Abstract
Materials and methods: The present study is the first to apply DNA barcoding on identifying 37 freshwater fish species from the rich Balkan ichthyofauna.
Results: The results are highly successful since in most cases barcodes cluster according to species, in agreement with morphological taxonomic studies. This is also evident based on mean conspecific and congeneric Kimura two-parameter distance values. The 5.6-fold difference between these values is lower than previous barcoding studies, possibly due to the restricted samplings and the recent taxonomy reevaluation for several species. A number of species were identified, where future work is needed: For the species Scardinius erythrophthalmus, Perca fluviatilis, and Rutilus rutilus, the divergence values found among conspecific populations could warrant their placement into different species; for Barbus and Rhodeus populations, the reported interspecific distances found were lower than expected; and for Cobitis species, the application of barcoding seems problematic, due to their complicated reproduction.
Conclusion: The extension of this work to other Greek or even Balkan freshwater systems should clarify the situation.
Keywords::
Introduction
The determination and the identification of species constitute some of the first basic steps for biodiversity monitoring and conservation (Dayrat Citation2005). This is even more critical now that human-mediated environment disturbance and correlated climate change have resulted in ecosystem deterioration, posing a threat to the survival of numerous species. Identification and assignment to species is usually carried out by specialists (taxonomists) who are, however, in many cases restricted by available morphological characteristics. To circumvent such problems, the “Consortium for the Barcode of Life” was established in 2004. It is devoted to the “DNA Barcode of Life”; that is, to develop DNA barcoding as a global standard for the identification of biological species (Costa and Carvalho Citation2007). The consortium aims to develop a reliable, automated, fast, and cheap method for the universal identification of eukaryotic species, to aid taxonomists and to be used by nonexperts as well (Frézal and Leblois Citation2008).
DNA barcoding efforts have shown that a short sequence of ∼650 bp from the mitochondrial subunit I of cytochrome c oxidase (COI) can be used for such purposes (Hebert and Gregory Citation2005). Indeed, this locus has been successful in identifying diverse species such as spiders (Barrett and Hebert Citation2005), butterflies (Hebert et al. Citation2004a), and fish (Ward et al. Citation2005). More than 79,000 species have now been assigned a DNA barcode (Barcode of Life Data Systems (BOLD); http://www.boldsystems.org).
Fish species identification also mainly relies on morphometric and meristic characters (Strauss and Bond Citation1990). However, it has been shown (Lleonart et al. Citation2006) that as high as 40% of fish catch is not identified at the species level. New reliable automated techniques are, therefore, also urgently needed for fish identification, especially for commercial fish. The use of DNA methods can circumvent such problems (Hebert and Gregory Citation2005). The project FISH-Barcode of Life was launched in 2005, aiming for a universal DNA barcode database and identification of the ∼30,000 estimated fish species. Until now, only one-quarter of these have been barcoded. Most of them concern marine fish of Australia and Asia (Ward et al. Citation2005, Citation2008a,Citationb; Ward and Holmes Citation2007; Zemlak et al. Citation2009), whereas in Europe 440 out of 2028 species have been barcoded until now (7 November 2010). DNA barcoding results have shown that their use is highly successful in fish and that genetic identification methods discriminate 98 and 93% of marine and freshwater fish, respectively (Ward et al. Citation2009).
The freshwater fish fauna of Greece is among the richest in the Balkan Peninsula, including several endemic species (Griffiths et al. Citation2004). According to the most recent taxonomic reevaluation of the existing taxa, a total of 161 freshwater (including euryhaline and diadromous species) are present in Greece (Bobori and Economidis Citation2006; Economou et al. Citation2007; Kottelat and Freyhof Citation2007): Forty seven (29.2%) of them are endemic to Greece, 14 (8.7%) are shared with neighboring countries, and 28 (17.4%) are Balkan endemics (Economou et al. Citation2007). Almost 40% of these are considered endangered or threatened (Bobori et al. Citation2001) whereas for several species their taxonomic status is still unclear. Therefore, a reliable assessment of the existing taxonomy as well as a closer look into the taxonomic problems regarding some species is urgently needed. In this paper, we applied the barcoding approach to 37 species from four lake systems in north Greece in order to verify possible uncertainties in the taxonomic position and distribution of freshwater fish species, and to contribute in the creation of fish barcodes for conserving and managing threatened species populations.
