Abstract
Aims:
Blood pressure (BP) reduction in hypertensive patients is more difficult to achieve in the elderly or in the presence of comorbidities. We aimed to investigate the efficacy of the single-pill combination (SPC) aliskiren/amlodipine in hypertensive elderly patients, patients with high body mass index (BMI), with at least one metabolic risk factor, and/or type 2 diabetes mellitus (DM).
Methods:
In an open-label non-randomized study, patients not adequately controlled by previous treatment with the SPC olmesarten 40/amlodipine 10 (phase 1) were switched to the SPC aliskiren 300/amlodipine 10 (phase 2). The present post-hoc analysis investigated BP reduction in phase 2 in the named subgroups. The EudraCT identifier was 2009-016693-33, ClinicalTrials.gov identifier NCT01113047.
Results:
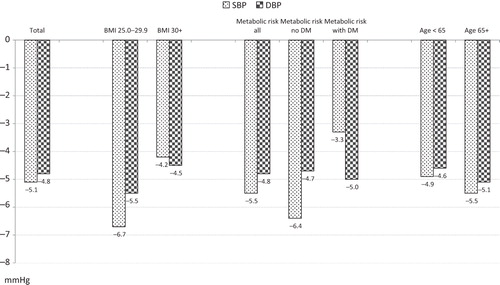
Of the 187 patients not adequately controlled in phase 1 and thus treated with the SPC aliskiren 300/amlodipine 10 in phase 2, 69 were of advanced age (≥65 years), 74 or 89 were overweight or obese (BMI 25.0–29.9 kg/m2 or ≥30 kg/m2, respectively), 91 had metabolic risk factors (without DM) and 41 had DM. At the beginning of phase 2, depending on the subgroup, baseline SBP was 168–169 mmHg and DBP 103–104 mmHg. After 4 weeks of treatment with aliskiren 300/amlodipine 10, SBP/DBP was lowered by −5.1/−4.8 mmHg in the total cohort, by −5.5/−5.1 mmHg in elderly patients, by −6.7/−5.5 in overweight and by −4.2/−4.5 mmHg in obese patients, by −6.4/−4.7 mmHg in patients with metabolic risk factors without DM, and by −3.3/−5.0 mmHg in DM patients. Limitations include low sample size, limited treatment duration and the fact that the post-hoc defined groups were not mutually exclusive.
Conclusions:
In this study reflecting clinical practice, the aliskiren/amlodipine combination achieved effective BP reduction in elderly patients or with metabolic comorbidities, including DM that might be more difficult to treat. This consistent BP lowering pattern facilitates everyday care of patients who receive aliskiren/amlodipine.
Introduction
Hypertension is an established major risk factor for an array of cardiovascular complications such as myocardial infarction or stroke, and is associated with a substantial increase in risk of premature deathCitation1. The great majority of hypertensive patients require drug treatment to control their condition, usually in the form of combinations with drugs of different mode of action and synergistic efficacyCitation2–5. Current guidelines advise the use of combination therapy not only in second-line, but also in first-line therapyCitation5–7. In addition, the ESH/ESC Guidelines recommend that in patients at higher risk, goal BP should be achieved promptly, which supports initial combination therapy and quicker adjustment of doses. Common clinical variables that influence prognosis should be used to stratify patient risk, including among others, higher age, abdominal obesity, diabetes mellitus (DM) or other metabolic risk factors.
The dihydropyridine calcium channel blocker (CCB) amlodipine, administered at doses of 5–10 mg once daily, is a direct peripheral arterial vasodilatator that reduces peripheral vascular resistance, and may reduce left ventricular hypertrophyCitation8–10. The basis for approval of the single-pill combination (SPC) olmesartan/amlodipine (Sevikar), was formed by a factorial dose-finding study in patients with mild-to-moderate hypertensionCitation11, a long-term safety studyCitation12 and two randomized, double-blind studies in patients with moderate-to-severe hypertension who were not adequately treated on one component aloneCitation13,Citation14.
The direct renin inhibitor aliskiren inhibits the renin–angiotensin–aldosterone system at its rate-limiting step. Aliskiren was tested in patients with essential hypertension in a large development program as monotherapy or in double and triple combination with other classes of anti-hypertensive drugsCitation15,Citation16. A number of studies with aliskiren investigated the drug in special patient groups (elderly, obese, components of the metabolic syndrome, see ).
