Abstract
Objective:
To review current available evidence for the role of renin–angiotensin system blockade in the management of atrial fibrillation.
Method:
We conducted a PubMed and Medline literature search (January 1980 through July 2011) to identify all clinical trials published in English concerning the use of angiotensin converting enzyme inhibitors or angiotensin II receptor blockers for primary and secondary prevention of atrial fibrillation. We also discussed renin–angiotensin system and its effects on cellular electrophysiology.
Conclusion:
The evidence from the current studies discussed does not provide a firm definitive indication for the use of angiotensin converting enzyme inhibitors or angiotensin II receptor blockers in the primary or secondary prevention of atrial fibrillation. Nevertheless, modest benefits were observed in patients with left ventricular dysfunction. In view of the possible benefits and the low incidence of side-effects with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, they can be given to patients with recurrent AF, specifically those with hypertension, heart failure and diabetes mellitus.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmiaCitation1. Prevalence of AF increases with ageCitation2–4. Individuals with AF have a two-fold increased mortality and five-fold increased stroke riskCitation3,Citation5. Some authors have indicated that AF could be causing those with a history of hypertension to have an even more elevated BPCitation6. Causes of AF are summarized in Citation1–5,Citation7.
Table 1. Causes of atrial fibrillation.
AF is generally thought to be caused by multiple re-entrant wavelets that propagate randomly throughout the atriaCitation8. Atrial tachycardias, which include atrial fibrillation, can cause electrical remodeling, that produce multi-circuit re-entry AFCitation9. This remodeling also acts on a cellular level resulting in decreased contractility and leading to atrial cardiomyopathy. Many other theories have been hypothesised for mechanisms contributing to development of AF. It has been suggested that high angiotensin II levels can cause atrial fibrosis, which may play a role in AFCitation10. Some studies suggest that paroxysmal AF is precipitated by premature beatsCitation11–13. Others propose some input from high vagal tone and a degree of subclinical myocarditisCitation14,Citation15. It is clear that electrical and mechanical remodeling occurs following arrhythmia that can lead to sustained AF.
The renin–angiotensin system (RAS) has been shown to play an important role in the onset of AF. RAS activation cause the electrical and structural remodeling of diseased atria by altering the balance between atrial currents, decreasing the cardiac action potential durationCitation16,Citation17 and causing fibrosis via disturbing the continuous cable-like arrangement of cardiomyocytesCitation18. Additionally, RAS activation may cause structural remodeling due to mitogen-activated protein kinase (MAPK) expression and reduction of collagenase activityCitation19. This can lead to fibrosis of the atrial myocardiaCitation20 and congestive heart failure (CHF)Citation21.
Thus lots of interest as to whether blocking RAS activity, with angiotensin converting enzyme inhibitors (ACE-I) or angiotensin II receptor blockers (ARBs), could be used to prevent electrical and structural remodeling has been generated.