Materials and methods
Study area
Fish samples were collected from three natural eutrophic lakes (Doirani, Volvi, and Mikri Prespa) and one dam-lake (Kerkini reservoir), all established in northern Greece (). Doirani, Mikri Prespa, and Kerkini reservoir are shallow (maximum depth < 8.5 m) whereas Lake Volvi is deep (maximum depth 21 m).
Fish were caught using multiple mesh gill nets (mesh sizes 8–90 mm knot to knot) and electrofishing. Sampling strategies involved analyzing three individuals per species where possible. A total of 145 individuals from 37 species were examined. Each specimen was expertly identified as an accurate representative from regional populations of each fish species and linked to a voucher specimen deposited at the School of Biology, Aristotle University of Thessaloniki. Taxonomic assignments follow Kottelat and Freyhof (Citation2007) and Froese and Pauly (Citation2010).
Analytical procedure
DNA extraction, PCR, and sequencing were performed following standard DNA barcoding methods for fish (Ward et al. Citation2005). Total DNA was extracted from frozen or ethanol-preserved muscle following classic phenol DNA extraction protocols.
Approximately, 700 bp were amplified from the 5′ region of the COI gene using in all cases the fish-specific primers described in Ward et al. (Citation2005): FishF1-TCAACCAACCACAAAGACATTGGCAC and FishR1-TAGACTTCTGGGTGGCCAAAGAATCA—with the exception of Gambusia holbrooki, where the following primers (Ward et al. Citation2005) were used: FishF2-TCGACTAATCATAAAGATATCGGCAC and FishR2-ACTTCAGGGTGACCGAAGAATCAGAA.
The 25 μl PCR mixes included 17.8 μl ultrapure water, 2.5 μl of 10 × PCR buffer, 1 μl MgCl2 (25 mM), 0.15 μl each primer (100 nmol), 0.5 μl each dNTP (10 mM), 0.625 U Taq polymerase, and 2.5 μl DNA template. The thermal regime consisted of an initial step of 2 min at 95°C followed by 35 cycles of 0.5 min at 94°C, 0.5 min at 52°C, and 1 min at 72°C, followed in turn by 10 min at 72°C, and then samples were held at 4°C.
Products were bidirectionally sequenced at Macrogen (Macrogen Inc., Gasan-dong, Seoul, South Korea). Sequences, electrophoregrams, and primer sequences are available in BOLD under project title “Barcoding Greek Freshwater Fish” and project code GRFRF. All sequences have also been deposited in GenBank (Table S1). A Kimura two-parameter (K2P) distance metric was employed for sequence comparisons (Kimura Citation1980), including genetic distance calculations and neighbor-joining analysis, using the BOLD Management and Analysis System (Ratnasingham and Hebert Citation2007).
Results and discussion
A total of 145 COI barcodes of 655 bp have been obtained for 37 species distributed among 27 genera and 10 families. Since, no stop codons (or other indels) were observed in the sequences and all amplified sequences were larger than 600 bp, the amplification of nuclear DNA sequences originating from mitochondrial DNA (NUMTs) seems not probable (Zhang and Hewitt Citation1996).
The average K2P distance of individuals within species was 0.98% compared with 4.69% for species within genera (). This is only a fivefold difference, much lower than the values observed in the comparison of congeneric species to conspecific individuals for Australian marine fish (25-fold difference; Ward et al. Citation2005) and for Canadian freshwater species (27-fold difference; Hubert et al. Citation2008). Results as regards mean values among species within families, orders, and classes are generally in agreement with those studies. When looking at the distribution of mean K2P distances among conspecific individuals and among congeneric species, a high overlap is evident with values ranging from 0 to 23.84 among conspecific individuals and from 0 to 28.44 among congeneric species ().
Table I. Summary of genetic divergences (% K2P distance) within various taxonomic levels.
