Abstract
Objectives:
The long-acting muscarinic antagonist (LAMA) glycopyrronium (NVA237) has recently been approved as a once-daily treatment for COPD. The objectives of this study were to determine the dose delivery characteristics of glycopyrronium and compare them with those of the LAMA tiotropium, both delivered by their respective capsule-based dry-powder inhalers (DPIs).
Research design and methods:
Seven inhalation profiles derived from patients with moderate and severe COPD were reproduced to determine the aerodynamic particle size distribution of glycopyrronium delivered by the Breezhaler device, a low-resistance DPI. Theoretical respiratory tract deposition was estimated using a semi-empirical model for healthy lungs. These results were compared with those of tiotropium delivered by the high-resistance HandiHaler device obtained in a previous study using the same set of inhalation profiles. Study limitations are that fine particle fraction (FPF) and particle size are generated by the inhalers are not a direct measure of lung deposition, and the bronchodilator effect of inhaled drugs does not depend solely upon the percentage of the total dose that reaches the lung.
Results:
The mean FPF (≤4.7 µm) was 42.6% of the nominal dose (which refers to the content of the capsule) for glycopyrronium and 9.8% for tiotropium while the mass median aerodynamic diameter (MMAD) was 2.8 µm and 3.9 µm for glycopyrronium and tiotropium, respectively. The mean estimated intrathoracic drug deposition as a percentage of the mean dose delivered to the Next Generation Impactor was 39% for glycopyrronium and 22% for tiotropium.
Conclusions:
The glycopyrronium capsule-based DPI delivered a higher FPF and greater and more consistent intrathoracic deposition irrespective of age and disease severity compared to the tiotropium capsule-based DPI, suggesting that it may be suitable for use by patients with a wide range of COPD severities.
Introduction
Chronic obstructive pulmonary disease (COPD), a preventable lung disease characterized by progressive airflow limitation, is a leading cause of mortality and morbidity world-wideCitation1. Bronchodilators are the mainstay of treatment of COPD, with inhalation preferred over other routes of administration because of greater efficacy and safetyCitation2. Pressurized metered-dose inhalers (pMDIs) and dry-powder inhalers (DPIs) are the main two types of inhaler currently availableCitation3. The Respimat inhaler, is a third type of device, which uses the mechanical energy of a spring to atomize the drug solution by forcing it through an extremely fine nozzle system, generating a slow-moving fine mistCitation5. The device requires the patient to coordinate inhalation with actuation.
DPIs, in which the patient’s inspiratory flow provides the force to generate the drug aerosol, have become increasingly popular in COPD as the lack of the need to co-ordinate actuation and inhalation is important in minimizing patient handling errors and optimizing drug deliveryCitation4.
The internal resistance of the DPI determines the effort patients have to make to achieve adequate inspiratory flows for effective and reproducible dose deliveryCitation6,Citation7. As high-resistance devices require greater effort to generate these flows, they may not be suitable for patients with significant airflow obstructionCitation8,Citation9.
In addition to providing consistent drug delivery over a wide range of inspiratory flow rates, an ideal inhaler for use in COPD should also generate optimal particle size for lung delivery and retention at the required siteCitation10. The aerodynamic size of drug particles generated by inhalers is one of the most important factors in defining the distribution and deposition of drug within the lung, with a particle size of less than 4.7 µm in diameter generally considered optimal for deposition in the bronchi and alveoliCitation11. A high fine particle fraction (FPF), defined as fraction of particles of less than 4.7 µm in diameter, indicates that a significant proportion of the inhaled dose is likely to reach the pulmonary region.
Inhaled long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) are two classes of bronchodilator widely used for the treatment of patients with moderate-to-severe COPDCitation1,Citation12,Citation13. Indacaterol maleate is a once-daily LABA delivered by the low-resistance DPI Breezhaler*Citation14. This device has been shown to deliver a consistent dose of indacaterol, irrespective of disease severity and associated inhalation flow profilesCitation15. The once-daily LAMA tiotropium is delivered by the high-resistance HandiHaler* device.