Table 1. Overview of studies with aliskiren in obese, elderly, or metabolic impaired patients.
Aliskiren and amlodipine have similarly long half-lives (aliskiren 40 h and amlodipine 30–50 h), which allows constant BP reduction throughout the entire dosing intervalCitation17 and beyondCitation18–20. Besides complimentary mechanisms of action, aliskiren is capable to compensatorily down-regulate the rise in plasma renin activity following the addition of amlodipineCitation21. The fixed combination of the two drugs (Rasilamlo) was investigated in a factorial studyCitation22, a long-term studyCitation23 and in add-on studies in patients not adequately responding to the monotherapyCitation24,Citation25, which led to the approval of the SPC aliskiren/amlodipineCitation26.
In the present post-hoc analysis of a previously published prospective non-randomized studyCitation27 we aimed to investigate the BP lowering effect of the SPC aliskiren/amlodipine in special patient groups with hypertension that typically present in everyday practice and may pose a particular challenge to treating physicians owing to comorbidity, comedication or complexity of the disease: patients of advanced age, high body mass index (BMI) (overweight, obesity), with metabolic risk factors and/or with DM.
Patients and methods
Study design
The AWESOME study (Aliskiren in Combination With Amlodipine in Hypertensive Patients Not Responding to Angiotensin Receptor Blocker Plus Amlodipine) was performed between May and October 2010 as a multicenter, open-label, non-randomized single-arm study in 38 centers in Germany. The study was approved by the ethics committee of the State Chamber of Physicians in Saxony in Dresden and the responsible health authority (BfArM, Federal Institute for Drugs and Medical Devices). All patients provided written informed consent prior to inclusion in the study. The study was conducted according to the ethical principles of the Declaration of Helsinki. It was registered in the ClinicalTrials.gov database under NCT01113047 and in EudraCT under 2009-016693-33.
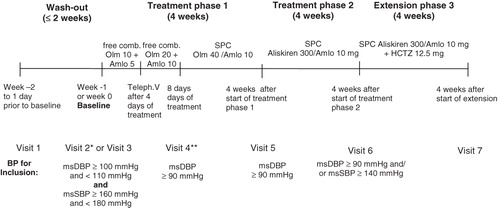
The study consisted of three phases, as shown in : after wash out, patients with mean sitting diastolic blood pressure (msDBP) 100–109 mmHg and mean sitting systolic blood pressure (msSBP) 160–179 mmHg at visit 3 (baseline) were included into a 4-week treatment phase 1 with forced titration to the SPC olmesartan 40 mg/amlodipine 10 mg; in non-responders (msDBP at trough ≥90 mmHg) this was followed by a subsequent 4-week treatment phase 2 with the SPC aliskiren 300 mg/amlodipine 10 mg once daily. This period was followed in non-responders (msDBP ≥90 mmHg and/or msSBP ≥140 mmHg at trough) by an optional 4-week extension with the SPC aliskiren 300 mg/amlodipine 10 mg/HCT 12.5 mg once daily. The aliskiren/amlodipine and aliskiren/amlodipine/HCT combinations were investigational formulations manufactured by Novartis for this study, whereas the olmesartan/amlodipine film tablets used were marketed products. The first 60 patients who were eligible and agreed to participate were included in the extension (results reported previously)Citation27. Study drugs were to be taken orally in the morning. Compliance was assessed by pill count.
Figure 1. Study design. *Visit 2 was performed only in patients with previous antihypertensive therapy. **Patients with an office mean sitting DBP<90 mmHg at an unscheduled visit after visit 4 or at visit 5 were discontinued from the study. Dotted box highlights study phase from which data were analyzed for this paper.
Patients
Male and female patients aged ≥18 years with uncomplicated moderate essential hypertension as defined above were eligible for participation. Patients were excluded from the core study, if they presented with DBP ≥110 mmHg or SBP ≥180 mmHg at any time between visit 1 and baseline, or if they could not discontinue all antihypertensive medication safely for a period of up to 2 weeks.
