Abstract
Objectives: The Lupus Academy was established as an independent, consortium-led continuing medical education (CME) initiative for physicians interested in the pathogenesis, diagnosis and management of patients with systemic lupus erythematosus (SLE) and related conditions, based on identification of an unmet clinical need. The Lupus Academy is intended to be a long-term enterprise that is committed to sharing best practice in the field of lupus. Challenge: European CME remains in its infancy and many regulatory aspects are yet to be finalised. Pharmaceutical company supporters must be willing to adopt an ‘arms length’ approach to funding to ensure that educational programmes are truly independent, whilst funding must be spent in an appropriate and ethical manner by education providers. This review manuscript describes the innovative approach of the Lupus Academy within the global CME environment; illustrates the interactions between pharmaceutical supporters, clinicians, education providers and support organisations, in adherence to international guidance; and shows how a pharmaceutical company can support a CME activity whilst satisfying the external regulatory environment. Project structure: The Lupus Academy was created by a Steering Committee of six international experts with funding from GlaxoSmithKline and Human Genome Sciences and support from three organisations to facilitate the delivery of logistics, medical education materials, and CME compliance and financial management. Together, these groups formed a Consortium in which all parties took equal and collective responsibility for the success of the programme, from content development to regulatory compliance, CME adherence and financial management. How and where the meeting took place: The Inaugural Meeting of the Lupus Academy took place in Barcelona, Catalonia, Spain in 2012. Programme content and learning objectives for the 1.5-day meeting were developed by a Steering Committee based on clinical training needs and advances in lupus, and the 17-strong expert faculty was selected by the Steering Committee. The Consortium worked with national and international lupus organisations to identify appropriate clinicians to attend the meeting; a broad range of delegates were attracted to the meeting from across a wide range of disciplines, including rheumatology, nephrology, dermatology, clinical immunology and internal medicine. Outcomes and results: The Inaugural Meeting included plenary sessions and interactive workshops with keypad voting to promote interactivity and delegate participation. The meeting was attended by 296 delegates from 30 countries across Europe, the Americas and the Asia Pacific region. Most delegates rated the educational content, meeting objectives and logistics of the meeting highly. Almost all delegates stated a preference for independent educational initiatives compared with pharmaceutical-controlled events and most delegates considered the absence of commercial bias to be important. Many of the delegates indicated that they would change their clinical practice based on what they learnt from the meeting. Conclusions and future objectives: The Inaugural Meeting of the Lupus Academy was considered a success by the Consortium members and financial supporters. The overall approach to the initiative, including the structure of the Consortium, the independence of the high-quality educational programme, the financial management and CME accreditation, was considered the key to its success. This long-term enterprise will continue as an annual event, with interactive workshops forming a greater part of future programmes to allow delegates to share clinical experiences. The second Annual Meeting of the Academy took place in Buenos Aires, Argentina in April 2013, the third is being planned for Berlin, Germany in March 2014.
Introduction
Improving patient outcomes relies on sustained and measurable improvements in Continuing Medical Education (CME) for healthcare professionals. Reassuringly, CME in Europe is developing at a rapid pace. It has been just 13 years since the concept was initiated on a pan- European basis, and it has become a commonly understood concept that is now perceived as ‘normal’ by healthcare professionals, an expectation by health authorities, and a regular activity for allied organisations; these include commercial organisations that manage and present educational activities and financial supporters, such as the pharmaceutical industry. There is an expectation across Europe that healthcare professionals engage in continuing professional activities to demonstrate that they are up to date with medical advances. Likewise, there is an expectation from healthcare professionals for independent/unbiased education, which may or may not be financially supported by commercial parties.
Undeniably, European CME has benefited from the experiences of CME in North America, notably the development of needs assessments, outcome measures and the avoidance of commercial bias; however, European CME is still developing and many regulatory aspects are yet to be finalised and achieve robustness. Moreover, the use of delegate feedback to shape educational programmes, in line with broader educational needs assessments, and to ensure measurable improvements in performance and patient outcomes is fundamental to the success of CME. One important aspect of CME is the appropriate distance of the financial supporter from the funded project, and the independent development of content.
The call for independent/unbiased education has led to a change in the way that educational programmes are funded by pharmaceutical companies. Educational meetings with solely promotional content are no longer well-received by healthcare professionals; there is an increasing requirement for the big clinical picture and for balanced updates on pathogenic mechanisms, diagnostic tools, evaluation methods, as well as all treatments that support improved clinical outcomes. Moreover, within pharmaceutical companies, there is a need to demonstrate corporate responsibility and an increased willingness to provide ‘arms length’ funding to support third parties in the development of independent educational programmes. Of course, this funding is not without conditions, including acknowledgement of the sponsor's willingness to support independent CME-accredited programmes and, importantly, acknowledgement that the funding is spent in an appropriate and ethical manner. This paper presents a case study in which several challenges were overcome to establish and deliver an independent, consortium-led CME initiative. The Lupus Academy project presented a number of issues in its establishment and operation. The authors believe that this is the first full CME initiative that GlaxoSmithKline (GSK) has supported under their arms-length Standard Operating Procedures (SOPs), outside the USA.
