower ADA activity may be involved in pathogenesis of PCOS by maintaining a higher concentration of adenosine affecting follicular growth. As a novel finding, we also showed great differences in genotype distribution and allele frequencies of A4223C polymorphism between groups indicating a protective role for C allele against PCOS.
AbbreviationsADA: adenosine deaminase PCOS: polycystic ovary syndrome PCR-RFLP: polymerase chain reaction–restriction fragment length polymorphism tADA: total adenosine deaminase
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women of reproductive age and its prevalence in various geographic regions ranges between 2.2% to 26% [Azziz et al. Citation2004; Tehrani et al. Citation2011]. PCOS as a heterogeneous syndrome has reproductive and metabolic dysfunctions including; luteinizing hormone (LH) hypersecretion, hyperandrogenism, polycystic ovaries, insulin resistance, and reduced infertility [Dumesic et al. Citation2005]. In addition to the clinical features such as oligomenorrhoea, hirsutism, acne, and hair loss [Mason et al. Citation2008], women with PCOS have follicular abnormalities in morphological features, and follicle number [Mason et al. Citation2008], development and maturation [Dumesic and Abbott Citation2008]. PCOS has attracted much attention during the last decade because of its worldwide prevalence and associated clinical complications.
Human follicle development is an ordered process, in which several hormones, growth factors, and enzymes are recruited [Dumesic et al. Citation2005; Mason et al. Citation2008; Norman et al. Citation2007]. Accordingly, an extensive body of research has recently been conducted to shed light on the underlying molecular mechanisms of PCOS. For instance, the importance of adenosine deaminase (ADA) which catalyzes the deamination of adenosine and deoxyadenosine to inosine and deoxyinosine [Nunes et al. Citation2011] in follicular growth and oocyte maturation has been investigated [Napolioni Citation2010]. Follicular fluid adenosine plays a crucial role in oocyte survival [Wen et al. Citation2010], therefore ADA is a pivotal enzyme in the metabolism and recycling of adenosine nucleotides in the human follicle [Napolioni Citation2010]. In this regard, a correlation has been found between maintenance of normal pregnancy and ADA activity [Napolioni Citation2010].
Two enzymes, ADA1 and ADA2, have been isolated with different characteristics and are known to possess ADA activity in humans [Zavialov et al. Citation2010]. ADA1 is a cytoplasmic and/or extracellular (ecto-ADA1) polymorphic enzyme widely distributed in human tissues [Khodadadi Citation2014; Spina et al. 2010; Zavialov et al. Citation2010]. ADA1 exists in all human tissues and accounts for the main ADA activity in most tissues. In comparison, ADA2, is the main ADA isoenzyme in serum originating mainly from the monocyte-macrophage system and specifically acts on the extracellular environment [Gakis Citation1996; Zavialov et al. Citation2010]. The ADA1 gene is located on human chromosome 20q13.11 and consists of 12 exons and 11 introns [Khodadadi Citation2014; Wiginton et al. Citation1986] whereas ADA2 is encoded by the CECR1 gene consisting of 9 exons located on chromosome 22q11.1 and belongs to a new family of adenosine deaminase growth factors [Zavialov et al. Citation2010]. Among the over 1,450 known single nucleotide polymorphisms of ADA1 gene (http://www.genecards.org/), the polymorphism resulting from the substitution of G by A at nucleotide 22 of exon 1 has attracted much more attention [Camargo et al. Citation2012; Dutra et al. Citation2010; Hettinger et al. Citation2008; Napolioni Citation2010; Napolioni and Predazzi Citation2010; Nunes et al. Citation2011; Riksen et al. Citation2008]. G22A polymorphism (rs73598374) replaces the Asp amino acid (G allele) with Asn amino acid (A allele) in position 8 of the enzyme protein. Consequently, individuals with the A allele express lower ADA1 activity compared with homozygous GG individuals [Nunes et al. Citation2011]. Studies have shown that the adenosine deaminase activity in subjects with the GG genotype are 15 and 30 percent higher than that of those carrying GA and AA genotypes, respectively [Battistuzzi et al. Citation1974; Napolioni and Lucarini Citation2010].
The A4223C polymorphism (rs452159) is another single nucleotide polymorphism resulting from an A/C substitution at position 4,223 in the first intron of the ADA1 gene. Recently, higher AA genotype frequency of A4223C polymorphism have been reported in obese people [Amoli et al. Citation2007]. Since obesity affects female reproduction by disturbing hormonal metabolism in the follicular microenvironment [Pantasri and Norman Citation2014], it is reasonable that obesity might be associated with PCOS [Barber and Franks Citation2013] and is prevalent in PCOS [Carmina Citation2013]. Therefore, it is plausible that the A4223C polymorphism may play a role in the development of PCOS. In addition, although the association of G22A polymorphism of ADA1 gene with many diseases including colon cancer [Spina et al. 2010], human longevity [Napolioni and Lucarini Citation2010], human reproduction [Napolioni Citation2010], fertility [Fattahi et al. Citation2015], schizophrenia [Dutra et al. Citation2010], and recurrent spontaneous abortions [Nunes et al. Citation2011] have been well established, no study has been conducted so far to determine the impact of the G22A polymorphism on PCOS. Therefore, in the present study we sought to investigate the possible association between the occurrence of PCOS with altered ADA activity and genetic distribution of G22A and A4223C polymorphisms of the ADA1 gene.
