Abstract
Objective:
Many medications have potential interactions with other drugs or substances when prescribed together. This study was intended to investigate the extent of poly-pharmacy, event of drug–drug interactions and associated ADRs in Adama Referral Hospital, Oromia regional State, Ethiopia to create awareness of potential drug interactions and for development of clinical strategies to prevent the occurrence of DDIs.
Methods:
A retrospective study was done at Adama Referral hospital, Adama city, Ethiopia during March–May 2014. Medscape online were used for DDIs and ADRs detection purposes.
Results:
The average number of drugs prescribed per person (encounter) in this study was found to be 2.6, showing the presence of poly-pharmacy prescribing practice based on WHO recommendations (1.4–2.4). With 788 medications prescribed, 267 DDIs were found in this study and 62 (20.7%) were categorized as serious DDIs, 95 (31.7%) as significant DDIs, and 110 (36.7%) as minor DDIs. DDIs occurrence was also categorized according to the mechanisms, Pharmacokinetic and pharmacodynamic interactions; the highest frequency of DDIs was observed in 85 (31.8%), attributable to metabolism interaction followed by Antagonistic effect in 51 (21.4%), and Synergistic/Additive effect in 44 (16.5%). It was observed that serious DDIs most often caused possible cardiovascular ADRs.
Conclusions:
The results of the study showed the high number of drugs per person compared to the WHO-reported average number of drugs per person and occurrence of DDIs associated with severe cardiovascular risk ADRs in the Adama Referral Hospital. This study recommends that the drug information center facilities and drug prescription validation is done by the pharmacist and the development of pharmacotherapeutic guidelines supporting selection of drugs in Ethiopian hospitals for preventing DDIs and ADRs.
Introduction
Pharmacists play a critical role in the medication use process. Throughout this process, there is the potential for unexpected adverse events, including errors in prescribing, dispensing, and administering medications, idiosyncratic reactions, and other adverse effects. These events can all be described as medication misadventures. Pharmacists need to realize the potential for numerous medication misadventures and be prepared to recognize and prevent such occurrences and minimize adverse outcomesCitation1. A medication misadventure may or may not result in an injury to a patient. All adverse drug events (ADEs), adverse drug reactions (ADRs), and medication errors fall under the umbrella of medication misadventures. ADRs refer to any unexpected, unintended, undesired, or excessive response to a medicine. Drug–drug interactions (DDIs) can also land in the category of ADRs. All medications, including the excipients of a product, are capable of producing adverse effects. Some of these are idiosyncratic and are unpredictable. However, many are predictable, based on understanding of their pharmacology, and, therefore, they can be anticipated and prevented. It is believed that 30–60% of ADRs are preventableCitation1.
Reporting of drug–drug interactions (DDIs) causing adverse drug reactions (ADRs) is neglected due to high work pressure, time, patient/physician ratio, consultation time/patient rates, etc. Recently, a few studies have been conducted in Ethiopia, one at Hawassa University teaching and referral hospital, south Ethiopia, about prescription patternCitation2, and another at Gondar teaching referral hospital, North West Ethiopia, about the extent of poly-pharmacy and occurrence of DDIs and ADRCitation3. Drug-related morbidity and mortality are often preventable, and pharmaceutical services can reduce the number of ADRs, the hospital stays, and the cost of care. Drug-related problems such as inappropriate prescription, clinically relevant drug–drug interactions, non-adherence, and adverse drug reactions are the most commonly experienced in practiceCitation3.These problems could be well prevented or minimized by initiating changes in the drug therapy through clinical pharmacy services. Awareness of potential drug interactions may permit development of clinical strategies to avoid their occurrence.
Prescribing multiple drugs to patients at once (called poly-pharmacy) is not generally recommended as problems such as dose missing, over dosing, DDIs, and ADRs may occur. Numerous medications have potential interactions with other drugs or substances when prescribed together. Studies have shown that increasing the number of drugs per patient increases the risk of DDIs and ADRs exponentiallyCitation4,Citation5. This study was intended to investigate the extent of poly-pharmacy, occurrence of drug–drug interactions and associated ADRs in Adama Referral Hospital, Oromia regional State, Ethiopia to create awareness of potential drug interactions and for development of clinical strategies to prevent the occurrence of DDIs.
Patients and methods
Study area and period
This study was done in Adama Hospital Medical College, Adama City, Oromia regional state, Middle East Ethiopia, 99 km away from Addis Abeba. The hospital serves as a referral hospital to the patients from different parts of Oromia region and other nearby regions such as Amhara, Somali and afar regional states. This study was carried out from March–May 2014.
Study design
A retrospective cross-sectional hospital-based study was conducted with hospitalized patients.