The roles of renin–angiotensin system
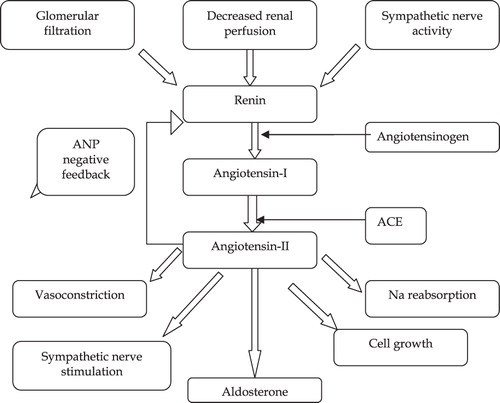
The RAS plays a vital physiological role. It promotes vascular growth and regeneration, salt retention and vasoconstrictionCitation22. Renin, a proteolytic enzyme, is secreted from the juxtaglomerular apparatus in the kidney in response to a decrease in blood pressure detected by baroreceptors in the vessels, activation of the sympathetic nervous system and when the macular densa responds to a decrease in the amount of NaCl in nephrons. Subsequently, renin is converted to angiotensin I by angiotensinogen, then broken down to angiotensin II by angiotensin converting enzyme (ACE). This then acts on angiotensin (AT1) and AT2 receptorsCitation23 (). All classic physiological effects of angiotensin II such as sodium and water retention, sympathetic activation, vasoconstriction, aldosterone and vasopressin release are mediated by the AT1 receptor. Via its AT1 receptor, angiotensin II is also involved in cell proliferation, left ventricular (LV) hypertrophy, nephrosclerosis, vascular media hypertrophy, endothelial dysfunction, neointima formation and processes leading to atherothrombosis, as well as in the modulation of various biological processes involved in development, cell differentiation, tissue repair and apoptosisCitation23,Citation24. Angiotensin II is also a powerful dipsogenCitation25 which acts on the subfornical organ in the brain to increase blood volume by stimulating the perception of thirstCitation26. The posterior pituitary gland is stimulated by the hypothalamus to secrete antidiuretic hormone (vasopressin). This acts on V2 receptors in the basolateral membrane of the distal tubules and collecting ducts in a nephron, resulting in an overall reduction in urinary outputCitation27. Furthermore, Weisinger and Cooper have suggested that body fat and obesity are also associated with increased levels of ACE and angiotensinogen, leading to an elevation in the blood pressureCitation28. The actions of the RAS are summarized in .
Aldosterone is secreted form the adrenal cortex and acts on a number of target organs, including the kidney and the transporting epithelia of bladder and colon. Aldosterone enhances sodium re-absorption from the collecting tubules, thus leading to increased blood pressureCitation29. Blockade of the aldosterone secretion has shown to restore the baroreceptor reflex, which tends to improve the variation in heart rate in heart failure patientsCitation30. Increased production of aldosterone is associated with hypertension and cardiac failureCitation31. In fact a recent study has shown that even mild plasma elevations of aldosterone concentration is associated with a higher risk of fatal cardiovascular diseaseCitation32. Spironolactone, a competitive aldosterone inhibitor, has shown benefits in the treatment of cardiovascular diseaseCitation33. It blocks aldosterone’s mineralocorticoid effects on the renal tubule. Eplerenone, a selective aldosterone blocker, is also proven to have cardiovascular benefitsCitation34. Different effects of aldosterone are summarized in .
Table 2. Actions of aldosterone.
Traditionally there are two ways of managing AF, rate or rhythm control. A recent study suggested that in a patient with AF after myocardial infarction (MI), there is nearly a two-fold excess in early mortality (0–45 days post-MI) with anti-arrhythmic drug-based rhythm control, as opposed to rate-control medicationCitation37. The AFFIRM trialCitation38 compared rate verses rhythm control for the treatment of AF. The results showed no survival advantage with either strategies, but potential drawbacks such as adverse drug effects and increased rate of hospitalization (80.1 vs. 73.0%, p < 0.001) in the rhythm group.
Interestingly, acute angiotensin II infusion has been shown to increase intra-atrial pressure and shorten both the atrial action potential duration and refractory periodCitation39, though this has not been the case in all studiesCitation40. In addition to influencing electrical remodeling, angiotensin II may augment the structural remodeling of AF through the endorsement of apoptosis and fibrosisCitation41,Citation42. Likewise, aldosterone may contribute to structural remodeling associated with AF by enhancing atrial fibrosisCitation43. Thus, theoretically, RAS inhibition should help to prevent atrial remodeling and AF development.
Effects RAS on cellular electrophysiology
Ion channels modulations secondary to dilatation of atria may play an important role in AF and its maintenanceCitation44,Citation45. Angiotensin II stimulation increase T-type calcium channel (ICa,T)Citation46 and L-type calcium channel (ICa,L) through protein kinase C (PKC) dependent pathwaysCitation47 and ICa,T blockade is reported to prevent AF-substrate developmentCitation48. Therefore, it is tempting to conclude that inhibition RAS might be beneficial by preventing angiotensin II-mediated ICa,T increases. Mibefradil, a drug with strong ICa,T blocking properties, prevents atrial tachycardia induced AF promoting electrophysiological remodellingCitation49 but no similar benefits observed with ACE-ICitation50.