Results
Adenosine deaminase activity and G22A and A4223C genotype frequencies
PCOS was associated with a concurrent reduction in serum total ADA catalytic activity (tADA), and ADA1 and ADA2 isoenzyme activities, as indicated in . The largest reduction was observed in ADA2 activity (40%) in PCOS patients compared with control individuals. The PCOS group also showed significantly reduced (30%) activity of tADA while ADA1 activity did not decline more than 5% compared with the control.
Table 1. Serum adenosine deaminase catalytic activity in control and PCOS women.
For G22A polymorphism analysis, the GG genotype was identified as a solo 397 bp fragment whereas the RFLP product was cleaved into 245 and 152 bp fragments for the AA genotype. In contrast, the heterozygous GA genotype was identified by three 397, 245, and 152 bp fragments (). In A4223C polymorphism analysis and apart from the 28 and 43 bp common fragments in all genotypes, the AA genotype was identified by the cleavage of RFLP product into two additional 108 and 333 bp fragments, while the CC genotype yielded three 108, 147, and 186 bp fragments. The CA heterozygous genotype was identified by a set of four fragments (108, 147, 186, and 333 bp) as shown in . The distribution of the ADA1 genotypes in the study population thus determined, was consistent with the predicted genotype by the Hardy-Weinberg equilibrium.The genotype frequencies of G22A polymorphism of ADA1 gene are described in detail in . The GG genotype was the predominant genotype in both the control and PCOS groups. In contrast the lowest frequency was observed for AA genotype in the study population (1%), controls (2%), and PCOS patients (0%). The G allele was the predominant allele in the study population (PCOS patients and controls) and had the highest frequency both in healthy subjects and PCOS patients (84.5% and 85.75%, respectively). In contrast, the frequency of the A allele was found as low as 15.5% in control women and 14.25% in PCOS patients (). However, our results indicated that the distribution of G22A genotypes and alleles did not significantly differ between control and PCOS individuals. Based on logistic regression analysis, none of ADA1-G22A genotypes are a risk factor for PCOS or give women protection against PCOS. Therefore, we showed that the presence of adenosine deaminase-1 G22A genotypes including GG, GA, and AA genotypes, and their relevant alleles are not risk factors for PCOS ().
Figure 1. Agarose electrophoresis of restriction fragment length polymorphism (RFLP) products for (A). G22A polymorphism and (B) A4223C polymorphism analysis. (A) For G22A polymorphism lane 1 represents DNA ladder, GG genotype is represented in lanes 6 and 7 as a solo 397 bp fragment, lanes 3 and 4 indicate AA genotype with 245 and 152 bp fragments. In contrast, the GA genotype was identified by three 397, 245, and 152 bp fragments in lane 2 and 5. (B) For A4223C polymorphism lane 3 represents DNA ladder, lane 4 shows AA genotype with 108 and 333 bp fragments, lane 1 and 2 represent AC genotype with a set of four fragments (108, 147, 186, and 333 bp), and lane 5 shows CC genotype with three 108, 147, and 186 bp fragments.
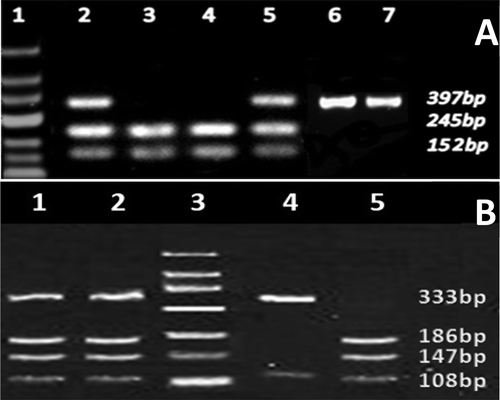
Table 2. Genotype distribution and allele frequencies of G22A and A4223C polymorphism in control and PCOS women.
