Abstract
Objective:
Hirsutism is the presence of excess terminal hairs in females in a male-like pattern. The most accepted hypothesis for the development of hirsutism is increased 5α-reductase activity in hair follicles of hirsute women. Finasteride partially blocks the conversion of testosterone to dihydrotestosterone through inhibition of 5α-reductase in hair follicles. This study was designed to determine the efficacy of finasteride gel 0.25% in management of idiopathic hirsutism and treatment of hirsutism with topical finasteride to lessen the side-effects.
Methods:
Women after puberty that have idiopathic hirsutism criteria are divided randomly in two groups; treatment and control. The number of patients in each group is 15 and received finasteride and placebo gel once a day on their skins. The patients were visited every month by a dermatologist and the amount of response to the treatment and the patient satisfaction was recorded. Ferriman–Gallwey score of the treated area was determined.
Results:
After 6-months, mean thickness hairs in treating group were decreased from 102.00 ± 9.58 µm to 86.4 ± 11.4 µm (p < 0.05), this difference was statistically significant. Gel application did not indicate any type of side-effects.
Limitations:
Inclusion and exclusion criteria.
Conclusion:
Finasteride partially blocks 5α-reductase. Because of the good absorption through the skin and good solubility of this medicine, the prepared gel formulation applied on the hirsutism area showed a significant decrease in hair growth locally, so finasteride gel is an efficient and harmless therapy in patients with idiopathic hirsutism.
Keywords: :
Introduction
Hirsutism is the presence of excess body or facial terminal hair growth in females in a male-like pattern, and affects 5–15% of women, depending on definitionCitation1. Hirsutism is often regarded as a purely esthetic problem, but its medical importance is highlighted by the high prevalence of androgen excess disorders reported among hirsute womenCitation2. Although there are objective methods of assessing the extent of hirsutism, the perception and impact of excess body hair in an individual woman depends not only on its extent and severity, but also on social and cultural influencesCitation3. Quality-of-life studies have indicated that severe hirsutism has a serious adverse effect on social interactions and that affected women have a high incidence of depressive symptomsCitation4–6. A commonly used method to grade hair growth is a modified scale of Ferriman and GallweyCitation7. A score of eight or more has been considered to represent hirsutismCitation7. Sex steroids and a number of local and systemic factors can act directly and indirectly on the dermal papilla to regulate hair growth. In response to the increased levels of androgens at puberty, vellus follicles in specific areas develop into terminal hairsCitation8,Citation9. Androgens increase hair follicle size, hair fiber diameter, the proportion of time terminal hairs spend in the anagen phase and sebum secretion. Therefore, not only does androgen action alter the type of present hair, but also they will increase the oiliness of skin and hairCitation9,Citation10.
Hirsutism is a sign of increased androgen action on hair follicles, from increased circulating levels of androgens (endogenous or exogenous) or increased sensitivity of hair follicles to normal levels of circulating androgens. The severity of the hirsutism does not directly correlate with the level of androgen plasma concentration, because the response of the androgen-dependent follicles to excess amount of androgen varies considerably between individualsCitation8,Citation11.
The term idiopathic hirsutism has been used to describe the circumstance in which hirsutism is present with circulating androgen levels within the normal rangeCitation9. Nearly all hirsute women have an increase in androgens, usually testosterone, but the increase may not be sufficient to raise the serum total testosterone concentration above the normal range because the carrier protein for testosterone, sex hormone-binding globulin, is suppressed when androgen production is increased. In the remaining women, the hirsutism may be due to increased conversion of testosterone to dihydrotestosterone by the enzyme 5α-reductase in peripheral tissue, including hair follicles in which this metabolite is more potent than testosteroneCitation12–17. Thus, elevated 5a-reductase activity has been demonstrated in the hair follicles of women with idiopathic hirsutism, and excess hair growth is likely to be due to an exaggerated response of the hair follicle to normal androgen levelsCitation18. Nearly all circulating testosterone is bound to sex hormone binding globulin and albumin, with free testosterone being the most biologically active formCitation11.
Different medical therapies, alone and in combination, have been used to treat idiopathic hirsutism. Oral contraceptives and anti-androgen therapy such as spironolactone, cyproterone acetate and flutamide inhibits ovarian or adrenal androgen production and androgen activity either by blocking androgen cytochrome P450 receptors or by inhibiting 5α-reductase activity. In addition, cosmetic hair removing procedures (camouflage by bleaching and various mechanical ways such as shaving, plucking, and using depilatory creams) achieve the desired result for only a brief periodCitation19,Citation20.