Further important exclusion criteria comprised: controlled BP levels on current antihypertensive medication (DBP <90 mmHg and SBP <140 mmHg) at visit 1; known hypertensive retinopathy; heart failure in NYHA II–IV; secondary hypertension; refractory angina pectoris; significant hepatic or renal disease; DM [type 1 or poorly controlled type 2, defined as a fasting blood glucose >200 mg/dL (>11.1 mmol/L)]; second- or third-degree heart block without a pacemaker; significant arrhythmia or valvular heart disease; history of transient ischemic attack, stroke, hypertensive encephalopathy, myocardial infarction; contraindications for the study medication, and use of concomitant medications known to significantly affect the metabolism of the study drug or BP, pregnant or breast feeding women. Main exclusion criteria for the extension study were premature discontinuation during the core study, hypersensitivity or contraindications to diuretics, in particular to HCT.
Efficacy and safety parameters
The primary efficacy parameter of this trial was the change in trough msDBP between visit 5 (start of treatment phase 2) and visit 6 (end of treatment phase 2), and in addition further BP parameters were assessed (including changes in SBP, responder rates, etc.) as well as safety and tolerability. BP was recorded using a calibrated standard sphygmomanometer and appropriate size cuff, in accordance with the 2005 AHA Committee Report on blood pressure measurementCitation28. BP values were measured three times at each visit, and the mean of all three sitting measurements recorded.
Patient aged ≥65 years were defined as elderly. The diagnosis DM in these hypertensive patients was according to physician’s individual assessment. Overweight was defined as BMI 25.0–29.9 kg/m2, obesity as BMI ≥30 kg/m2. The condition ‘at least one metabolic risk factor’ was present, if serum glucose was ≥5.56 mmol/l, LDL cholesterol ≥4.16 mmol/l, or triglycerides were ≥2.28 mmol/l.
Safety and tolerability
The safety information included frequency of adverse events (AE), results of physical examinations, data on body weight, and laboratory evaluations. Vital signs were assessed as part of the efficacy evaluations. All laboratory samples, obtained in a fasting state, were sent to a central laboratory for analysis.
Statistical analysis
The safety population of the respective phase comprised the sample of all patients who took at least one dose of the study medication, the intention-to-treat population (ITT) of all patients from the safety population in phase 2 who had at least one evaluation of the primary efficacy parameter after visit 5 (baseline phase 2).
The present post-hoc analysis was performed in subgroups of ITT patients with certain diagnoses as provided by demographic criteria (elderly patients), physician diagnoses (DM) in the context of clinical and laboratory criteria (metabolic risk factors including obesity). Patients suitable for several subgroups were analyzed in all these groups. The main analysis was performed descriptively on the mean change in trough SBP/DBP between visit 5 and visit 6 (treatment phase 2). The interpretation of all secondary parameters was explorative.
Results
Patient flow
A total of 439 patients were screened of which 97 were not eligible (e.g., BP criterion not fulfilled) and 342 patients were enrolled in phase 1. A total of 187 patients were switched to aliskiren 300 mg/amlodipine 10 mg at the beginning of phase 2. In that phase, 74 patients were overweight, 89 were obese, 69 were aged 65 years and above (elderly), and 132 had at least one metabolic risk factor (91 without DM and 41 with DM).
Baseline characteristics in the total cohort and in the subgroups are shown in . With the exception of two patients, all were Caucasians, mean age overall was 60.5 years, and 58.3% of patients were male. Mean age was higher in the group of patients with DM (64 years), while the gender distribution did not vary much across subgroups.
Table 2. Baseline characteristics in all 3 phases, including subgroups in phase 2.
Blood pressure
SBP and DBP at the beginning of the treatment phases are displayed in , bottom. Mean baseline BP at the beginning of phase 2 in the total patient cohort was 168/104 mmHg, and it was nearly identical in the various subgroups. SBP and DBP reduction at end of phase 2 was −5.1/−4.8 mmHg in the total cohort, −5.5/−5.1 in the elderly, −6.7/−5.5 in overweight patients, −4.2/−4.5 in obese patients, and −5.5/−4.8 mmHg in metabolic risk patients (−6.4/−4.7 without DM, −3.3/−5.0 with DM; ).