Statistical analysis of the A4223C polymorphism to determine its genotype frequencies showed that AC genotype is the predominant genotype in the study population (52.75%) as well as in controls (54%) and PCOS patients (51.5%). Moreover, while CC genotype was the second most frequent genotype (28%) in healthy women, the AA genotype was observed in 26.5% of PCOS patients as the second highest genotype (). Based on Chi square test, statistical analysis did not show any significant difference in genotype distribution between groups (p=0.09). However, the sum of AC and CC genotype frequencies (AC+CC) was different between the control and PCOS groups (p=0.041), as indicated in . In addition, 55% of healthy women had the C allele while PCOS patients mostly (52.25%) had the A allele. The allele distribution significantly differed between the two groups (p=0.04) being that the A and C alleles were more frequent in patients and controls, respectively. Furthermore, logistic regression analysis showed a protective role for CC genotype and C allele. In summary, the CC genotype provided 1.876 times protection against PCOS compared with the AA genotype. In the same way, 1.337-fold protection against PCOS resulted from C allele compared to A allele ().
In both the control and PCOS groups, subjects with GG genotype of G22A polymorphism were more likely to have the AC or CC genotype of A4223C polymorphism, as indicated in . However, while healthy women with the GA genotype (G22A) mostly had the AC genotype (A4223C), PCOS patients with the GA genotype were more likely to have AA genotype of A4223C polymorphism. Collectively, in contrast to the more frequent GA/AA genotypes combinations, the GA/CC combination of genotype had the lowest frequency in PCOS patients ().
Table 3. Interaction of G22A genotypes with A4223C genotypes in control and PCOS women.
Serum adenosine deaminase activity
A significant difference was observed in tADA activity between different genotypes of the G22A polymorphism in the study population (healthy subjects and patients), as indicated in . Similarly, significant differences were also observed between GG, GA, and AA genotype carriers for ADA1 activity in the study population. Overall, a remarkable reduction in adenosine deaminase activity was observed from the GG genotype to GA and from GA to AA genotype. GG genotype carriers showed the highest tADA activity while the lowest tADA activity was observed in subjects carrying AA genotype (). Similarly, significantly higher activity of ADA1 was detected in GG genotypes whereas carriers of AA genotype showed the lowest ADA1 activity. Unlike different enzyme activities observed in various genotypes of G22A polymorphism, genotypic varients of A4223C polymorphism (AA, AC, and CC genotypes) did not cause any significant changes in tADA, ADA1, or ADA2 enzyme activity in the study population (healthy women and patients), as indicated in .
Table 4. Serum adenosine deaminase catalytic activities based on genotype distribution of G22A and A4223C polymorphisms of ADA1 gene in control and PCOS women.
Statistical ANOVA analysis to compare enzyme activities between healthy women and women with PCOS based on genotype distribution showed that healthy individuals with GG and GA genotypes, but not AA genotype, had remarkably (p<0.001) higher tADA activity than PCOS patients (). In comparison ADA1 activity was only higher in controls with the GA genotype compared to patients. None of enzyme activities differed between controls and PCOS patients carrying AA genotypes. Significant alteration in tADA, ADA1, or ADA2 enzyme activity was observed in control women based on genotype distribution being higher in GG genotype than in other genotypes (). However, genotypic differences did not alter enzyme activities in PCOS patients.
Statistical analysis to compare enzyme activities of healthy women with PCOS patients based on A4223C genotype distribution showed that tADA, ADA1, or ADA2 enzyme activities did not differ between carriers of AA, AC, and CC genotypes (). However, significant reduced tADA activity was observed (p<0.001) in women with PCOS compared with control individuals in all three genotype (). Similarly, significant reduction in ADA1 activity was found in PCOS patients with AA or CC (but not in AC) genotypes ().
One-Way-ANOVA analysis followed by post-hoc test showed that both tADA and ADA1 activities significantly differed between different combinations of G22A and A4223C genotypes in the study population (control and patients). The least tADA and ADA1 activities were found in subjects with GA/AA genotype combination while the greatest enzyme activities were observed in GG/AA and GA/CC genotype combinations (). Our results also showed no significant differences in enzyme activities between different combinations of genotypes in controls or in PCOS patients, except for ADA1 activity in PCOS patients. We found that tADA, ADA1, and ADA2 enzyme activities significantly declined in PCOS patients carrying GA/AA or GA/AC genotype combinations compared with corresponding control women (p<0.001), as shown in . Similarly, carrying the GA/CC genotype combination resulted in significant reduction in tADA activity (37.54%) in PCOS patients.
Table 5. Serum adenosine deaminase activities based on different combination of G22A and A4223C genotypes in control and PCOS women.