Glycopyrronium, a once-daily LAMA recently approved as a dry-powder formulation for the treatment of COPD, is also delivered via the same type of device currently used with indacaterol. To evaluate the dose delivery characteristics of glycopyrronium by this capsule-based DPI, the aerodynamic particle size distribution was determined and the theoretical respiratory tract deposition estimated under simulated inhalation conditions. Inspiratory profiles were obtained from seven patients representative of a wide range of COPD severities, ages, and degrees of airflow obstruction. The results for glycopyrronium obtained in this study were also compared with those for tiotropium delivered by its capsule-based DPI generated in a previous study using the same set of inhalation profilesCitation16.
Methods
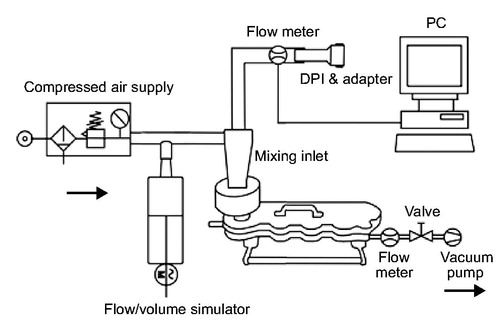
Seven patient inhalation profiles were selected in a previous study from a group of 26 patients to cover a range from moderate to severe COPD and represent different degrees of airflow obstructionCitation16. The generation of the inhalation flow profiles was performed with an empty device for glycopyrronium and with a device containing the drug product for tiotropium. The profiles were reproduced in a flow/volume simulator (Hans Rudolph, Inc., USA) coupled through a mixing inlet to a Next Generation Impactor (NGI, Copley Scientific, UK) with pre-separator and induction port (). Selected flow profiles were limited to a peak inspiratory flow of 100 L/min at maximum due to technical limitations of the experimental design (e.g., NGI calibration for a maximum flow rate at 100 L/min, limited maximum flow rate generated by flow/volume simulator). The flow/volume simulator was used to reproduce flow profiles by balancing and modulating the air flow through the DPI and auxiliary air to maintain a constant flow rate of 100 L/min through the NGI. The patient’s breathing patterns were reproduced at the mouthpiece of the DPIs by modulating the air flow using the computer-controlled flow volume simulator. At the impactor outlet, a flow rate of 100 L/min was generated by means of a vacuum pump whereas an air supply of 100 L/min leading into the mixing inlet assured a neutral sum flow at experimental rest as previously described by Chapman et al.Citation16. Three replicate measurements were performed for each simulated patient flow profile, using a new DPI for each determination. Each capsule contained 50 µg of glycopyrronium (nominal dose).
Figure 1. Experimental set-up with flow/volume simulator. DPI, dry-powder inhaler. Data for the tiotropium capsule-based DPI were obtained Chapman et al.Citation16.
To determine glycopyrronium deposition, the NGI was disassembled and samples from each component were analyzed by high-performance liquid chromatography. Data evaluation was carried out using validated software from Copley (C.I.T.D.A.S., Version 2.0). The delivered dose (DD) to the NGI was defined as drug amount from mouth piece adapter, USP throat, mixing inlet, pre-separator and all NGI collection cups. The fine particle mass (FPM) was calculated for an aerodynamic diameter of ≤4.7 μm to allow comparison with data obtained for tiotropiumCitation16. The fine particle fraction (FPF) was calculated by expressing the quotient of FPM relative to the nominal dose (contents of the capsule), which was 50 μg for glycopyrronium. The mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD), a measure of the variability of the particle diameters within the aerosol, were also determined.
Based on the results of the particle size analysis, theoretical respiratory tract depositions were estimated for each of the patient’s breathing profiles using the mathematical ICRP-lung model ICRP 66, which estimates extrathoracic and intrathoracic deposition in healthy subjectsCitation17. Extrathoracic deposition equals the deposition in the mouth cavity and larynx. Intrathoracic deposition equals the deposition in trachea, bronchial tree and alveolar region.