Finasteride is a 5α-R inhibitor which can be used systemically or locally. Finasteride decreases hair growth by causing less exposure of hair follicles to androgen stimulationCitation21,Citation22. Although the efficacy of systemic finasteride has been reported in different studies, there are a few articles in which the efficacy and tolerability of topical gel of finasteride has been evaluated. The aim of this study was to examine the efficacy and tolerability of topical finasteride in females with idiopathic hirsutism.
Materials and methods
Materials
Hydroxy propyl methyl cellulose (HPMC) and sodium carboxy methyl cellulose (CMC) were purchased from Pastor chemical company (Japan). Sodium hydroxide (NaOH), potassium dihydrogen phosphate (KH2PO4), methyl paraben, and propylene glycol were purchased from Merck (Germany). Finasteride was provided by Iran hormone pharmaceutical company (Tehran, Iran), and ethanol and dialysis membrane were purchased from Touba azma (Tehran, Iran).
Clinical study
A double blind and randomized study which was controlled by dermatologist in comparison to placebo gel was conducted for 6 months. The inclusion criteria for the subject selection were the following:
Ferriman-Gallwey Score > 8Citation7;
Normal serum androgen (total testosterone, free testosterone, androstenedione and DHEA-S);
Normal serum level of thyroid hormone, prolactin and cortisol;
No chemical or biochemical evidence of polycystic ovarian syndrome, which is ruled out by regular menstrual cycles, normal ultrasound exam, and serum LH/FSH ratio < 1 and normal serum SHBG;
Normal basal and ACTH-stimulated serum 17-hydroxyprogestrone level;
Absence of chronic renal disease, diabetes mellitus, and hepatic disease; and
The subjects that did not use any other drugs for treatment of hirsutism
The selected women, after signing a written consent form, are divided randomly in two groups; treatment group (with finasteride) and control group (with placebo). The number of patients in each group was 15.
This study was approved by the ethical committee of Kermanshah University of medical sciences. All the patients gave informed consent for their participation in our study after reading the protocol of this experiment. They were informed that finasteride could affect a male fetus and consequently pregnancy was contraindicated during the treatment and so effective contraceptive must be used. They were also informed that the potential side-effects of finasteride were unknown in women and they should report any possible side-effects during the medication. The patients were advised not to use any other drug for idiopathic hirsutism at the same time. Moreover, electrolysis, waxing, and plucking were not permitted during the treatment, whereas shaving was permitted for subjective evaluation of hair growth by patients. The degree of hirsutism in the skin area was determined by Ferriman-Gallwey score. The scale is from 0 (absence of terminal hairs) to 4 (extensive terminal hair growth). Premature scores were determined by two examiners and mean scores were calculated for each patients. Three hairs of the skin area were plucked from each patient. Each hair was then fixed on a slide with a transparent resin that solidifies with air and was covered with another slide. Hair caliber was measured with a micrometer applied to an optical microscope (×10 magnification). Then they received finasteride gel 0.25% on their skins once a day for 6 months. They were advised to clean the skin area before usage and to avoid using powder, lotions and sprays 3 h after gel application.
The patients were seen in consultation at 1 month intervals. Questions were asked about the side-effects, menstrual abnormalities and also patients self-evaluation of the clinical effects of the treatment. After 6 months, rate of hair growth of the skin area, the mean caliber of three plucked hairs, and the Ferriman-Gallwey score of the skin area was evaluated.
Statistical analyses
Data are presented as mean ± SD or a percentage. Statistical analyses were performed using SPSS software version 16:0:0 and paired T-test for comparison of quantitative variables was used to compare the hair caliber before and after medication. p Values less than 0.05 were statistically significant.
Preparation of Finastride gel
According to previous studies related to the clinical effect of finasteride on hirsutism, for preparing finasteride gel 0.25%, pure finasteride was used. Hydroxy propyl methyl cellulose and sodium carboxymethyl cellulose as polymer gel formulations were used by the specified amount. Propylene glycol as a wetting agent and ethanol as a co-solvent for finasteride and methyl paraben as a preservative agent were used. A 15 g aluminum tube was selected as an appropriate package. The placebo gel consisted of the derma base alone in the same size and type of tube. No difference in color or texture was evident between the placebo and medication containing gels.