Transcriptional transient outward (Ito) down regulation is a hallmark of AF-induced electrical remodellingCitation51. The AT1-receptor regulates Ito cell-surface expressionCitation52 and also contributes to intracellular Ito reduction. Furthermore, angiotensin II increased the rapid delayed-rectifier current (IKr) in guinea pig ventricular myocytes, while the slow component (IKs) is decreasedCitation53.
In vitro studies showed different effects of ARBs. Losartan blocks currents carried by HERG-related gene corresponding to IKr, KvLQT1, IKs and hKv1.5 subunitsCitation54. Candesartan, eprosartan, and irbesartan inhibit currents carried by hKv1.5, KvLQT1 + mink, and rKv4.3 subunitsCitation55. Whereas, irbesartan blocks rKv4.3 and hKv1.5 at therapeutic concentrations, blockade of HERG and KvLQT1 + minK currents required supra-therapeutic concentrationsCitation56,Citation57. ARBs may be beneficial in acute atrial remodeling by directly inhibiting repolarizing K+ currents. Some ARBs inhibit Kv1.5 currents, which stimulate the atrially expressed sustained outward potassium current (Kur)Citation58,Citation59, an emerging target for AF therapyCitation59. Overall, these observations imply that the renin–angiotensin system could play an important role if the cellular electrophysiology of AF via ion-channel modulation, impulse propagation and facilitate re-entry and inhibiting RAS may have possible advantages.
Primary prevention of AF using ACE-I/ARB
Left ventricular dysfunction
The randomized TRACE trialCitation60 used trandolapril in patient with LV dysfunction and sinus rhythm following an acute MI. The results showed a significant reduction (2.8 vs. 5.3%) in the incidence of AF in the 2–4 years follow-up period in the trandolapril group. There was a similar picture in the retrospective analysis of the SOLVD randomized trialCitation61 and Val-HeFTCitation62, both studies recruited patient with chronic LV dysfunction. The SOLVD trial showed that only 5.4% of those treated with enalapril developed AF, by the mean follow-up point of 2.9 years, compared to 24% developing new onset AF in the placebo group.
The CHARM study also supported the above trials conclusion. It enlisted over 7000 patients, the majority of which did not have AF at baseline. Patient with both diastolic and systolic dysfunction were recruited. At an average follow-up period of 37.7 months, an episode of AF had been reported in 5.55% in the candesartan group compared to 6.74% in the placebo group (p = 0.048)Citation63.
A meta-analysis of 11 trials by Healey in 2005Citation64, summarized that both ARB and ACE-I are effective in the primary prevention of AF with a relative risk reduction [RRR] of 0.28 (95% CI 0.15–0.40), but only in patients with systolic LV dysfunction or hypertrophy. These clinical trials are summarized in .
Table 3. Summary of clinical trials.
Hypertension
L'Allier et al.Citation65 performed a retrospective study on a medical and pharmacy claims database. They compared the incidence of AF in 10,926 hypertensive patients who were prescribed an ACE-I vs. a calcium channel blocker (CCB). At an average follow-up of 4.5 years, there was a significantly reduced incidence of AF in the ACE-I group (17.9 per 1000 patient-years) than the CCB group (18.9 per 1000 patient-years). The hazard ratio for the patient treated with ACE-I was 0.85 (95% CI 0.74–0.97). These results were supported by a post hoc analysis from the LIFE trialCitation66,Citation67. Hypertensive patients with electrocardiograph (ECG) evidence of LV hypertrophy and a mean BP of 177/97 mmHg were randomly given losartan or atenolol. New onset AF occurred in 150 patients randomized to losartan and 221 patients in the atenolol arm. Relative risk reduction using losartan was 0.67 (95% CI 0.55–0.83, p < 0.001).
Heckbert and colleaguesCitation68 observed hypertensive patients without heart failure to determine if treatment with ACE-I and/or ARB’s, as opposed to diuretics, is associated with fewer occurrences of AF. The results showed that single users of ACE-I/ARB produced fewer episodes than single users of a diuretic (adjusted OR 0.63, 95% CI 0.44–0.91).