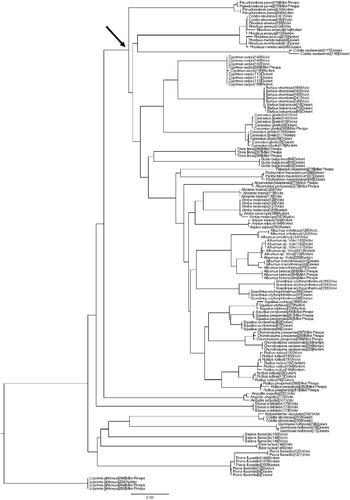
The entire K2P/neighbor-joining tree derived from the study is shown in . Almost all designated species can be differentiated based on COI barcoding. However, the placement of Cobitis species in the tree poses a serious problem; the genus seems polyphyletic since its populations are found in three different positions with genetic distances among the three groups at least 22%. Cobitis strumicae individuals from Lake Kerkini are grouped with Knipowitschia caucasica individuals (genetic distance of 0.77). C. strumicae individuals from Lake Volvi are grouped with Rhodeus amarus individuals from the same lake (with identical sequences). Finally, Cobitis vardarensis individuals from Lake Doirani formed a well-defined group, although they were the only non-Cyprinidae individuals that actually clustered within the Cyprinidae family group ().
Figure 2. Neighbor-joining tree of 145 COI sequences from the 37 freshwater fish species sampled as obtained in BOLD, using K2P distances (arrow shows the base of the Cyprinidae family clade).
If we omit this genus and recalculate mean, minimum, and maximum values, there is considerable improvement as regards overlap of values (since maximum values are greatly reduced; ) but the overall difference among mean conspecific and congeneric values is still 5.6-fold. This could be possibly attributed to the recent reevaluation of the taxomonic status for several species (Kottelat and Freyhof Citation2007) that has not been confirmed for some of them, as well as to the narrow geographic distribution of the populations used in this study.
The two Cobitis species belong to different lineages, but the actual uncorrected p genetic distances between them based on sequencing analysis of cytochrome b would not be expected to exceed 11% (Bohlen et al. Citation2006). This discrepancy could be attributed to their complicated reproduction (Choleva et al. Citation2008). Cobitis species produce all-female hybrid asexual lineages and in some cases have incorporated additional genomes through mating with parental or non-parental species. This has resulted in a variety of diploid and polyploid biotypes, sometimes resulting in tri-genomic hybrids (Bohlen and Rab Citation2001; Janko et al. Citation2005). Recent results (Janko et al. Citation2005) have shown that the ability of loaches to incorporate unrelated genomes is more common than previously thought. Although evidence until now supports the mixing of genomes only within the Cobitis genus, the possibility of mitochondrial DNA introgressive hybridization in the past from other species could not be ruled out. A phenomenon of intergeneric mitochondrial introgressive hybridization, although not so frequent, has previously been shown for Scardinius dergle that possessed a Squalius mitochondrial genome (Freyhof et al. Citation2005). Misidentification of the original Cobitis specimens should also be ruled out as a possible explanation of the obtained results, since they are clearly morphologically different from species with which they group in the tree.
It must be stressed that the Balkan fish fauna is very rich (Griffiths et al. Citation2004) and its status, as regards species taxonomy, is constantly revised. The present work helps in this direction by identifying species where more taxonomic work is needed. Thus, Barbus individuals from Lakes Doirani and Volvi have been assigned to different species (Barbus balcanicus and Barbus strumicae, respectively) based on a recent taxonomy work (Kottelat and Freyhof Citation2007). Additionally, the Rhodeus genus taxonomy is complicated (Kottelat and Freyhof Citation2007; Bryja et al. Citation2010), and two species are found in Greek territory (Rhodeus meridionalis in the Vardar System and Lake Doirani, and Rhodeus amarus in Lakes Kerkini and Volvi). However, K2P distances for both Barbus and Rhodeus genera are 0 and 0.46, respectively, not supporting such a distinction. This could be ascribed to several factors, such as erroneous taxonomy; low sister-species divergence; cases of introgressive hybridization; and some non-recorded human translocations of these populations have been done in the past. In any case, the inclusion of more specimens from other areas hosting these species will further contribute to the clarification of their taxonomic status.