Discussion
The importance of adenosine, and therefore adenosine deaminase activity (ADA), in follicular growth and oocyte maturation has previously been reported [Wen et al. Citation2010] indicating a crucial role of ADA in the metabolism and recycling of adenosine nucleotides in human follicle [Napolioni Citation2010] and oocyte survival [Wen et al. Citation2010]. However, the importance of adenosine deaminase activity in polycystic ovaries and its association with the risk of PCOS has not been studied. The results of the present study showed that serum total adenosine deaminase catalytic activity (tADA) as well as ADA1, and ADA2 enzyme activities were significantly reduced in PCOS compared to control healthy women. A large reduction of tADA activity (30%) in PCOS patients compared to controls was observed. The report by Kurdoglu et al. [Citation2012] is the only available data in the literature on ADA activity in PCOS. They did not observe any significant difference in total adenosine deaminase activity between healthy women and PCOS subjects. The discordance between our results and the results of the previous study might be due to the low number of subjects recruited (40 control and 45 patients) [Kurdoglu et al. Citation2012] compared with 200 subjects enrolled in each group in the present study. Reducing adenosine deaminase activity in PCOS patients, as observed in this study will probably leave a higher concentration of adenosine in serum and follicular fluid. Since the concentration of adenosine in follicular fluid plays a crucial role in oocyte survival, growth, and maturation [Wen et al. Citation2010], ADA activity is pivotal in follicular microenvironment and it is plausible that ADA may be involved in the pathogenesis of PCOS. This finding can be supported, albeit in part, by a previous study indicating a critical correlation between the maintenance of normal pregnancy and ADA activity [Lee et al. Citation2007; Napolioni Citation2010]. Our report is the first report confirming declined tADA activity as well as the activity of ADA1 and ADA2 isoenzymes in women with PCOS. However, further studies are required to simultaneously investigate ADA activity and the concentration of adenosine in serum or follicular fluid and to confirm the role of lower adenosine deaminase activity in the onset or in the development of PCOS.
Apart from the important role of ADA activity in diseases such as severe combined immunodeficiency [Bose and Nandagopal Citation2013], pulmonary tuberculosis [Salmanzadeh et al. Citation2015], HIV [Abdi et al. Citation2013], breast cancer [Mahajan et al. Citation2013], multiple sclerosis [Polachini et al. Citation2014], arthritis [Doudkani-Fard et al. Citation2014], and infertility [Fattahi et al. Citation2015], ADA polymorphic genotype variations have also been found as key players in the development of many pathological conditions. Among over 1,450 known single nucleotide ADA1 gene polymorphisms, the G22A polymorphism has attracted much more attention. In this respect, the association of G22A polymorphism with many diseases including colon cancer [Spina et al. 2010], rheumatoid arthritis [Sebastiani et al. Citation2010], human longevity [Napolioni and Lucarini Citation2010], human reproduction [Napolioni Citation2010] and fertility [Fattahi et al. Citation2015], schizophrenia [Dutra et al. Citation2010], autism [Hettinger et al. Citation2008], coronary artery diseases [Safranow et al. Citation2007], and recurrent spontaneous abortions [Nunes et al. Citation2011] has previously been reported. Similarly, the correlation of A4223C, the lesser studied polymorphism of ADA1 gene, with diseases such as chronic heart failure [He et al. Citation2014], maternal neural tube defects [Pangilinan et al. Citation2012; Pangilinan et al. Citation2014], and obesity [Amoli et al. Citation2007] has recently been reported. However, no study has been conducted so far to determine the impact of G22A and A4223C polymorphisms on PCOS.
In the present study, we have investigated the genotype frequencies of G22A and A4223C polymorphisms of ADA1 in healthy control and PCOS patients. Our results for the first time showed that there was no significant different distribution of G22A genotypes between healthy women and PCOS patients. Frequency of GG, GA, and AA genotypes did not differ between PCOS and healthy individuals. In addition, G was the predominant allele in both the control and PCOS groups and no statistical differences in allele distribution or frequencies were observed between groups. Therefore, none of the G22A genotypes or alleles showed a protective role or acted as a risk factor for PCOS. Although association of G22A polymorphism of ADA1 gene with many diseases has been previously well established, intriguingly we showed no association between G22A polymorphism and PCOS. Since this is the first study investigating the role of G22A polymorphism in PCOS it is not possible to compare our results with the data in literature.
Unlike the G22A polymorphism, the genotype distribution and allele frequencies of A4223C polymorphism significantly differed between the control and PCOS groups. The homozygous CC genotype provided 1.876 times protection for PCOS and carriers of C allele showed 1.337-fold protection against PCOS. Therefore, a strong association was observed between A4223C polymorphism of the ADA1 gene and PCOS in this study and it can be concluded that while the carriers of A allele are more susceptible to PCOS, the existence of the C allele protects women from PCOS. Since there is no previous study in the literature investigating the association of A4223C polymorphism with PCOS it is not clear how this polymorphism contributes to PCOS. It is postulated that the observed correlation of A4223C polymorphism and PCOS might be in part due to the association of A4223C polymorphism with obesity [Barber and Franks Citation2013] since obesity is normally prevalent in PCOS patients [Carmina Citation2013] and the fecundity of obese women is lower than normal weight women [Pantasri and Norman Citation2014]. However, further studies are needed to prove this hypothesis since BMI was not been measured in the present study.