The original model was designed for continuous drug delivery during inhalation. It was adapted for inhalation with bolus drug delivery by simulating three different depositions using total inhaled volume (D1), volume until the beginning of the bolus (D2) and volume until the end of the bolus (D3). The deposition of the bolus (Dbolus) was calculated using the following equation:
Input variables were MMAD, GSD, mean inhalation flow, inhalation volume, onset time of aerosol bolus, aerosol bolus length, functional residual capacity (FRC), age and breath hold after inhalation. As the model assumes a constant inhalation flow rate, the patients’ actual inhalation flow rate was converted to a constant mean inhalation flow rate over the entire flow rate values. Mean flow rate until the beginning of the bolus and bolus volume were assumed to be 12 L/min and 500 ml, respectively. These parameters were determined empirically by Chapman et al.Citation16 but not confirmed in vitro for the two inhalers. The in vitro dose delivery study was carried out at Inamed Research GmbH and Co. KG, Gauting, Germany.
Results
Seven breathing patterns derived from patients with moderate and severe COPD were reproduced to determine the dose delivery characteristics of glycopyrronium and tiotropium ( and ). The mean inhalation time (IT) was 2.2 s with the glycopyrronium capsule-based DPI and 4.2 s for the tiotropium capsule-based DPI () while mean peak inspiratory flow (PIF) was 72 L/min and 36 L/min for the glycopyrronium and tiotropium capsule-based DPIs, respectively ( and ).
Figure 2. Individual inhalation flow profiles for the selected patients through the glycopyrronium capsule-based DPI (a) and tiotropium capsule-based DPI (b). Reproduced with permission from Chapman et al.Citation16.
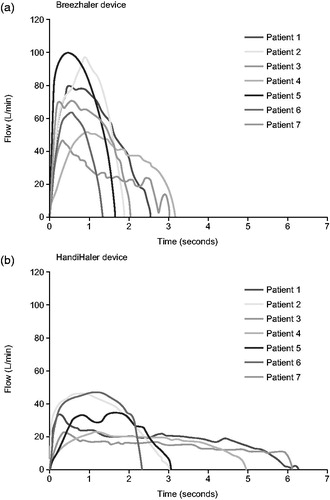
Table 1. Patient demographics and derived inhalation variables through the two inhalers.
The FPF ranged from 36.3% to 49.3% for glycopyrronium while it ranged between 7.6% and 10.9 % for tiotropium (). The mean FPF was 42.6% of the 50 µg nominal dose for glycopyrronium (RSD 10.68) and 9.8% of the 18 µg nominal dose for tiotropium (RSD 11.64) (). The MMAD was 2.8 µm for glycopyrronium (RSD 2.85) and 3.9 µm for tiotropium (RSD 6.33) ().
Table 2. Characteristics of aerosols generated using patient inhalation profiles representative of moderate to severe COPD.
The mean estimated intrathoracic drug deposition as a percentage of the mean delivered dose was 39% (RSD 4.20) versus 22% (RSD 8.78) for glycopyrronium and tiotropium, respectively ( and ). The mean estimated extrathoracic drug deposition was 45% (RSD 3.02) for glycopyrronium and 71% (RSD 2.67) for tiotropium ().
Figure 3. Theoretical intrathoracic drug deposition as percentage of delivered dose. Data for tiotropium were obtained from Chapman et al. 2011.
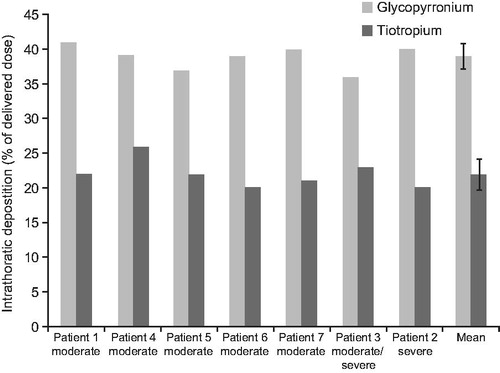
Table 3. Theoretical intrathoracic and extrathoracic drug deposition as a percentage of delivered dose.