Tolerability and safety
Overall, 19 patients (10.1%) in phase 2 experienced at least one AE. No deaths or serious adverse events occurred. The most frequently observed AEs were ‘peripheral edema’ in seven (3.7%) patients, ‘edema’ in three (1.6%) patients and ‘eczema’ in one (0.5%) patient. All other AEs occurred only in one or two patients at maximum. Changes in laboratory values generally were infrequent, in accordance with the known profiles of the drugs. Details have been reported elsewhereCitation27. AE were not analyzed by subgroups in view of the low patient numbers.
Discussion
The present subgroup analysis shows that treatment with the aliskiren/amlodipine SPC achieves meaningful BP reductions in elderly or obese patients, in metabolic risk patients or DM patients. These BP reductions were in line with the BP reduction in the overall study population.
Such patients are frequent in everyday care, and they may present a particular challenge to treating physicians. In a cross-sectional point prevalence study of 45,000 unselected consecutive patients in a representative nationwide sample of primary care physicians in Germany (HYDRA), 28% had a BMI ≥30.0 kg/m2 Citation29. Overall prevalence of hypertension, defined as BP ≥140/90 mmHg or on antihypertensive medication, was 60.6%, in grade 1 obesity 72.9%, in grade 2 obesity 77.1%, and in grade 3 obesity 74.1%. The odds ratio for good BP control (<140/90 mmHg) in diagnosed and treated patients was 0.8 in overweight patients compared with normal weight, 0.6 in grade 1, 0.5 in grade 2, and 0.7 in grade 3 obese patientsCitation29. While patients with overweight/obesity are not specifically considered in the current ESC hypertension guidelines, those with metabolic syndrome are. Although no implication is made that it is a pathogenetic entity, metabolic syndrome is specifically mentioned as it constitutes a cluster of risk factors often associated with high BP which markedly increases cardiovascular riskCitation30–35. At least five definitions with slight or moderate deviations from each other have been proposed by international organizations or by expert groupsCitation30–35. In our study, a more general approach was chosen, as patients with at least one ‘metabolic risk factor’ were analyzed in the respective group.
With respect to age, in elderly patients with uncomplicated hypertension, treatment should be initiated gradually, while in those with higher risk, goal BP should be achieved more promptly, which favors initial combination therapy and quicker adjustment of dosesCitation5.
As high age, high BMI and metabolic problems including DM are highly prevalent, the aliskiren monotherapy and aliskiren/amlodipine combination studies have included substantial fractions of such patients and found conclusive and consistent effects on BP in the various subgroups ().
In obese patients, aliskiren-based treatment was highly effective and well-tolerated in patients who failed first line-treatment with a thiazideCitation36, and (with optional addition of amlodipine) had a substantially lower incidence of hypokalemia (1 vs. 14%) compared with thiazide-based therapyCitation37. In the same study, aliskiren provided a stronger antihypertensive effect compared with HCTCitation37. In the ATTAIN study, the aliskiren/HCT combination was more effective than ramiprilCitation38, and a subanalysis of an aliskiren/amlodipine combination study showed similar BP lowering in obese or non-obese patientsCitation39. Recently, Boschmann highlighted aliskiren's prolonged BP-lowering effect following discontinuation, and showed that the drug penetrates adipose and skeletal muscle tissue at levels that are apparently sufficient to reduce tissue RAS activityCitation40.
With regard to patients with metabolic syndrome, the Krone et al. study suggested that aliskiren 300 mg may offer advantages to irbesartan 300 mgCitation41. In the pooled analysis by Gradman et al. no differences in the BP lowering effects between women with or without metabolic syndrome were notedCitation42. Weinberger et al. reported that the aliskiren/amlodipine combination was more effective than amlodipine monotherapy in African-Americans with obesity or metabolic syndromeCitation43. In a subgroup analysis of the ASCENT study, the dual aliskiren/amlodipine combination was as effective as the triple aliskiren/amlodipine/HCT combination in lowering BP among high-risk US minority patients with cardiometabolic syndromeCitation44.
In patients with DM, studies on aliskiren (alone or in combination with angiotensin receptor blockers) mainly focused on the assessment of antiproteinuric/nephroprotective effects rather than the antihypertensive effectsCitation45,Citation46. However, the studies by Uresin et al. (aliskiren vs. ramipril)Citation47 and Townsend et al. (aliskiren/HCT vs. amlodipine)Citation48 showed beneficial BP lowering effects of aliskiren in diabetic patients. Also, a pooled analysis of aliskiren depicted the same effect in diabetic versus non-diabetic patientsCitation49. Interestingly, prospective registry data from the 3A Registry and the DRIVER study supported this finding in unselected patientsCitation50,Citation51.