We have also investigated the possible linkage between two G22A and A4223C ADA1 gene polymorphisms and the likelihood of a specific genotype combination being a risk factor for PCOS. The results showed that none of the GG genotype combinations from G22A polymorphism with AA, AC, or CC genotypes from A4223C polymorphism increases the risk of PCOS or protects women from disease. Interestingly, we found that the GA/AA genotype combination likely increases susceptibility to PCOS whereas the existence of the GA/CC combination of genotypes may protect women from PCOS. Although our observation is preliminary and requires further investigation, it is the first report on the involvement of G22A and A4223C polymorphisms in PCOS.
There is an extensive body of literature reporting that serum adenosine deaminase activity is influenced by the G22A ADA1 gene polymorphism contributing greater in the GG genotype than both GA and AA genotypes [Battistuzzi et al. Citation1974; Lucarini and Borgiani Citation1989; Napolioni and Lucarini Citation2010]. In line with previous studies we showed that within the study population (healthy women and PCOS patients) serum ADA1 and total ADA catalytic activity are greater in subjects with the GG genotype than individuals carrying GA and AA genotypes. This observation confirmed that replacement of Asp amino acid (G allele) with Asn amino acid (A allele) in position 8 of the enzyme protein leads to lower ADA1 activity [Nunes et al. Citation2011] and probably leaves higher levels of adenosine both in circulation and intra-cellular environment. An increased concentration of adenosine may have in turn a number of consequences as the adenosine present in plasma and follicular fluid is an important local hormone which may lead to several effects on the follicular growth and oocyte maturation.
A similar pattern of reduction in enzyme activity (tADA and ADA1) was also found in healthy women, but not in PCOS patients. It seems that the G22A polymorphism-induced reduction in enzyme activity is abrogated in PCOS by other contributors such as inflammation since a low-grade chronic inflammation which is modulated by adenosine level has been reported in PCOS [Spritzer et al. Citation2015].
Unlike the impact of the G22A polymorphism on serum adenosine deaminase catalytic activity, the genotypic distribution of A4223C polymorphism of ADA1 gene had no effect on tADA, ADA1, or ADA2 activities. This observation is not surprising since the A4223C polymorphism is not located in exons or in the promoter region of ADA1 gene and therefore the substitution of A to C is not as effective as replacement of G by A in G22A polymorphism. However, a significant reduction in tADA, ADA1, and ADA2 activities was observed in PCOS patients compared with control subjects in all three AA, AC, and CC genotypes. The highest tADA activity was observed in healthy women carrying homozygote CC genotype which explains the protective effect of C allele against PCOS.
In conclusion, for the first time we showed that ADA activity is lower in PCOS which confirms the involvement of adenosine deaminase in the pathogenesis of PCOS. We have also shown that ADA1 A4223C polymorphism provides protection against PCOS. In contrast, the distribution of G22A genotypes did not differ between the control and PCOS groups and none of the G or A alleles showed protection or acted as a risk factor for PCOS.
The limitations of this study should be considered when interpreting its findings. First, adenosine is produced or degraded by several enzymes, including 5’-nucleotidase, ADA, and adenosine kinase [Khodadadi Citation2014] and therefore the role of all enzymes should be taken into account in investigating adenosine metabolism. Second, simultaneous determination of serum ADA catalytic activity and the level of adenosine is required. One limitation of the present study is the lack of a more comprehensive genetic analysis of the polymorphisms potentially associated with adenosine levels. In addition, ADA activity both in serum and follicular fluid should be determined. Finally, we were unable to establish the mechanisms underlying the effects of the two selected SNPs on PCOS.
Materials and methods
Subjects
Two hundred women with PCOS who were admitted to the Fertility Centre of Fatemieh Hospital (Hamadan-Iran) to receive assisted reproductive technology treatment during September 2013 to December 2014 and 200 age-matched healthy women were recruited in this study. The mean age of PCOS patients (29.52±3.1) and control women (29.70±5.4) did not differ significantly (p<0.820). PCOS was diagnosed by the presence of two or more of: chronic anovulation, clinical or biochemical signs of hyperandrogenism, and polycystic ovaries, according to the 2003 Rotterdam criteria [Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Citation2004]. Control individuals had regular menstruation with no sign of hirsutism and acne. Subjects with the history of cancer, diabetes, and hypertension, and individuals with known liver, kidney, thyroid, and coronary heart diseases as well as subjects with any diseases related to the adenosine metabolism or ADA activity such as rheumatoid arthritis and brucellosis were excluded from the study. In addition, smoking and age of over 40 years were set as exclusion criteria. Written informed consent for participation was obtained and the project was approved by the Research Ethics Committee of Hamadan University of Medical Sciences (DP16-35-9-403).
Blood sample collection and analysis
Ten milliliters of peripheral blood was collected from each subject in heparin containing tubes. After centrifugation at 3,000×g for 10 min, plasma was separated and stored at -70°C for biochemical measurements while cell pellet was used for DNA extraction and genotype analysis using Polymerase Chain Reaction-Restriction Fragment Length Polymorphisms (PCR-RFLP) method.