In contrast, the CAPPP trialCitation69 randomized 10985 hypertensive patients to either a Captopril or a beta blocker and diuretic group. The results showed no difference in new onset AF between the two groups. The VALUE trialCitation70 randomized 15,245 patients with hypertension to received either valsartan or amlodipine, there were no difference in the occurrence of AF. The STOP Hypertension-2Citation71 studied new onset AF in a population of 6614 hypertensive patients. The patients were randomly assigned to CCB, ACE-I or beta blocker and/or diuretics. The study found no difference in new onset AF between all 3 groups. The J-RHYTHM IICitation72 studied 318 hypertensive patients who were randomly assigned candesartan or amlodipine. There was no significant difference in AF frequency between the two. Amlodipine had a greater effect on lowering the blood pressure.
Over all, the hypertensive trials gave inconsistent results. Possible reasons for this includes the fact that none of the final 3 trials were placebo controlled and patients in CAPP and STOP Hypertension-2 had less severe hemodynamic abnormalities as determined from echocardiography parameters. In the VALUE study there were significantly higher initial blood pressures in the valsartan group. The CAPPP trial administered captopril as a once or twice daily dose, however, it is now well recognized that captopril has a short duration of action, implying the possibility of an inadequate dose.
In the Study on Cognition and Prognosis in the Elderly (SCOPE), elderly hypertensive patients were investigated to determine the effect of candesartan on reducing the risk of cerebrovascular events. It demonstrated a significant reduction in nonfatal strokes in those treated with an candesartan compared with a placeboCitation73. A possible theory is reduction in AF in these patients could have contributed to reducing nonfatal strokes, however there is no evidence to support this.
Post-myocardial infarction and coronary artery bypass graft
There have been two studies that observed the risk of AF following an MI in patients on ACE-I. The GISSI-3 studyCitation74 looked at the effects of lisinopril and/or transdermal glycerol nitrate on mortality and LV function after acute MI. There was no significant reduction in AF with 6 weeks of treatment. The TRACE studyCitation60 looked at patients given trandolapril 3–7 days following MI. Unlike the previous trial, TRACE study showed a 48% relative risk reduction in incidence of AF. The TRACE study enrolled only patients with LV dysfunction, whereas the GISSI-3 had mainly patients with no evidence of heart failure. This, as shown in the previous section on LV hypertrophy, may hold an important key to the difference in results.
A further multicenter study analyzed 4657 patients who were undergoing coronary artery bypass graft (CABG). Those treated with ACE-I had significantly less postoperative AF occurrences and furthermore in those who had post-operative withdrawal of ACE-I had increased AF occurrenceCitation75.
Secondary Prevention
Madrid et al.Citation76 analyzed 154 patients who had been electrically cardioverted for persistent AF, were then randomly prescribed amiodarone alone or in combination with irbesartan. The results showed those additionally treated with irbesartan had a significantly higher probability of remaining in sinus rhythm at 2 months (84.75% compared to 63.17%, p = 0.008), and at a median follow-up of 254 days (79.52% to 55.91%, p = 0.007).
A Similar trial by Ueng et al.Citation77 randomized patients to amiodarone with or without enalapril 4 weeks prior to electric cardioversion. Those treated with the ACE-I had a lower rate of immediate recurrence (4.3% vs. 14.7%, p = 0.067) and a higher probability of remaining in sinus rhythm at a median follow-up of 270 days (74.3% vs. 5703%, p = 0.021).
A prospective randomized trialCitation78 compared the incidence of AF in 177 patient with lone paroxysmal AF. Patients were randomized to amiodarone alone, amiodarone plus losartan or amiodarone plus perindopril. The data showed a significant reduction in AF recurrence in both the losartan (p = 0.006) and perindopril (p = 0.04) groups compared to the amiodarone alone group. At the 2 year follow-up, the left atrial size in those randomized to losartan or perindopril was significantly smaller. In summary, these results demonstrate a significant reduction in AF recurrence when amiodarone is combined with an ACE-I or ARB, rather than given alone.