In elderly patients, aliskiren exposure is modestly increasedCitation52. A significant dose relationship for the BP lowering effect was found in the elderly, with no evidence of dose-related increases in adverse eventsCitation53,Citation54. Compared with a ramipril-based regime, aliskiren lowered BP significantly stronger in elderly patients with systolic hypertensionCitation55. A substantial BP reduction over the long term has further been substantiated in the 3A Registry with 15,000 unselected patientsCitation56.
Overall, the present analyses of the various subgroups in the AWESOME study, taken together with evidence from the aliskiren/amlodipine study program and other randomized studies indicate that the use of the combination leads to comparable efficacy in various subgroups. This could facilitate clinical management of the diverse hypertensive population. The overall results of the study show that the switch of patients not adequately controlled by the SPC olmesartan 40 mg/amlodipine 10 mg once daily to aliskiren 300/amlodipine 10 mg benefits about one-third of patients (36.4%) that can be treated to target at the end of phase 2 (week 8). For non-normalizers in phase 2, the addition of a thiazide diuretic in a SPC further led to a significant reduction of SBP and DBP.
Some methodological limitations should be considered when interpreting the results of the present analysis. Patients could be part of more than one subgroup (populations were not mutually exclusive). An open-label study, in particular without a control arm, can be compromised by unknown bias of various typesCitation57. The multiple subgroup analyses were specified post-hoc and carried out descriptively, and performed to assess the consistency of a treatment effect among various patient characteristics. Thus, the typical problems of subgroup analyses (in particular multiplicity) do not apply hereCitation58. However, the sample size of the current study was low and the treatment duration limited. Larger and longer-term studies are needed for definitive conclusions. Also for the assessment of safety, much larger datasets are neededCitation59.
Conclusion
The SPC aliskiren 300 mg/amlodipine 10 mg had consistent BP lowering effects in various subgroups of patients with stage 2 hypertension. The BP lowering effect was in line with the achieved BP lowering effect in the total population of this study, and with various reports of other randomized controlled trials on this combination.
Transparency
Declaration of funding
The study was funded by Novartis Pharma GmbH.
Declaration of financial/other relationships
C.S. and F.M. are employees of Novartis Pharma GmbH Germany. E.K. is a former employee of Novartis. D.P. has received consultancy honoraria from Novartis.
Acknowledgments
We acknowledge the cooperation and commitment of all investigators and their staff, who made the present trial possible.
These results were presented, among others, at the 21st Annual European Meeting of Hypertension 17–20 June 2011, Milan, Italy.
Notes
*Sevikar is a registered trademark of Daiichi Sankyo, Parsippany, NJ, USA
†Rasilamlo is a registered trademark of Novartis, Basel, Switzerland
References
- Wong ND, Dede J, Chow VH, et al. Global cardiovascular risk associated with hypertension and extent of treatment and control according to risk group. Am J Hypertens 2012;25:561-7
- Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895-906
- Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363:2022-31
- Ruschitzka F. Evidence for improvement in survival with antihypertensive combination treatment. J Hypertens 2011;29(Suppl 1):S9-14
- Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;28:1462-536
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-71
- Deutsche Hypertonie Gesellschaft - Deutsche Hochdruckliga e.V. (DHL). S2 Guideline: Treatment of Arterial Hypertension. Bonn, 2008. http://www.awmf.org/uploads/tx_szleitlinien/046-001_S2_Behandlung_der_arteriellen_Hypertonie_06-2008_06-2013.pdf. Accessed on 27 October 2011.;34:481-98
- Amlodipinbesilat-Sandoz (Manufacturer: Sandoz Pharmaceuticals GmbH, Holzkirchen). Fachinformation (Prescribing Information). Update July 2010. Internet: www.fachinfo.de. Accessed on 22 July 2011