Adenosine deaminase activity assay
Adenosine and erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA) were purchased from Sigma-Aldrich (Sigma, MO, USA). Serum total ADA (tADA) activity was determined according to the Giusti method [Giusti Citation1974]. In brief, ADA hydrolyzed its substrate (adenosine) into inosine and ammonia. The ammonia liberated from adenosine was then converted to colored indophenol complex in the presence of an alkaline solution of phenol and hypochlorite in a process called Bertholet reaction. Finally, the colored indophenol complex was quantified spectrophotometrically at 630 nm. To determine ADA2 isoenzyme activity, serum ADA1 activity was selectively inhibited by the addition of EHNA into the serum samples and estimated ADA1 activity was calculated by subtracting of ADA2 from tADA activity, as we have previously described [Khodadadi et al. Citation2011]. Finally, all activities were expressed in unit per liter (U/L).
ADA1 genotyping
Genomic DNA was extracted from peripheral venous blood using ethanol-chloroform method and stored at -20°C for further analysis. Forward F: 5´-GCCCGGCCCGTTAAGAAGAGC-3´ and reverse R: 5´-GGTCAAGTCAGGGGCAGAAGCAGA-3´ primers were used to amplify a 397 bp PCR product containing G22A polymorphism site [Napolioni and Lucarini Citation2010] whereas to amplify a 512 bp product containing A4223C polymorphism site a pair of forward 5’-TATCTCACGGAATCCTCTGG-3’ and reverse 5’-TGCATCAGAGAGGGACAGTT-3’ primers was used [Amoli et al. Citation2007]. Amplifications were carried out using AccuPower® HotStart PCR PreMix Kit (Bioneer Inc., Seoul, Korea), according to manufacturer’s instruction in a Eppendorf DNA thermal cycler (Eppendorf Ltd., Stevenage, UK). The PCR cycles consisted of an initial denaturation step (95°C for 5 min) followed by 36 cycles of denaturation (95°C for 15 sec), annealing (60°C for 30 sec) and extension (72°C for 30 sec), and a final extension at 72°C for 5 min. The size of G22A and A4223C related PCR products was then confirmed by 2% agarose gel electrophoresis and the G22A and A4223C related PCR products were digested by TaqI and MspI restriction enzymes, respectively (Fisher Scientific Ltd., Paisley, UK) for 90 min at 65°C. The RFLP products were then electrophoresed on 2.5% agarose gel and the bands were visualized by SYBR Safe staining (Invitrogen, Paisley, UK).
The GG genotype was identified as a solo 397 bp fragment whereas the RFLP product was cleaved into 245 and 152 bp fragments for AA genotype. In contrast, the heterozygous GA genotype was identified by three 397, 245, and 152 bp fragments. A4223C polymorphism analysis and apart from the 28 and 43 bp common fragments in all genotypes, the AA genotype was identified by the cleavage of RFLP product into two additional 108 and 333 bp fragments, while the CC genotype yielded three 108, 147, and 186 bp fragments. The CA heterozygous genotype was identified by a set of four fragments (108, 147, 186, and 333 bp).
Statistical analysis
All statistical analyses were carried out using the Statistical Package for Social Sciences version 16 (SPSS, Inc., Chicago, IL, USA). Values were presented as mean ± SD and statistical significance was defined as p values less than 0.05 (p<0.05). The ADA genotypic distribution was tested for accordance with the Hardy-Weinberg equilibrium. Statistically significant differences in mean between genotypes were assessed by t test and differences between cases and controls in frequencies of genotypes were evaluated using Chi-square (χ2) test. One-Way ANOVA analysis followed by post-hoc Tukey and Dunnett tests were used as appropriate. In addition, logistic regression test was applied to estimate the relative risk of ADA1 genotypes. STATA software version 11.2 (StataCorp LP., TX, USA) was used to determine the likelihood of interaction between two G22A and A4223C polymorphisms.
Declaration of interest
The authors report no declarations of interest. This research was supported by Hamadan University of Medical Sciences, as a MSc thesis, and did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector. It is declared that none of the authors are directly funded or employed by the Government of Iran.
Acknowledgments
We are very grateful to Dr. Fattahi for his valuable assistance in statistical analysis.
Additional information
Notes on contributors
Mahshid Salehabadi
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
Marzieh Farimani
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
Heidar Tavilani
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
Marzieh Ghorbani
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
Faranak Poormonsefi
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
Jalal Poorolajal
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
Gholamreza Shafiei
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
Neda Ghasemkhani
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
Iraj Khodadadi
Clinical evaluation of patients in Fertility Research Centre (Fatemieh Hospital): MF; Enrolment of subjects and sample collection: MG, FP; Performed all experiments and laboratory tests and collected data: MS; Performed the initial statistical analysis: JP; Prepared first draft of manuscript: GS, NG; Provided valuable information for conducting the project and preparing manuscript: HT; Planned and conducted project as well as prepared manuscript: IK.