Van Noord et al.Citation79, studied 107 consecutive patients undergoing electrical cardioversion. Those who had ACE-I started before cardioversion showed significantly greater acute success of the treatment (95% CI 1.3–26.1, p > 0.05), although it did not help to reduce recurrence. In contrast another studyCitation80, suggested that the use of ACE-I/ARBs or diuretics before ablation resulted in a significantly lower rate of AF recurrence post treatment (p = 0.04; RR 0.55; 95% CI 0.31–0.97 and p < 0.01; RR 0.28; 95% CI 0.13–0.63, respectively). However, a retrospective analysis by Richter et al.Citation81 showed conflicting results. They looked at recurrence following AF ablation and discovered no improvement to recurrence rates with routine ACE-I, ARB and statin use.
The CAPRAF trialCitation82 randomized 124 patients with AF, prior to electrical cardioversion, to receive placebo or candesartan. Candesartan was associated with improved cardioversion outcome, nonetheless it decreased the fibrillatory rate, but this effect was restricted to patients with high baseline fibrillatory rates. FogariCitation83 studied the recurrence rate of AF in hypertensive patients with well-controlled type II diabetes mellitus. One arm received valsartan and amlodipine while the other was treated with atenolol and amlodipine. Both groups showed a statistically significant decrease in blood pressure with no difference between the two groups. Over a year’s follow-up, the valsartan/amlodipine combined group showed a statistically lower recurrence rate (20.3%) than the atenolol and amlodipine combination (34.1%). The group also investigated the recurrence of AF in 369 patients with mild hypertension. All patients were in sinus rhythm at but had at least two recorded episodes of AF in the preceding 6 monthsCitation84. They were randomized to receive a year of amlodipine, valsartan or ramipril. All three groups showed a significant reduction in the BP (p < 0.001). There was a significant decrease in AF recurrence in the valsartan (16.1%) and ramipril (27.9%) groups when compared to amlodipine (47.4%). Fogari’s two studiesCitation83,Citation84 support the theory that an ARB or an ACE-I leads to a lower rate of recurrence. It also suggests that an ARB maybe more effective.
A retrospective analysis of the AFFIRM study demonstrated no difference in recurrence rates of AF between the 421 patients treated with ARB/ACE-ICitation38, and the 732 patients without. The study, however, showed that those with congestive heart failure or impaired LV dysfunction did have a lower risk of recurrence of AF if treated with either an ARB or ACE-I.
The GISSI-AFCitation85 study looked at 1442 patients with recurrent AF. They were randomized to a placebo or valsartan group. At 1 year, there was no recurrence difference in the two groups. There was a slight but non-significant beneficial effect in those with LV dysfunction or HF. A possible reason for the lack of effect in the valsartan group could be due to optimal background therapy.
The active-I trialCitation86 looked at a group of 9016 subjects with AF who were randomized to placebo or irbesartan. The patients receiving irbesartan showed a significant decrease in hospitalization from HF (HR 0.86, 95% CI 0.76–0.98, p = 0.018), and also a 40% reduction in stroke.
Discussion
The evidence from the current studies discussed does not provide a firm definitive indication for the use of ARB/ACE-I to prevent new-onset AF, recurrence of AF or treat established AF. Inconsistent findings between different studies are likely to be related to variation in the number of individuals in each trial and trial methodology. Modest benefits in terms of reduction in AF onset were seen in those with LV dysfunctionCitation60–63. A couple of meta-analyses estimated the magnitude of RAS blocking on AF and aimed at identifying patient subgroups most likely to benefitCitation64,Citation84 revealed that patients with LV dysfunction or LVH tend to benefit the most.
Conclusion
In view of the possible benefits and general low incidence of side-effects, ACE-I and ARB can be used in patients with recurrent AF and those at risk of AF, specifically those with hypertension, heart failure and diabetes mellitus. Future randomized control studies should aim to clarify the benefits from the use of ARB/ACE-I and determine a definitive indication for their use.