- Haria M, Wagstaff AJ. Amlodipine. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disease. Drugs 1995;50:560-86
- Bruder O, Jensen C, Bell M, et al. Effects of the combinations of amlodipine/valsartan versus losartan/hydrochlorothiazide on left ventricular hypertrophy as determined with magnetic resonance imaging in patients with hypertension. J Drug Assessment 2012;1:1-10
- Chrysant SG, Melino M, Karki S, et al. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther 2008;30:587-604
- Chrysant SG, Oparil S, Melino M, et al. Efficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertension. J Clin Hypertens (Greenwich) 2009;11:475-82
- Barrios V, Brommer P, Haag U, et al. Olmesartan medoxomil plus amlodipine increases efficacy in patients with moderate-to-severe hypertension after monotherapy: a randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig 2009;29:427-39
- Volpe M, Brommer P, Haag U, et al. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig 2009;29:11-25
- Duggan ST, Chwieduk CM, Curran MP. Aliskiren: a review of its use as monotherapy and as combination therapy in the management of hypertension. Drugs 2010;70:2011-49
- Billecke SS, Marcovitz PA. Aliskiren/amlodipine combination for the treatment of hypertension. Drugs Today (Barc) 2011;47:403-17
- Black HR, Weinberger MH, Purkayastha D, et al. Comparative efficacy and safety of combination aliskiren/amlodipine and amlodipine monotherapy in African Americans with stage 2 hypertension. J Clin Hypertens (Greenwich) 2011;13:571-581
- Palatini P, Jung W, Shlyakhto E, et al. Maintenance of blood-pressure-lowering effect following a missed dose of aliskiren, irbesartan or ramipril: results of a randomized, double-blind study. J Hum Hypertens 2010;24:93-103
- Dusing R, Brunel P, Baek I, et al. Sustained decrease in blood pressure following missed doses of aliskiren or telmisartan: the ASSERTIVE double-blind, randomized study. J Hypertens 2012;30:1029-40
- Smilde J. A comparison of amlodipine and felodipine extended release in the treatment of hypertension at steady state and after two missed doses. Curr Ther Res 1997;58:141-53
- Brown MJ, McInnes GT, Papst CC, et al. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial. Lancet 2011;377:312-20
- Littlejohn TW, 3rd, Jones SW, Zhang J et al. Efficacy and safety of aliskiren and amlodipine combination therapy in patients with hypertension: a randomized, double-blind, multifactorial study. J Hum Hypertens 2012 (online first). Accessed on 14 January 2013
- Chrysant SG, Murray AV, Hoppe UC, et al. Long-term safety and efficacy of aliskiren and valsartan combination with or without the addition of HCT in patients with hypertension. Curr Med Res Opin 2010;26:2841-9
- Glorioso N, Thomas M, Troffa C, et al. Antihypertensive efficacy and tolerability of aliskiren/amlodipine single-pill combinations in patients with an inadequate response to aliskiren monotherapy. Curr Vasc Pharmacol 2012;10:750-7
- Pfeiffer D, Rennie N, Papst CC, et al. Efficacy and tolerability of aliskiren/amlodipine single-pill combinations in patients who did not respond fully to amlodipine monotherapy. Curr Vasc Pharmacol 2012;10:775-82
- European Medicines Agency (EMA). Rasilmalo (Aliskiren/Amlodipine, EMEA/H/C/002073). Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002073/WC500107200.pdf. Accessed on 6. October 2011
- Axthelm C, Sieder C, Meister F, et al. Efficacy and tolerability of the single-pill combination of aliskiren 300 mg/amlodipine 10 mg in hypertensive patients not controlled by olmesartan 40 mg/amlodipine 10 mg. Curr Med Res Opin 2012;28:69-78
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005;45:142-61
- Bramlage P, Pittrow D, Wittchen HU, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens 2004;17:904-10
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva; 1999
- Kerner W, Brückel J, Böhm B. Definition, Klassifikation und Diagnostik des Diabetes mellitus. Deutsche Diabetes Gesellschaft DDG, November 2004 Herausgeber: W. A. Scherbaum, W. Kiess. Aktualisierung der 1. Auflage vom Juli 2001
- Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract 2003;9:237-52
- Grundy SM, Brewer HB Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation 2004;109:433-8
- Bloomgarden ZT. American Association of Clinical Endocrinologists (AACE) consensus conference on the insulin resistance syndrome: 25-26 August 2002, Washington, DC. Diabetes Care 2003;26:933-9
- Internation Diabetes Federation. IDF Worldwide Definition of the Metabolic Syndrome. http://www.idf.org/idf-worldwide-definition-metabolic-syndrome. Accessed on 22 December 2010
- Jordan J, Engeli S, Boye SW, et al. Direct renin inhibition with aliskiren in obese patients with arterial hypertension. Hypertension 2007;49:1047-55
- Schmieder RE, Philipp T, Guerediaga J, et al. Aliskiren-based therapy lowers blood pressure more effectively than hydrochlorothiazide-based therapy in obese patients with hypertension: sub-analysis of a 52-week, randomized, double-blind trial. J Hypertens 2009;27:1493-501
- Whaley-Connell A, Purkayastha D, Yadao A, et al. Central pressure and biomarker responses to renin inhibition with hydrochlorothiazide and ramipril in obese hypertensives: The ATTAIN study. Cardiorenal Med 2011;1:53-66
- Braun-Dullaeus R, Shustov S, Alvarez C, et al. Aliskiren/amlodipine combination lowers BP in obese and non-obese patients with moderate-to-severe hypertension [PO-339]. Annual Meeting of the American Society of Hypertension (ASH), New York, USA, 21-24.5.2011
- Boschmann M, Nussberger J, Engeli S, et al. Aliskiren penetrates adipose and skeletal muscle tissue and reduces renin-angiotensin system activity in obese hypertensive patients. J Hypertens 2012;30:561-6
- Krone W, Hanefeld M, Meyer HF, et al. Comparative efficacy and safety of aliskiren and irbesartan in patients with hypertension and metabolic syndrome. J Hum Hypertens 2010;25:186-95
- Gradman AH, Weir MR, Wright M, et al. Efficacy, safety and tolerability of aliskiren, a direct renin inhibitor, in women with hypertension: a pooled analysis of eight studies. J Hum Hypertens 2010;24:721-9
- Weinberger MH, Izzo JL Jr, Purkayastha D, et al. Comparative efficacy and safety of combination aliskiren/amlodipine and amlodipine monotherapy in African Americans with stage 2 hypertension and obesity or metabolic syndrome. J Am Soc Hypertens 2011;5:489-97
- Ferdinand KC, Weitzman R, Purkayastha D, et al. Aliskiren-based dual- and triple-combination therapies in high-risk US minority patients with stage 2 hypertension. J Am Soc Hypertens 2012;6:219-27
- Persson F, Rossing P, Reinhard H, et al. Optimal antiproteinuric dose of aliskiren in type 2 diabetes mellitus: a randomised crossover trial. Diabetologia 2010;53:1576-80
- Persson F, Lewis JB, Lewis EJ, et al. Aliskiren in combination with losartan reduces albuminuria independent of baseline blood pressure in patients with type 2 diabetes and nephropathy. Clin J Am Soc Nephrol 2011;6:1025-31
- Uresin Y, Taylor AA, Kilo C, et al. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. J Renin Angiotensin Aldosterone Syst 2007;8:190-8
- Townsend RR, Forker AD, Bhosekar V, et al. Comparison of aliskiren/hydrochlorothiazide combination therapy and amlodipine monotherapy in patients with stage 2 systolic hypertension and type 2 diabetes mellitus. J Clin Hypertens (Greenwich) 2011;13:889-97
- Taylor A, Anderson D, Arora V, et al. Antihypertensive efficacy of the direct renin inhibitor aliskiren in patients with diabetes: a pooled analysis of 10 randomized trials [0483-P]. Presentation at the meeting of the American Diabetes Association, June 22 - 26, 2007, Chicago, IL, USA
- Zeymer U, Dechend R, Deeg E, et al. Superior blood pressure reduction with aliskiren in hypertensive patients with diabetes mellitus in real life. results of the 3A registry [PP 27.364]. Annual Meeting of the European Society of Hypertension. 19 June 2011
- Verpooten GA, Aerts A, Coen N, et al. Antihypertensive effectiveness of aliskiren for the 'real-world' management of hypertension: multilevel modelling of 180-day blood pressure outcomes (the Belgian DRIVER Study). Int J Clin Pract 2011;65:54-63