References
- Abdi, M., Ahmadi, A., Roshany, D., Khodadadi, I., Javid, S., Shahmohammad-Nezhad, S., et al. (2013) Diagnostic value of serum adenosine deaminase activity in HIV infected patients of Kurdish population. Clin Lab 59:757-762.
- Amoli, M.M., Amiri, P., Namakchian, M., Saeid Nejad, R., Fakhrzadeh, H., Heshmat, R., et al. (2007) Adenosine deaminase gene polymorphism is associated with obesity in Iranian population. Obesity Research & Clinical Practice 1:173-177.
- Azziz, R., Woods, K.S., Reyna, R., Key, T.J.,Knochenhauer, E.S. and Yildiz, B.O. (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745-2749.
- Barber, T.M. and Franks, S. (2013) Genetics of polycystic ovary syndrome. Front Horm Res 40:28-39.
- Battistuzzi, G., Scozzari, R., Santolamazza, P., Terrenato, L. and Modiano, G. (1974) Comparative activity of red cell adenosine deaminase allelic forms. Nature 251:711-713.
- Bose, R. and Nandagopal, K. (2013) Genetic and biochemical consequences of adenosine deaminase deficiency in humans. Indian J Biochem Biophys 50:345-356.
- Camargo, U., Toledo, R.A., Cintra, J.R., Nunes, D.P., Acayaba de Toledo, R., Brandao de Mattos, C.C., et al. (2012) Lack of association of the G22A polymorphism of the ADA gene in patients with ankylosing spondylitis. Genet Mol Res 11:1178-1184.
- Carmina, E. (2013) Obesity, adipokines and metabolic syndrome in polycystic ovary syndrome. Front Horm Res 40:40-50.
- Doudkani-Fard, M., Ziaee, V., Moradinejad, M.H., Sedaghat, M., Haghi-Ashtiani, M.T. and Ahmadinejad, Z. (2014) Sensitivity and specificity of adenosine deaminase in diagnosis of juvenile idiopathic arthritis. Med J Islam Repub Iran 28:113.
- Dumesic, D.A. and Abbott, D.H. (2008) Implications of polycystic ovary syndrome on oocyte development. Semin Reprod Med 26:53-61.
- Dumesic, D.A., Schramm, R.D. and Abbott, D.H. (2005) Early origins of polycystic ovary syndrome. Reprod Fertil Dev 17:349-360.
- Dutra, G.P., Ottoni, G.L., Lara, D.R. and Bogo, M.R. (2010) Lower frequency of the low activity adenosine deaminase allelic variant (ADA1*2) in schizophrenic patients. Rev Bras Psiquiatr 32:275-278.
- Fattahi, A., Khodadadi, I., Amiri, I., Latifi, Z., Ghorbani, M. and Tavilani, H. (2015) The role of G22A adenosine deaminase 1 gene polymorphism and the activities of ADA isoenzymes in fertile and infertile men. Urology 80(4):730–734.
- Gakis, C. (1996) Adenosine deaminase (ADA) isoenzymes ADA1 and ADA2: diagnostic and biological role. Eur Respir J 9:632-633.
- Giusti, G. (1974) Adenosine deaminase. In:. ed. Bergmeyer H.U. Methods of Enzymatic Analysis, 2nd edition. Academic Press, NY, pp. 1092–1099.
- He, H.R., Li, Y.J., He, G.H., Wang, Y.J., Zhai, Y.J., Xie, J., et al. (2014) The adenosine deaminase gene polymorphism is associated with chronic heart failure risk in Chinese. Int J Mol Sci 15:15259-15271.
- Hettinger, J.A., Liu, X. and Holden, J.J. (2008) The G22A polymorphism of the ADA gene and susceptibility to autism spectrum disorders. J Autism Dev Disord 38:14-19.
- Khodadadi, I. (2014) Mini Review From the Molecular Base to the Diagnostic Value of Adenosine Deaminase. Avicenna Journal of Medical Biochemistry 2:e24310.
- Khodadadi, I., Abdi, M., Ahmadi, A., Wahedi, M.S., Menbari, S., Lahoorpour, F., et al. (2011) Analysis of serum adenosine deaminase (ADA) and ADA1 and ADA2 isoenzyme activities in HIV positive and HIV-HBV co-infected patients. Clin Biochem 44:980-983.
- Kurdoglu, Z., Ozkol, H. and Kurdoglu, M. (2012) Serum myeloperoxidase and adenosine deaminase activities in polycystic ovary syndrome. Gynecol Endocrinol 28:115-118.
- Lee, S.J., Hwang, H.S., Kim, B.N., Kim, M.A., Lee, J.W., Park, Y.W., et al. (2007) Changes in serum adenosine deaminase activity during normal pregnancy. J Korean Med Sci 22:718-721.