Transparency
Declaration of funding
The author was supported by the Manchester Wellcome Trust Clinical Research Facility and the Lipid Disease Fund.
Declaration of financial/other relationships
The author declares no conflict of interest and has received no payment in preparation of this manuscript.
Acknowledgment
The peer reviewers on this manuscript have disclosed any relevant financial relationships.
References
- Chen L. Epidemiology of atrial fibrillation: a current perspective. Heart Rhythm. 2007;4:S1-6
- Fuster V, Rydén LE, Cannom DS, et al. American College of Cardiology; American Heart Association; European Society of Cardiology. [ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation – executive summary. Eur Heart J 2006;27:1979-2030
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5
- Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 1995;98:476-84
- Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-56
- Masood SO, Wasmund SL, Akoum NW, et al. The effects of rate and rhythm control on blood pressure and antihypertensive drug usage in patients with atrial fibrillation and hypertension enrolled in the AFFIRM trial. J Cardiovasc Electrophysiol (Epub ahead of print) 2010;21:1094-8
- Lubitz SA, Yi BA, Ellinor PT. Genetics of atrial fibrillation. Heart Fail Clin 2010;6:239-47
- Moe GK, Abildskov AJ. Atrial fibrillation as a self-sustained arrhythmia independent of focal discharge. Am J Cardiol 1959;77:10-23A
- Khairy P, Nattel S. New insights into the mechanism and management of atrial fibrillation. CMAJ 2002;167:1012-20
- McEwan PE, Gray GA, Sherry L, et al. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation 1998;98:2765-73
- Kolb C, Nürnburger S, Ndrepepa G, et al. Modes of initiation of paroxysmal atrial fibrillation from analysis of spontaneously occurring episodes using a 12-lead Holter monitoring system. Am J Cardiol 2001;88:853-7
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-66
- Tsai CF, Tai CT, Hsieh MH, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation 2000;102:67-74
- Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997;96:1180-4
- Fuster V, Rydén LE, Asinger RW, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation); North American Society of Pacing and Electrophysiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology, American Heart Association task force on practice guidelines and the European Society of Cardiology committee for practice guidelines and policy conference. Circulation 2001;104:2118-50
- Nattel S, Maquy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 2007;87:425-56
- Yue L, Feng J, Gaspo R, et al. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 1997;81:512-25
- van der Velden HM, Ausma J, Rook MB, et al. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc Res 2000;46:476-86
- Novo G, Guttilla D, Fazio G. The role of the renin-angiotensin system in atrial fibrillation and the therapeutic effects of ACE-Is and ARBS. Br J Clin Pharmacol 2008;66:345-51
- Hanatani A, Yoshiyama M, Kim S, et al. Inhibition by angiotensin II type 1 receptor antagonist of cardiac phenotypic modulation after myocardial infarction. J Mol Cell Cardiol 1995;27:1905-14
- Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res 1993;27:341-8
- Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol 2002;89:3A-9A
- Rang H, Dale MM, Ritter J, Moore P. Pharmacology. 5. Edinburgh: Churchill Livingstone, 2003
- Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 2003;12:70-88
- Fitzsimons JT. The Physiology of Thirst and Sodium Appetite: Cambridge: Cambridge University Press, 1979
- McKinley MJ, Johnson AK. The Physiological Regulation of Thirst and Fluid Intake. News Physiol Sci 2004;19:1-6
- Thornton SN. Thirst and hydration: physiology and consequences of dysfunction. Physiol Behav 2010;100:15-21
- Cooper R, McFarlane-Anderson N, et al. ACE, angiotensinogen and obesity: a potential pathway leading to hypertension. J Hum Hypertens 1997;11:107-11
- Booth R, Johnson JP, Stockand JD. Aldosterone. Adv Physiol Educ 2002;26:8-20
- Ovaert P, Elliott J, Bernay F, et al. Aldosterone receptor antagonists – how cardiovascular actions may explain their beneficial effects in heart failure. J Vet Pharmacol Ther 2010;33:109-17