Discussion
In this study the impact of inhalation profiles representative of a range of COPD severity on the dose delivery characteristics of the LAMA glycopyrronium was evaluated and compared with results previously reported for tiotropium. Both glycopyrronium and tiotropium capsule-based DPIs delivered consistent doses across these inhalation profiles in terms of DD and FPF. However, delivery efficiency across COPD severity and ages for glycopyrronium (FPF from 36.3 to 49.3%) was higher than that for tiotropium (FPF from 7.6 to 10.9%). This finding is particularly important in light of the results of recent studies suggesting that older patients and patients with moderate-to-severe COPD have difficulty generating the necessary inspiratory flow to achieve an efficient and reproducible drug delivery from DPIsCitation8,Citation9. The internal resistance determines the inspiratory effort required to obtain consistent and efficient dose delivery from the inhalersCitation6,Citation7. Low-resistance devices allow air to flow through them easily as opposed to high-resistance devices. Therefore, they do not require a forceful and prolonged inhalation to achieve optimal drug delivery. As inspiratory flow is inversely proportional to inhaler resistance, the glycopyrronium capsule-based DPI (specific airflow resistances of 2.2 10−2 kPa½ L−1 min), may be more suitable for the majority of patients with COPD than the tiotropium capsule-based DPI (5.1 × 10−2 kPa½ L−1 min)Citation16. Moreover, it has also been suggested that low-resistance DPIs have a greater patient acceptabilityCitation18.
The predicted intrathoracic deposition of glycopyrronium (39%, RSD 4.20) was higher and more consistent than that previously estimated for tiotropium (22%, RSD 8.78) while the extrathoracic deposition was lower. In addition, the MMAD of the particles for glycopyrronium was smaller than that for tiotropium (2.8 µm and 3.9 µm, respectively). The higher FPF and intrathoracic deposition of glycopyrronium are not only determined by the device used to deliver this drug, but are also dependent on the formulation, which plays an important role in ensuring efficient delivery to the lungsCitation19. The glycopyrronium formulation contains the excipient magnesium stearate as a ‘Force Control Agent’ which increases the dispersibility of drug particles thereby enabling optimal aerosolizationCitation20.
Particle size affects lung deposition, with a particle size of less than 4.7 µm in diameter generally considered optimal for deposition in the bronchi and alveoliCitation11, The aerodynamic particle size distribution and the theoretical intra and extrathoracic deposition obtained for glycopyrronium in this study suggest that, compared with tiotropium, a higher proportion of the dose of glycopyrronium is delivered to the lower airways whereas a lower fraction deposits in the mouth and could be swallowed resulting in systemic exposure. This could in principle affect the clinical effectiveness of glycopyrronium as well as its tolerability profile. Nevertheless, it must be noted that FPF and the size of the particles generated by the inhalers are not a direct measure of lung deposition as they only provide an estimate of the dose likely to reach the lower airways. Moreover, the bronchodilator effect of inhaled drugs does not depend solely upon the percentage of the total dose that reaches the lung, but is the result of the combination of several physiological factors including airway geometry and lung clearance mechanismsCitation21. Despite these limitations in extrapolating the current results to the clinical situation, a correlation between in vitro and in vivo data has previously been reported. A review of studies comparing the aerodynamic particle size measured in vitro at a constant air flow rate with lung deposition data obtained by gamma scintigraphy revealed that the aerodynamic particle size can predict the real distribution of inhaled drugs in the lung with reasonable accuracyCitation22. Further, agreement between the delivered dose estimated in an in vivo study, which investigated the performance of pMDIs by simulating patient breathing profiles, and ex vivo data has previously been shownCitation23. Thus the use of real inhalation profiles derived from patients with COPD in our study make the results obtained by this methodology even more predictive of the real drug delivery profiles in patients with COPD.
Conclusions
This study provides new evidence to suggest that deposition in the lung is not always greater with high-resistance devices, as suggested for some DPIsCitation24,Citation25. The low-resistance glycopyrronium capsule-based DPI delivers a higher FPF and generates a greater and more consistent intrathoracic deposition compared with the tiotropium capsule-based DPI. Therefore, it may be suitable for patients with COPD of different severities, including severe COPD.
Transparency
Declaration of funding
This study was funded by Novartis Pharma AG, Basel, Switzerland.
Declaration of financial/other relationships
P.C., T.K., E.C. and J.J. are employees of Novartis and declare no competing interests. T.V. has received reimbursement for attending scientific conferences and/or fees for presentations and/or consultations and/or educational programs from Boehringer Ingelheim, Chiesi, Janssen-Cilag, GlaxoSmithKline, Novartis, Teva and Mundipharma.