Study population
Medical record charts of patients who were admitted to all wards during the study period were included in the study.
Inclusion criteria
Patents who were taking two or more drugs concurrently for at least 48 hours were included.
Exclusion criteria
Patients who were taking two or more drugs but not concurrently;
Incomplete patient medical records;
Hospital stay of fewer than 48 h; and
Patients on single drug therapy.
Data processing and analysis
Medscape online was used for DDIs and ADRs detection purposes. Data were presented as mean and percentages.
Results
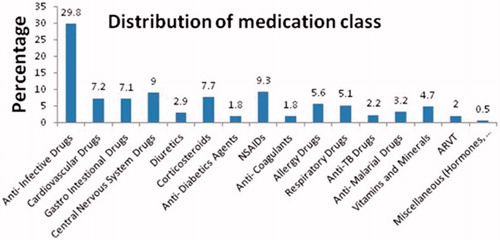
A total of 300 patient records were complete and were enrolled in the study. Among the 300 patients, 149 (49.7%) were male and 151 (50.3%) female. The age distribution of the patients included in the study were less than 1 year (n = 1, 0.3%), 1–17 years (n = 41, 13.7%), 18–55 years (n = 215, 71.7%), and more than 55 years (n = 43, 14.3%). The total number of medications prescribed was 788. Thus, the average number of medication per person or mean was 2.6 (SD = 0.26), with a range between 2–6. shows the incidence of poly-pharmacy. The commonly prescribed medication classes were antibiotics (235; 29.8%), followed by NSAIDs (74; 9.3%), and central nervous system drugs (71; 9%). The distribution of medication classes is shown in .
Table 1. Incidence of polypharmacy in Adama referral hospital.
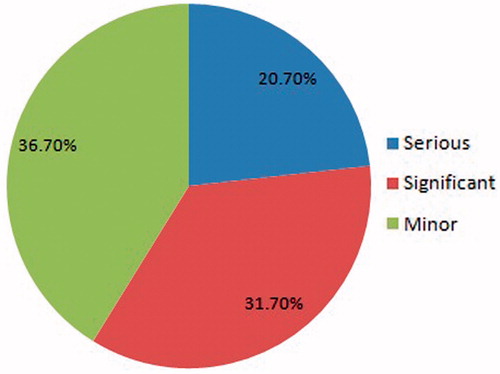
DDIs were evaluated using the Medscape online drug–drug interaction checker. The occurrence of DDIs was divided into three categories; severe, significant, and minor. The percentage of DDIs is shown in the pie chart (). With 788 medications prescribed, 267 DDIs were found in our study and categorized as serious DDIs (62/267; 23.2%), significant DDIs (95/267; 35.6%), and minor DDIs (110/267; 41.2%). The degree of DDIs classified as serious, significant, and minor with a number of drugs and prescriptions are shown in .
Table 2. Degree of DDIs categorized as serious, significant, and minor.
DDIs were divided into two groups according to the mechanisms involved, DDIs with a pharmacokinetics basis and a pharmacodynamics basis. The occurrence of DDIs according to the mechanisms involved was shown in the . Dangerous ADRs, such as increasing the QTc intervals, Torsade de pointes, and risk of sudden cardiac arrest were associated with CNS agents causing serious DDIs. Possible ADRs attributable to a combination of the drugs were recognized by using medscape online, and ADRs were categorized systematically, listed in the . Drugs that cause serious and significant DDIs and its effects are set out in Tables 5 and 6 (included in the supplementary material).
Table 3. The occurrence of DDIs according to the mechanisms involved in the Adama Referral Hospital.
Table 4. Possible ADRs due to a combination of the drugs were recognized by using medscape online in the Adama Referral Hospital.
Discussion
Many medications have potential interactions with other drugs or substances when prescribed together. According to the WHO definitions, the average number of drugs per person was within the range of 1.4–2.4. In this study, the average number of drugs prescribed per patient was found to be 2.6, showing the presence of poly-pharmacy prescribing practiceCitation4. No doubt, a high number of drugs prescribed to a patient increase the risk of DDIs, contraindications, and adverse drug reactions. Studies have shown that increasing the number of drugs increases the risk of DDIs and ADRs exponentiallyCitation4,Citation5.
The use of electronic data resources and decision support to screen for DDIs is now the standard of practice for healthcare providersCitation6. For this study, the online electronic data resource used for the DDIs assessment was medscape online that provides evidence-based drug information about DDIs and possible ADRs categorizing into three classes, Serious, Significant, and Minor. In total, 267 DDIs were found in this study and over one-half of identified DDIs were serious or significant (). The study conducted recently in Gondar referral teaching hospital, North West Ethiopia, showed a total of 1324 DDIs were detected in the 711 drugs of the 2180 prescriptions containing two or more drug regimensCitation3. The DDIs found in this study were categorized according to the mechanisms, pharmacokinetic, and pharmacodynamic interactions. The highest frequency of DDIs was observed as 85 (31.8%), owing to metabolism interaction, followed by antagonistic effect in 51 (21.4%) and synergistic/additive effect in 44 (16.5%), shown in .