- Weber KT. Heart-hitting tales of salt and destruction. J Lab Clin Med 2000;136:7-13
- Tomaschitz A, Pilz S, Ritz E, et al. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J 2010;31:1237-47
- Pitt B, Zannad F, Remme WJ, et al; for the Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709-17
- Pitt B, Remme W, Zannad F, et al. for the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-21
- Gekle M, Grossmann C. Actions of aldosterone in the cardiovascular system: the good, the bad, and the ugly? Pflugers Arch 2009;458:231-46
- Vinson GP, Coghlan JP. Expanding view of aldosterone action, with an emphasis on rapid action. Clin Exp Pharmacol Physiol 2010;37:410-16
- Nilsson KR, Al-Khatib SM, Zhou Y, et al. Atrial fibrillation management strategies and early mortality after myocardial infarction: results from the Valsartan in Acute Myocardial Infarction (VALIANT) Trial. Heart 2010;96:838-42
- Olshansky B, Rosenfeld LE, Warner AL, et al. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol 2004;43:1201-8
- Nakashima H, Kumagai K, Urata H, et al. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation 2000;101:2612-17
- Kistler PM, Davidson NC, Sanders P, et al. Absence of acute effects of angiotensin II on atrial electrophysiology in humans. J Am Coll Cardiol 2005;45:154-6
- Li D, Shinagawa K, Pang L, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation 2001;104:2608-14
- Cardin S, Li D, Thorin-Trescases N, et al. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res 2003;60:315-25
- Milliez P, Deangelis N, Rucker-Martin C, et al. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J 2005;26:2193-9
- Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J 2006;27:512-18
- Schotten U, Neuberger HR, Allessie MA. The role of atrial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol 2003;82:151-62
- Ferron L, Capuano V, Ruchon Y, et al. Angiotensin II signaling pathways mediate expression of cardiac T-type calcium channels. Circ Res 2003;93:1241-8
- De Mello WC, Monterrubio J. Intracellular and extracellular angiotensin II enhance the L-type calcium current in the failing heart. Hypertension 2004;44:360-4
- Fareh S, Bénardeau A, Thibault B, Nattel S. The T-type Ca(2+) channel blocker mibefradil prevents the development of a substrate for atrial fibrillation by tachycardia-induced atrial remodeling in dogs. Circulation 1999;100:2191-7
- De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension 1998;32:976-82
- Shinagawa K, Mitamura H, Ogawa S, Nattel S. Effects of inhibiting Na(+)/H(+)-exchange or angiotensin converting enzyme on atrial tachycardia-induced remodeling. Cardiovasc Res 2002;54:438-46
- Bosch RF, Scherer CR, Rüb N, et al. Molecular mechanisms of early electrical remodeling: transcriptional downregulation of ion channel subunits reduces I(Ca,L) and I(to) in rapid atrial pacing in rabbits. J Am Coll Cardiol 2003;41:858-69
- Doronin SV, Potapova IA, Lu Z, Cohen IS. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. J Biol Chem 2004;46:48231-7
- Daleau P, Turgeon J. Angiotensin II modulates the delayed rectifier potassium current of guinea pig ventricular myocytes. Pflugers Arch 1994;427:553-5
- Caballero R, Delpón E, Valenzuela C, et al. Losartan and its metabolite E3174 modify cardiac delayed rectifier K(+) currents. Circulation 2000;101:1199-205
- Delpón E, Caballero R, Gómez R, et al. Angiotensin II, angiotensin II antagonists and spironolactone and their modulation of cardiac repolarization. Trends Pharmacol Sci 2005;26:155-61
- Caballero R, Delpón E, Valenzuela C, et al. Direct effects of candesartan and eprosartan on human cloned potassium channels involved in cardiac repolarization. Mol Pharmacol 2001;59:825-36
- Moreno I, Caballero R, González T, et al. Effects of irbesartan on cloned potassium channels involved in human cardiac repolarization. J Pharmacol Exp Ther 2003;304:862-73
- Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res 1993;73:1061-76
- Feng J, Wible B, Li GR, et al. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res 1997;80:572-9
- Pedersen O, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation 1999;100:376-80