On the other end of the scale, we detected deep divergences among individuals that had been assigned to single species. These are cases of conspecific populations where their divergence could warrant placement into different species. This holds for the populations of Scardinius erythrophthalmus, Perca fluviatilis and Rutilus rutilus, which showed lake-specific haplotypes with minimum distances of 3.63, 2.66, and 2.84%, respectively. These results give evidence to intraspecific divergences and support a careful reappraisal of the current taxonomy. According to Hebert et al. (Citation2004b), specimens showing >10 times the average intraspecies distance should be flagged as provisional new species. If we use the estimate of Hubert et al. (Citation2008) calculated for 190 Canadian freshwater species of 0.3% intraspecies distance, our results are close to (above or below) the 3% cut-off value. It is likely, therefore, that three undescribed species exist.
More analytically—although the most recent taxomy (Kottelat and Freyhof Citation2007) identifies the occurrence of one Rutilus species in Lakes Doirani, Kerkini and Volvi—Economidis (Citation1991) has recognized three Rutilus subspecies, one of which, R. rutilus doiranensis, was present in Lake Doirani and the Axios river basin. As regards P. fluviatilis, although autochthonous populations were present in the past, the situation is hampered by repeated restocking and movement of fish between lakes (a recorded one took place in 1996 and 1998 with fish originating from Lake Doirani transferred to both Lakes Kerkini and Volvi; Economidis et al. Citation2000). Results, however, point to a separate species status for Lake Volvi population. Finally, as regards S. erythrophthalmus, which shows the highest divergence, it is the first time some data indicate such divergence between populations and more work is (urgently) needed. However, as previously mentioned (Ward et al. Citation2009), barcoding studies do not imply a “mitochondrial DNA species concept”, but they recognize the role that molecular analyses can play in contemporary taxonomic studies.
In conclusion, the results of the present work are in support of previous analyses as regards the success of barcoding to identify fish species (Ward et al. Citation2009). Moreover, it should also be noted that our data generally support the recent taxonomic reappraisal of some fish genera such as Squalius and Alburnus proposed by Kottelat and Freyhof (Citation2007). It is of course, inevitable, due to human errors or incomplete research that problems will arise even after DNA barcoding analyses. The extension, however, of this work with other freshwater systems in Greece or the Balkans should clarify the situation.
supplementary Material
Supplementary materials available online at: www.informahealthcare.com/mdn
Supplementary Material
Download MS Excel (48 KB)Acknowledgements
The authors would like to thank G. Economidis for his help in sampling and an anonymous reviewer for constructive comments.
Declarations of interest: The Goulandris Natural History Museum-Greek Biotope/Wetland Centre and the Hellenic Ministry of Foreign Affairs are acknowledged for financially supporting part of this research.
References
- Barrett RDH, Hebert PDN. 2005. Identifying spiders through DNA barcodes. Can J Zool. 83:481–491.
- Bobori DC, Economidis PS. 2006. Freshwater fishes of Greece: Their biodiversity, fisheries and habitats. Aquat Ecosyst Health Manag. 9:407–418.
- Bobori DC, Economidis PS, Maurakis EG. 2001. Freshwater fish habitat science and management in Greece. Aquat Ecosyst Health Manag. 4:381–391.
- Bohlen J, Rab P. 2001. Species and hybrid richness in spined loaches of the genus Cobitis (Teleostei: Cobitidae), with a checklist of European forms and suggestions for conservation. J Fish Biol. 59:75–89.
- Bohlen J, Perdices A, Doadrio I, Economidis PS. 2006. Vicariance, colonisation, and fast local speciation in Asia minor and the Balkans as revealed from the phylogeny of spined loaches (Osteichthyes; Cobitidae). Mol Phylogenet Evol. 39:552–561.
- Bryja J, Smith C, Konečný A, Reichard M. 2010. Range-wide population genetic structure of the European bitterling (Rhodeus amarus) based on microsatellite and mitochondrial DNA analysis. Mol Ecol. 19:4708–4722.
- Choleva L, Apostolou A, Rab P, Janko K. 2008. Making it on their own: Sperm-dependent hybrid fishes (Cobitis) switch the sexual hosts and expand beyond the ranges of their original sperm donors. Philos Trans R Soc B: Biol Sci. 363:2911–2919.
- Costa F, Carvalho G. 2007. The Barcode of Life initiative: Synopsis and prospective societal impacts of DNA barcoding of fish. Genomics Soc Policy. 3:29–40.
- Dayrat B. 2005. Towards integrative taxonomy. Biol J Linn Soc. 85:407–415.