Physicochemical and microbial stability tests
To study the chemical and physical stability of the formulation, pharmaceutical products was placed at 40°C and 70% humidity in the germinator and each month, viscosity, color, and amount of drug were studied. Microbial and preservative effectiveness testing was based on the US Pharmacopoeia guidelines.
In vitro evaluation of drug release
Preparation of solutions
Isotonic phosphate buffered saline pH 5.75 (PBS-buffer) was prepared by dissolving 1.36 g KH2PO4 and 0.028 g NaOH in 200 ml distilled water. PBS-buffer was used as the receptor solution.
Experiments with Franz diffusion cells
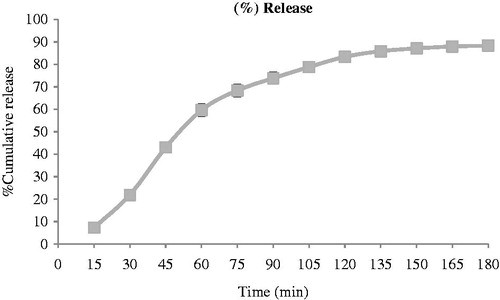
Franz diffusion cell with a volume of 78 ml was used for the drug release evaluation. The Franz diffusion cell consisted of a donor and receptor compartment. The membrane was mounted between the cell compartment and an O-ring was used to position the membrane. The two cell compartments were held together with a clamp. The temperature of Franz diffusion cell chamber was adjusted to 37°C by a water bath circulation and 1 g of gel was applied to the cell that was put on dialysis membrane. The receptor solution was continuously stirred by means of a spinning bar magnet, at 200 rpm; 2.0 ml aliquots were withdrawn through the sampling port of the receptor compartment at specified time intervals. The cells were refilled with receptor solution to keep the volume of receptor solution constant during the experiment. The experiments were run for 3 h. Sample absorbance was read by a spectrophotometer at a wavelength of 210 nm. The linearity of calibration curve with concentration range from 0.6125–10 µg/ml and linear equation y = 0.1669x − 0.0196 has been verified. The drug release profiles of the drug formulations were plotted according to the calibration curve.
Results
Patient compliancy
None of the women reported any problems with irregularity of menstrual periods, changes in libido and changes in energy level, nausea, vomiting, diarrhea, abdominal pain or headache. Allergic reaction to the medication or skin eruption in the areas in which the gels were applied was observed in one patient. All of the patients showed a good compliancy related to the finasteride gel application and there were no incompliance reports about the viscosity, odor, color and filling during the remaining time of gel on the skin.
Clinical effects
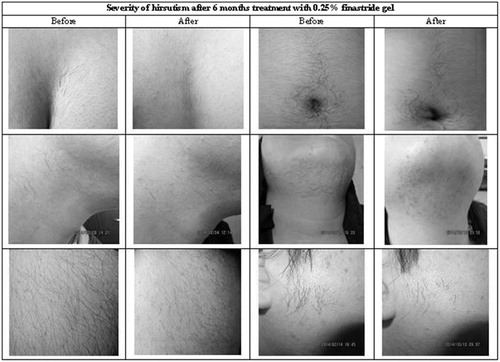
All of the patients in treating group noted a considerable diminished rate of hair growth on the areas in which the gel were applied (at least fewer times needed for shaving) and hair follicles became looser and easier to pluck, but patients in placebo group didn’t mention any difference rate of hair growth. The pictures of some patients before and after treatment are shown in .
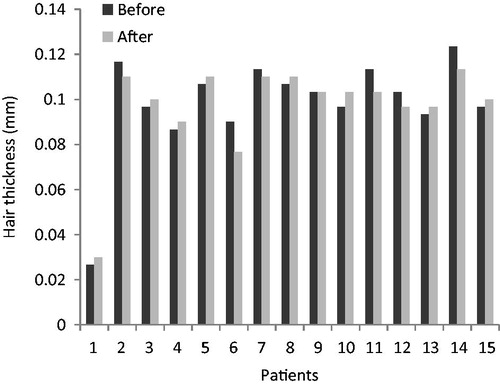
Hair thickness in the treating group, before and after treatment, are shown in and and hair thickness in the placebo group, before and after treatment, are shown in and . Comparison Chart for mean thickness hairs before and after 6 months in both groups are shown in and .