- Lucarini, N. and Borgiani, P. (1989) Enzyme activity and distribution of adenosine deaminase types in a population of central Italy. Hum Hered 39:322-326.
- Mahajan, M., Tiwari, N., Sharma, R., Kaur, S. and Singh, N. (2013) Oxidative stress and its relationship with adenosine deaminase activity in various stages of breast cancer. Indian J Clin Biochem 28:51-54.
- Mason, H., Colao, A., Blume-Peytavi, U., Rice, S., Qureshi, A., Pellatt, L., et al. (2008) Polycystic ovary syndrome (PCOS) trilogy: a translational and clinical review. Clin Endocrinol (Oxf) 69:831-844.
- Napolioni, V. (2010) ADA (22G>A) polymorphism: a possible genetic marker for predictive medicine of human reproduction? Metabolism 59:e9–e10.
- Napolioni, V. and Lucarini, N. (2010) Gender-specific association of ADA genetic polymorphism with human longevity. Biogerontology 11:457-462.
- Napolioni, V. and Predazzi, I.M. (2010) Age- and gender-specific association between ADA (22G>A) and TNF-alpha (-308G>A) genetic polymorphisms. Tissue Antigens 76:311-314.
- Norman, R.J., Dewailly, D., Legro, R.S. and Hickey, T.E. (2007) Polycystic ovary syndrome. Lancet 370:685-697.
- Nunes, D.P., Spegiorin, L.C., Mattos, C.C., Oliani, A.H., Vaz-Oliani, D.C. and Mattos, L.C. (2011) The ADA*2 allele of the adenosine deaminase gene (20q13.11) and recurrent spontaneous abortions: an age-dependent association. Clinics (Sao Paulo) 66:1929-1933.
- Pangilinan, F., Molloy, A.M., Mills, J.L., Troendle, J.F., Parle-McDermott, A., Kay, D.M., et al. (2014) Replication and exploratory analysis of 24 candidate risk polymorphisms for neural tube defects. BMC Med Genet 15:102.
- Pangilinan, F., Molloy, A.M., Mills, J.L., Troendle, J.F., Parle-McDermott, A., Signore, C., et al. (2012) Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Med Genet 13:62.
- Pantasri, T. and Norman, R.J. (2014) The effects of being overweight and obese on female reproduction: a review. Gynecol Endocrinol 30:90-94.
- Polachini, C.R., Spanevello, R.M., Casali, E.A., Zanini, D., Pereira, L.B., Martins, C.C., et al. (2014) Alterations in the cholinesterase and adenosine deaminase activities and inflammation biomarker levels in patients with multiple sclerosis. Neuroscience 266:266-274.
- Riksen, N.P., Franke,B., van den Broek, P., Naber, M., Smits, P. and Rongen, G.A. (2008) The 22G>A polymorphism in the adenosine deaminase gene impairs catalytic function but does not affect reactive hyperaemia in humans in vivo. Pharmacogenet Genomics 18:843-846.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19-25.
- Safranow, K., Rzeuski, R., Binczak-Kuleta, A., Czyzycka, E., Skowronek, J., Jakubowska, K., et al. (2007) ADA*2 allele of the adenosine deaminase gene may protect against coronary artery disease. Cardiology 108:275-281.
- Salmanzadeh, S., Tavakkol, H., Bavieh, K. and Alavi, S.M. (2015) Diagnostic Value of Serum Adenosine Deaminase (ADA) Level for Pulmonary Tuberculosis. Jundishapur J Microbiol 8:e21760.
- Sebastiani, G.D., Bottini, N., Greco, E., Saccucci, P., Canu, G., Lucarelli, P., et al. (2010) A study of Adenosine-Deaminase genetic polymorphism in rheumatoid arthritis. Int J Immunopathol Pharmacol 23:791–795.
- Spina, C., Bottini, E., Cozzoli, E., Khodadadi, I., Gloria-Bottini, F., (2010) A study of three polymorphic sites of ADA gene in colon cancer. Cancer Invest 28:989-992.
- Spritzer, P.M., Lecke, S.B., Satler, F. and Morsch, D.M. (2015) Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 149:R219–227.
- Tehrani, F.R., Simbar, M., Tohidi, M., Hosseinpanah, F. and Azizi, F. (2011) The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol 9:39.
- Wen, X., Perrett, D., Jones, N., Tozer, A.J., Docherty, S.M. and Iles, R.K. (2010) High follicular fluid adenosine levels may be pivotal in the metabolism and recycling of adenosine nucleotides in the human follicle. Metabolism 59:1145-1155.
- Wiginton, D.A., Kaplan, D.J., States, J.C., Akeson, A.L., Perme, C.M., Bilyk, I.J., et al. (1986) Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry 25:8234-8244.
- Zavialov, A.V., Gracia, E., Glaichenhaus, N., Franco, R., Zavialov, A.V. and Lauvau, G. (2010) Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol 88:279-290.