- . 1991. Checklist of the freshwater fish of Greece (recent status of threat and protection)Economidis PS. Athens: Bulletin of the Hellenic Society for the Protection of Nature, Special Publication.
- Economidis PS, Dimitriou E, Pagoni R, Michaloudi E, Natsis L. 2000. Introduced and translocated fish species in the inland waters of Greece. Fish Manag Ecol. 7:239–250.
- Economou AN, Giakoumi S, Vardakas L, Barbieri R, Stoumboudi M, Zogaris S. 2007. The freshwater ichthyofauna of Greece: An update based on a hydrographic basin survey. Mediterr Mar Sci. 8:91–166.
- Freyhof J, Lieckfeldt D, Pitra C, Ludwig A. 2005. Molecules and morphology: Evidence for introgression of mitochondrial DNA in Dalmatian cyprinids. Mol Phylogenet Evol. 37:347–354.
- Frézal L, Leblois R. 2008. Four years of DNA barcoding: Current advances and prospects. Infect Genet Evol. 8:727–736.
- . 2010. FishBaseFroese R, Pauly D Version (9.2010) [Online] Available at http://www.fishbase.org. (accessed 7 November 2010).
- . 2004. Balkan biodiversity: Pattern and process. In: Griffiths HI, Krystufek B, Reed JM. editors. The European hotspot. Dordrecht: Kluwer Academic Publishers.
- Hebert PDN, Gregory TR. 2005. The promise of DNA barcoding for taxonomy. Syst Biol. 54:852–859.
- Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 2004a; 101:14812–14817.
- Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM. Identification of birds through DNA barcodes. PLoS Biol. 2004b; 2:1657–1663.
- Hubert N, Hanner R, Holm E, Mandrak NE, Taylor E, Burridge M, Watkinson D, Dumont P, Curry A, Bentzen P, Zhang J, April J, Bernatchez L. 2008. Identifying Canadian freshwater fishes through DNA barcodes. PLoS One. 3:e2490.
- Janko K, Kotlik P, Culling MA, Rab P. 2005. Ice age cloning: Comparison of quaternary evolutionary histories of sexual and clonal forms of European loaches (Cobitis; Teleostei) using the analysis of mitochondrial DNA variation. Mol Ecol. 14:2991–3004.
- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 15:111–120.
- . 2007. Handbook of European freshwater fishesKottelat M, Freyhof J. Berlin: Kottelat, Cornol and Freyhof.
- Lleonart J, Taconet M, Lamboeuf M. 2006. Integrating information on marine species identification for fishery purposes. Mar Ecol Prog Ser. 316:231–238.
- Ratnasingham S, Hebert P. 2007. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol Ecol Notes. 7:355–364.
- Strauss RE, Bond CE. 1990. Taxonomic methods: Morphology. In: Schreck CB, Moyle PB. editors. Methods for fish biology. Bethesda, MD: American Fisheries Society109–140.
- Ward RD, Holmes BH. 2007. An analysis of nucleotide and amino acid variability in the barcode region of cytochrome c oxidase I (COI) in fishes. Mol Ecol Notes. 7:899–907.
- Ward R, Zemlak T, Innes B, Last P, Hebert P. 2005. DNA barcoding Australia's fish species. Philos Trans R Soc B: Biol Sci. 360:1847–1857.
- Ward RD, Costa FO, Holmes BH, Steinke D. DNA barcoding shared fish species from the North Atlantic and Australasia: Minimal divergence for most taxa but a likely two species for both Zeus faber (John dory) and Lepidopus caudatus (silver scabbardfish). Aquat Biol. 2008a; 3:71–78.
- Ward RD, Holmes BH, White WT, Last PR. DNA barcoding Australian chondrichthyans: Results and potential uses in conservation. Mar Freshw Res. 2008b; 59:57–71.
- Ward RD, Hanner R, Hebert PDN. 2009. The campaign to DNA barcode all fishes, FISHBOL. J Fish Biol. 74:329–356.
- Zemlak TS, Ward RD, Connell AD, Holmes BH, Hebert PDN. 2009. DNA barcoding reveals overlooked marine fishes. Mol Ecol Resources. 9:237–242.
- Zhang D, Hewitt M. 1996. Nuclear integrations: Challenges for mitochondrial DNA markers. Trends Ecol Evol. 11:247–251.