DDIs detected in this study were carefully reviewed for the occurrence of possible ADRs. The occurrences of ADRs were recognized using medscape online and systematically categorized based on the risk of the body system where these ADRs could occur (shown in ). We observed that serious DDIs most often caused possible cardiovascular ADRs. The study conducted at Gondar Hospital discloses that more than half (61.22%) of the patients were at risk for cardiovascular effects, such as hypertension, hypovolemia, cardiac arrhythmias, and QT prolongation, from DDIsCitation3.
Other potential ADRs detected in this study were increased toxicity caused by pharmacodynamic synergism or effects on hepatic CYP3A4 and 2D6 metabolism. Other DDIs risks included were pharmacodynamic antagonism, chlorpromazine and amitriptyline increase sedation, decreasing renal clearance, increased serum potassium, and inhibition of GI absorption. Moreover, administration of the combinations ibuprofen + hydrocortisone, diclofenac + dexamethasone, and diclofenac + hydrocortisone cause GI ulceration; and furosemide + digoxin and enalapril + furosemide might cause Hypokalemia that increases digoxin effects; possible digoxin toxicity might cause nausea, vomiting, and cardiac arrhythmias, Risk of acute hypotension and renal insufficiency found with furosemide. Other significant ADRs included were diclofenac + ibuprofen and indomethacin both increase anticoagulation, increased risk of CNS stimulation, and seizures with high doses of fluoroquinolones; Allopurinol may increase potential for allergic or hypersensitivity reactions to amoxicillin; Sulfadiazine + pyrimethamine may increase the risk of anemia; Lovastatin might cause theophylline toxicity, which leads to increased palpitation, vomiting, and Lovastatin administration, with insulin changes in blood glucose and increased risk of hypoglycemia or hyperglycemiaCitation7.
Limitations of the study
Since the current study was retrospective in nature, we could not evaluate a clinical effect of DDIs, but future prospective studies will follow patients to allow evaluation of the clinical effect of DDIs. We evaluated the occurrence of DDIs at a single hospital that may not occur in another hospital, so a pilot study needs to be conducted throughout the country. ADRs found in this study with the help of electronic software may not occur in patients taking medications, and DDIs and ADRs can differ between patients based on the disease condition, genetic characteristics, etc. The potential DDIs and ADRs should be used as a precaution to use alternative drugs, monitor the patient closely, and adjust the patient’s treatment regimen if needed.
Conclusion
The results of the study showed the high number of drugs per person compared to the WHO-reported average number of drugs per person and occurrence of DDIs associated with severe cardiovascular risk ADRs in the Adama Referral Hospital. We suggest that pharmacists review drug prescriptions for potential DDIs and ADRs and that Ethiopian hospitals develop guidelines to assist in drug selection to prevent DDIs and ADRs.
Transparency
Declaration of funding
This study wasn’t funded.
Declaration of financial/other relationships
The authors have no relevant financial relationships to disclose.
Acknowledgements
We appreciate the support of medical doctors, AMHC, Adama and we would like to thank also the Department of Pharmacy for consistent support of materials and appropriate guidance.
References
- Malone P. Relationship among medication misadventures, adverse drug events, medication errors, and adverse drug reactions. Am J Health-Syst Pharm 1998;55:165-6
- Desalegn. Assessment of drug use pattern using WHO prescribing indicators at Hawassa University teaching and referral hospital, south Ethiopia: a cross-sectional study. BMC Health Serv Res 2013;13:170
- Admassie E, Melese T, Mequanent W, et al. Extent of poly-pharmacy, occurrence and associated factors of drug-drug interaction and potential adverse drug reactions in Gondar Teaching Referral Hospital, North West Ethiopia. J Adv Pharm Technol Res 2013;4:183-9
- Wochenschr K. Poly-pharmacy, inappropriate prescribing and adverse drug reactions in Austria. Mid Euro J Med 2008;120:713-4
- Cadieux RJ. Drug interactions in the elderly. How multiple drug use increases risk exponentially? Postgrad Med 1989;86:179-86
- Wong K, Yu S, Holbrook A. A systematic review of medication safety outcomes related to drug interaction software. J Pop Ther Clin Pharmacol 2010;17:243-55
- Heininger-Rothbucher D, Bischinger S, Ulmer H, et al. Incidence and risk of potential adverse drug interactions in the emergency room. Resuscitation 2001;49:283-8