Figure 2. Comparison chart for mean thickness hairs before and after 6 months in the treating group.
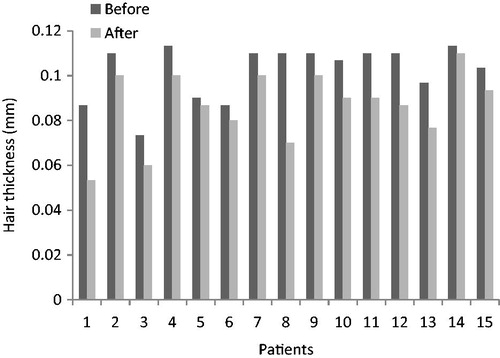
Table 1. Hair thickness (mm) before treatment in the treating group.
Table 2. Hair thickness (mm) after treatment in the treating group.
Table 3. Hair thickness in the placebo group, before treatment.
Table 4. Hair thickness in the placebo group, after treatment.
After 6-months, mean thickness hairs in the treating group were decreased from 102.00 ± 9.58 µm to 86.4 ± 11.4 µm (p < 0.05), this difference was statistically significant.
After 6-months, mean thickness hairs in placebo group were decreased from 98.22 ± 22.32 µm to 96.88 ± 20.75 µm (p < 0.05), this difference was not statistically significant.
In vitro drug release
Sample absorbance was read by a spectrophotometer at a wavelength of 210 nm. The linearity of calibration curve with concentration range from 0.6125–10 µg/ml has been proved. The drug release profiles of the drug formulations were plotted according to the calibration curve. The percentage of drug release can be seen in and .
Table 5. The percentage of drug release from finastride gel.
Discussion
Hyperactivity of 5α-reductase in the skin is considered a major mechanism of excessive hair growth in hirsute women with normal levels of serum androgens. Androgens increase hair follicle size, hair fiber diameter, the proportion of time terminal hairs spend in the anagen phase, and sebum secretion. Therefore, not only does androgen action alter the type of present hair, also it they will increase the oiliness of skin and hair, since finasteride is a 5α-reductase inhibitor, with no androgenic, anti-androgenic, or steroid hormone-related properties, and affinity for androgen receptors, the use of finasteride for the treatment of hirsutism is rational because of its specific effect on 5α-reductase, the enzyme responsible for sensitizing the hair to testosteroneCitation22–26. In previous studies, orally administered finasteride has been successfully used in the treatment of hirsutism, but has major side-effectsCitation27–29. Notably there have been fewer investigations about topical application of finasteride. In fact its effects as a topical drug in the treatment of hirsutism are still debated, so this study was designed to determine the efficacy of finasteride gel 0.25% in management of idiopathic hirsutism and treatment of hirsutism with topical finasteride to lessen the side-effects. Because the majority of patients with hirsutism have oily skin, the oily base used for production pharmaceutical formulations may cause unpleasant feeling on the skin and can even lead to acne. Therefore, usage of the water-based gel formulations, in addition to the lack of acne, is more suitable for washing and cleansing of the skin, and has greater acceptance by patients. Heydari et al.Citation30 studied 40 women with idiopathic hirsutism received finasteride cream 0.25% twice a day for 6 months on their chins and reported that acne was reported by eight patients (20%) during the therapy, while in this study with gel application, acne was not reported by patients. In a previous study, LucasCitation22 showed a significant reduction in mean hair count and the thickness of the hair in eight women with hirsutism treated with finasteride cream 0.25%, while another study by Heydari et al.Citation30 indicated that mean hair thickness and mean Ferriman–Gallwey score were decreased. This study confirms the results of these two studies.
Conclusions
The current study, designed to assess the clinical effects of finasteride gel on hirsutism, showed significant improvement in the area treated by topically applied finasteride.
In our study no adverse effect, except a rash in one person, was reported. This indicates that topical finasteride is a promising therapy for idiopathic hirsutism, with less side-effect in comparison with the orally administered route.
In this study, hair follicles became looser and easier to pluck. These effects helped the patients to have fewer problems with their hirsutism, as they managed to pluck hairs in longer intervals. One of the most important reasons for patient satisfaction from this dosage form was reduction in shaving time.