Acknowledgments
The authors were assisted in the preparation of the manuscript by Roberta Sottocornola, a professional medical writer contracted to CircleScience (Macclesfield, UK) and Mark J. Fedele (Novartis). Writing support was funded by the study sponsor Novartis. The authors thank Inamed GmbH and Co. KG, Gauting, Germany who carried out the in vitro dose delivery study and analyzed the results. The authors also thank Dilraj Singh and Richard Pavkov from Novartis for the generation of the patient inhalation flow profiles.
Notes
*These data were presented at the Drug Delivery to the Lungs conference in Edinburgh, Scotland (5–7 December 2012)
†Breezhaler is a registered trade name of Novartis Pharma AG, Basel
‡HandiHaler is a registered trade name of Boehringer-Ingelheim, Ingelheim, Germany
*Respimat is a registered trade name of Boehringer-Ingelheim, Ingelheim, Germany
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the diagnosis, management and prevention of COPD. Updated December 2011. www goldcopd org 2011 [cited 2012 Aug 30]
- Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol 1996;42:697-705
- Geller DE. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir Care 2005;50:1313-21
- Girodet PO, Raherison C, Abouelfath A, et al. [Real-life use of inhaler devices for chronic obstructive pulmonary disease in primary care]. Therapie 2003;58:499-504
- Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011;37:1308-31
- Terzano C. Dry powder inhalers and the risk of error. Respiration 2008;75:14-15
- Wieshammer S, Dreyhaupt J. Dry powder inhalers: which factors determine the frequency of handling errors? Respiration 2008;75:18-25
- Al-Showair RA, Tarsin WY, Assi KH, et al. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med 2007;101:2395-401
- Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J 2008;31:78-83
- Byron PR. Drug delivery devices: issues in drug development. Proc Am Thorac Soc 2004;1:321-8
- United States Pharmacopeial Convention. Aerosols, metered dose inhalers, and dry powder inhalers. USP30-NF25. Rockville, MD: 2009
- Montuschi P. Pharmacological treatment of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2006;1:409-23
- Montuschi P, Macagno F, Valente S, et al. Inhaled muscarinic acetylcholine receptor antagonists for treatment of COPD. Curr Med Chem 2012; Sep 3. [Epub ahead of print]
- Cazzola M, Matera MG, Lotvall J. Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs 2005;14:775-83
- Pavkov R, Mueller S, Fiebich K, et al. Characteristics of a capsule based dry powder inhaler for the delivery of indacaterol. Curr Med Res Opin 2010;26:2527-33
- Chapman KR, Fogarty CM, Peckitt C, et al. Delivery characteristics and patients' handling of two single-dose dry-powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis 2011;6:353-63
- ICRP. ICPR publication 66: Human respiratory tract model for radiological protection. A report of a task group of the International commission on radiological protection. Ann ICRP 1994;24:1-482
- Van Der PJ, Eijsvogel MM, Kuipers BF, et al. Comparison of the Diskus inhaler and the Handihaler regarding preference and ease of use. J Aerosol Med 2007;20:38-44
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 2003;56:600-12
- Kumon M, Machida S, Suzuki M, et al. Application and mechanism of inhalation profile improvement of DPI formulations by mechanofusion with magnesium stearate. Chem Pharm Bull (Tokyo) 2008;56:617-25
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 2003;56:588-99
- Newman SP, Chan HK. In vitro/in vivo comparisons in pulmonary drug delivery. J Aerosol Med Pulm Drug Deliv 2008;21:77-84
- Berg E, Madsen J, Bisgaard H. In vitro performance of three combinations of spacers and pressurized metered dose inhalers for treatment in children. Eur Respir J 1998;12:472-6
- Svartengren K, Lindestad P, Svartengren M, et al. Added external resistance reduces oropharyngeal deposition and increases lung deposition of aerosol particles in asthmatics. Am J Respir Crit Care Med 1995;152:32-7
- Weuthen T, Roeder S, Brand P, et al. In vitro testing of two formoterol dry powder inhalers at different flow rates. J Aerosol Med 2002;15:297-303