- Vaidyanathan S, Reynolds C, Yeh CM, et al. Pharmacokinetics, safety, and tolerability of the novel oral direct renin inhibitor aliskiren in elderly healthy subjects. J Clin Pharmacol 2007;47:453-60
- Villa G, Le Breton S, Ibram G, et al. Efficacy, safety, and tolerability of aliskiren monotherapy administered with a light meal in elderly hypertensive patients: a randomized, double-blind, placebo-controlled, dose-response evaluation study. J Clin Pharmacol 2012;52:1901-11
- Verdecchia P, Calvo C, Mockel V, et al. Safety and efficacy of the oral direct renin inhibitor aliskiren in elderly patients with hypertension. Blood Press 2007;16:381-91
- Duprez DA, Munger MA, Botha J, et al. Aliskiren for geriatric lowering of systolic hypertension: a randomized controlled trial. J Hum Hypertens 2010;24:600-8
- Schmieder R, Lehmann M, Dechend R, et al. Blood pressure reduction in 7000 elderly hypertensive patients: results from the 3A registry. J Hypertens 2011;29(e-Supplement A):e136
- Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health 2004;58:635-41
- Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189-94
- European Agency for the Evaluation of Medicinal Products (EMA). ICH E1 Population Exposure: The Extent of Population Exposure to Assess Clinical Safety (CPMP/ICH/375/95). London, June 1995. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002747.pdf. Accessed on 14 January 2013
- Weir M, Prescott M, Bush C, et al. Antihypertensive efficacy of the direct renin inhibitor aliskiren in patients with diabetes, metabolic syndrome or obesity: a pooled analysis of 10 randomized trials [abstract no. P888]. Circulation 2008;118:162
- Yarows S, Oparil S, Patel S, Wright M, Yadao A, Zhang J. Initial use of combination aliskiren/valsartan is more effective than either component monotherapy in elderly and non-elderly hypertensive patients [PO--71]. Presented at the Annual Meeting of the American Society for Hypertension 2010. J Clin Hypertens (Greenwhich) 2010;12 (Suppl. 1):A--48.
- Gradman A, Weir M, Arora V, et al. Aliskiren provides highly effective blood pressure reduction independent of age in patients with hypertension (p. 38). Annual Meeting of the ASH 2008. J Clin Hypertens 2008;10:A21-2
- Littlejohn III T, Trenkwalder P, Hollanders G, et al. Aliskiren in combination with amlodipine provides blood pressure reduction with good tolerability in hypertensive patients regardless of age over 54 weeks of treatment. Presentation at the American Society of Hypertension (ASH) 23rd Annual Scientific Meeting. May 14–17, 2008. New Orleans, Louisiana, USA. J Clin Hypertens (Greenwich) 2008;10(5 Suppl A):A1-169
- Basile J, Babazadeh S, Lillestol M, et al. Comparison of aliskiren/hydrochlorothiazide combination therapy with hydrochlorothiazide monotherapy in older patients with stage 2 systolic hypertension: results of the ACTION study. J Clin Hypertens (Greenwich) 2011;13:162-9
- Sowers J, Severin T, Maboudian M, et al. Blood pressure lowering effects of the direct renin inhibitor aliskiren in patients with diabetes: a pooled analysis of 16 randomised trials [abstract 1106). Diabetologia 2011;54(Suppl):S1-542
- Braun-Dullaeus R, Zappe D, Papst C, et al. Aliskiren/amlodipine combination therapy lowers blood pressure more effectively than amlodipine alone in patients with type 2 diabetes mellitus or metabolic syndrome [PP 43.392]. Presentation at the Annual Meeting of the European Society of Hypertension (ESH).e551
- White W, Anderson D, Arora V, Bush C, Keefe D. Antihypertensive effectiveness of the direct renin inhibitor aliskiren in patients with metabolic syndrome: a comparative analysis of 7219 patients from 10 randomized trials [P4845]. Presentation at the Annual Meeting of the ESC September 2007, Vienna, Austria. Eur Heart J 2007;28 (Abstract Supplement):868.
- Lacourciere Y, Taddei S, Konis G, Salko T, Fang H, Zhang J. Aliskiren/amlodipine/hydrochlorothiazide combination lowers blood pressure more effectively than component dual combinations in moderate to severe hypertensive patients, regardless of metabolic syndrome status. Poster presentation at High Blood Pressure Research Scientific Sessions Oct 13--16, 2010. Washington, DC, USA.