Transparency
Declaration of interest
The authors have no relevant financial relationships to disclose.
Acknowledgments
We gratefully acknowledge the Vice Chancellor for Research and Technology, Kermanshah University of Medical Sciences for financial support. This work resulted from pharm D thesis of Sahahr Masoud in Faculty of Pharmacy, Kermanshah University of Medical Sciences, Kermanshah, Iran.
References
- DeUgarte CM, Woods KS, Bartolucci AA, et al. Degree of facial and body terminal hair growth in unselected black and white women: toward a populational definition of hirsutism. J Clin Endo Metab 2006;91:1345-50
- Carmina E, Rosato F, Janni A, et al. Relative Prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab 2006;91:2-6
- Yildiz BO, Bolour S, Woods K, et al. Visually scoring hirsutism. Hum Reprod Update 2010;16:51-64
- Barth JH, Catalan J, Cherry CA, et al. Psychological morbidity in women referred for treatment of hirsutism. J Psychosom Res 1993;37:615-19
- Archer JS, Chang RJ. Hirsutism and acne in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2004;18:737-54
- Jones GL, Hall JM, Balen AH, et al. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update 2008;14:15-25
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961;21:1440-7
- Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med 2005;353:2578-88
- Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev 2000;21:347-62
- Messenger A. The control of hair growth: an overview. J Invest Dermatol 1993;101:4-9S
- Hunter MH, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Physician 2003;67:2565-72
- Samojlik E, Kirschner MA, Silber D, et al. Elevated production and metabolic clearance rates of androgens in morbidly obese women. J Clin Endocrinol Metab 1984;59:949-54
- Kirschner MA, Samojlik E, Silber D. A comparison of androgen production and clearance in hirsute and obese women. J Steroid Biochem 1983;19:607-14
- Matteri RK, Stanczyk FZ, Gentzschein EE, et al. Androgen sulfate and glucuronide conjugates in non hirsute and hirsute women with polycystic ovarian syndrome. Am J Obstet Gynecol 1989;161:1704-9
- Labrie F. Intracrinology. Mol Cell Endocrinol 1991;78:C113-18
- Hatch R, Rosenfield RL, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 1981;140:815-30
- Oláh KS. The modern management of hirsutism. Rev Gynecol Prac 2004;4:211-20
- Nikolaou D, Gilling-Smith C. Hirsutism. Cur Obstet Gynecol 2005;15:174-82
- Callan A. Management of hirsutism. Australas J Dermatol 1982;23:97-104
- Tartagni M, Schonauer MM, Cicinelli E, et al. Intermittent low-dose finasteride is as effective as daily administration for the treatment of hirsute women. Fertil Steril 2004;82:752-5
- Price TM, Allen S, Pegram GV. Lack of effect of topical finasteride suggests an endocrine role for dihydrotestosterone. Fertil Steril 2000;74:414-15
- Lucas KJ. Finasteride cream in hirsutism. Endocr Pract 2001;7:5-10
- Rittmaster RS. Treating hirsutism. Endocrinologist 1993;3:211-18
- Rittmaster RS, Loriaux DL. Hirsutism. Ann Intern Med 1987;106:95-107
- Akalin S. Effects of ketoconazole in hirsute women. Acta Endocrinol (Copenh) 1991;124:19-22
- Moghetti P, Tosi F, Tosti A, et al. Comparison of spironolactone, flutamide, and finasteride efficacy in the treatment of hirsutism: a randomized, double blind, placebo controlled trial. J Clin Endocrinol Metab 2000;85:89-94
- Sahin Y, Dilber S, Kelestimur F. Comparison of Diane 35 and Diane 35 plus finasteride in the treatment of hirsutism. Fertile Steril 2001;75:496-500
- Falsetti L, Gambera A, Legrenzi L, et al. Comparison of finasteride versus flutamide in the treatment of hirsutism. Eur J Endocrinol 1999;141:361-7
- Falsetti L, Gambera A. Comparison of finasteride and flutamide in the treatment of idiopathic hirsutism. Fertil Steril 1999;72:41-6
- Heydari I, Amiri A, Razmjou S, et al. The efficacy of topical Finasteride in the treatment of Idiopathic Hirsutism. J Turk Acad Dermatol 2008;2