- Vermes E, Tardif JC, Bourassa MG, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction. Circulation. 2003;107:2926-31
- Maggioni A, Latini R, Carson PE, et al. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am Heart J 2005;149:548-57
- Ducharme A, Swedberg K, Pfeffer MA, et al. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J 2006;152:86-92
- Healey JS, Baranchuk A, Crystal E, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol 2005;45:1832-9
- L'Allier PL, Ducharme A, Keller PF, et al. Angiotensin-converting enzyme inhibition in hypertensive patients is associated with a reduction in the occurrence of atrial fibrillation. J Am Coll Cardiol 2004;44:159-64
- Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995-1003
- Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. Am Coll Cardiol 2005;45:712-19
- Heckbert SR, Wiggins KL, Glazer N, et al. Antihypertensive treatment with ACE inhibitors or beta-blockers and risk of incident atrial fibrillation in a general hypertensive population. Am J Hypertens 2009;22:538-44
- Hansson L, Lindholm LH, Niskanen L, et al. For the Captopril Prevention Project study group. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999;353:611-16
- Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363:2022-31
- Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999;354:1751-6
- Yamashita T, Inoue H, Okumura K, et al. Randomized trial of angiotensin II-receptor blocker vs. dihydropiridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension (J-RHYTHM II Study). Europace 2011;13:473-9.
- Lithell H, Hansson L, Skoog I, et al.; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003;21:875-86
- Pizzetti F, Turazza FM, Franzosi MG, et al. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction the GISSI-3 data. Heart 2001;86:527-32
- Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004;291:1720-9
- Madrid A, Bueno MG, Rebollo JM, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation 2002;106:331-6
- Ueng K, Tsai TP, Yu WC, et al. Use of enalapril to facilitate sinus rhythm maintenance after external cardioversion of long-standing persistent atrial fibrillation. Results of a prospective and controlled study. Eur Heart J 2003;24:2090-8
- Yin Y, Dalal D, Liu Z, et al. Prospective randomized study comparing amiodarone vs. amiodarone plus losartan vs. amiodarone plus perindopril for the prevention of atrial fibrillation recurrence in patients with lone paroxysmal atrial fibrillation. Eur Heart J 2006;27:1841-6
- Van Noord T, Crijns HJ, van den Berg MP, et al. Pretreatment with ACE inhibitors improves acute outcome of electrical cardioversion in patients with persistent atrial fibrillation. BMC Cardiovasc Disord 2005;5:3
- Anné W, Willems R, Van der Merwe N, et al. Atrial fibrillation after radiofrequency ablation of atrial flutter: preventive effect of angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and diuretics. Heart 2004;90:1025-30
- Richter B, Derntl M, Marx M, et al. Therapy with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and statins: no effect on ablation outcome after ablation of atrial fibrillation. Am Heart J 2007;153:113-19
- Bollmann A, Tveit A, Husser D, et al. Fibrillatory rate response to candesartan in persistent atrial fibrillation. Europace 2008;10:1138-44
- Fogari R, Zoppi A, Mugellini A, et al. Comparative evaluation of effect of valsartan/amlodipine and atenolol/amlodipine combinations on atrial fibrillation recurrence in hypertensive patients with type 2 diabetes mellitus. J Cardiovasc Pharmacol 2008;51:217-22
- Fogari R, Derosa G, Ferrari I, et al. Effect of valsartan and ramipril on atrial fibrillation recurrence and P-wave dispersion in hypertensive patients with recurrent symptomatic lone atrial fibrillation. Am J Hypertens 2008;21:1034-9
- Disertori M, Latini R, Barlera S, et al. GISSI-AF Investigators, Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med 2009;360:1606-17
- Yusuf S, Healey JS, Pogue J, et al; ACTIVE I Investigators. Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011;364:928-38
- Anand K, Mooss AN, Hee TT, Mohiuddin SM. Meta-analysis: inhibition of renin-angiotensin system prevents new-onset atrial fibrillation. Am Heart J 2006;152: 217-22