This publication aims to demonstrate some fundamental aspects of best practice in CME that will help to inform future initiatives. The experiences of the Lupus Academy illustrate how best to work within a financially supported programme with support organisations and educational providers in utilising funding from pharmaceutical companies using recently published guidance.Citation1 Moreover, such experiences show how a pharmaceutical company can support a CME activity financially, whilst satisfying both its internal SOPs and the external regulatory environment. The Lupus Academy, now in its second year of providing CME, is a long-term initiative that is committed to sharing best practice in its clinical area; this paper will describe its innovative approach within the European and global CME environment.
Lupus Academy: an educational initiative from inception to delivery
The Lupus Academy was created and is led by a Steering Committee of six international experts in systemic lupus erythematosus (SLE), who are committed to establishing an independent and highly interactive global educational forum through which clinicians can enhance their clinical approaches to the management of lupus. The Steering Committee provides insights into unmet clinical needs and leading edge developments in clinical practice in Europe, South America and Asia. It provides the Lupus Academy with a diverse and balanced expertise in the clinical management of lupus. Each member has a different relationship with industry and there is no overall bias towards any individual company or interest in a single product. In future, the Committee intends to invite additional clinical experts to both expand the expertise of the group and represent further geographical areas.
In early 2011, the members who formed the Steering Committee (2011 - 2012) attended and participated in the 8th European Lupus Conference in Porto, Portugal, during which discussions on the need for an independent CME-accredited educational initiative began to form. Independently, several of the Steering Committee members had already approached GSK, as the company with the highest likelihood of having a product approved with an indication in lupus, for funding for several different academic/CME activities, including national meetings and an online postgraduate course on autoimmune diseases for Latin American physicians. This request resulted in GSK and Human Genome Sciences (HGS) offering combined funding for a global CME initiative, the Lupus Academy, to address unmet educational needs in lupus on a global scale. Despite launching the first treatment for lupus in 50 years, GSK/HGS were keen to support the Lupus Academy with arms-length funding, taking no part in the creation of the programme, selection of speakers, geographical location or presentation of content. GSK/HGS's interests in supporting this programme were to endorse the motivations of a Steering Committee committed to improving patient outcomes in systemic lupus erythematosus (SLE) and allied diseases. For the Steering Committee, these motivations were to enhance, both directly and indirectly, the clinical training of physicians from a wide variety of specialities involved in the management of patients with lupus, including those working in the fields of internal medicine, rheumatology, nephrology, dermatology and clinical immunology. It was agreed that the most effective format for this educational programme would be a long-term global CME initiative created around a framework of annual meetings and enduring materials. With improving patient outcomes as its core mission, the Lupus Academy aims to become a self-sustaining, credible and reliable source of multidisciplinary education for the growing number of clinical experts in lupus.
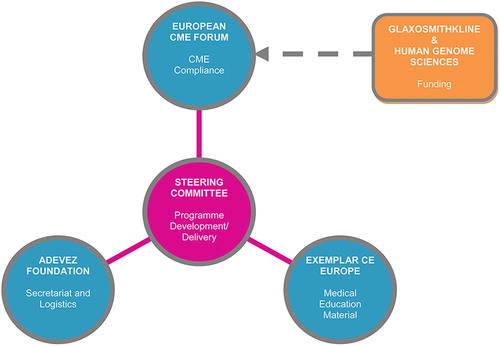
In mid 2011, the Steering Committee selected and appointed three support organisations to facilitate the delivery of the logistics, medical education materials and CME compliance for the Inaugural Meeting of the Lupus Academy ().
Lupus Academy grant proposal
An important consideration for the initiative was the funding structure. The financial supporters considered it inappropriate to fund a commercial entity to provide this independent activity; the preferred route would have been via a hospital or university. The Lupus Academy Steering Committee investigated potential options within their own institutions; however this was not possible for a number of reasons, mostly related to the high fees required to manage the fund. Some institutions were asking for significant levels of fees as a percentage of the project, without offering professional services, which could have resulted in a detrimental effect on the finances of the project. Consequently, an alternative route for receiving the funds was sought.
The financial supporters required that funding should go to a ‘not-for-profit’ organisation. One of the support organisations, the European CME Forum, as a ‘company limited by guarantee’ is a ‘not-for-profit’ organisation in the UK and fulfilled this requirement. However, despite heritage in presenting medical education meetings, it was not a recognised educational institution. In order for it to be acceptable for the European CME Forum to manage the funding, some additional provisions needed to be established.
The first was to demonstrate that the programme would be developed and managed independently. Pharmaceutical industry regulations provide no guidance to indicate what constitutes an acceptable distance between a financial supporter and an independent, educational programme that is CME accredited, whether from the European Federation of Pharmaceutical Industries and Associations (EFPIA)Citation2 or the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA),Citation3 even though the most recent version of the IFPMA guidelines has started to address this topic. As the meeting was to be CME accredited, the process was described to the supporting companies; although the process demonstrated some controls, it was still felt that CME accreditation alone was not a sufficiently robust demonstration of independence to satisfy the financial supporters’ internal compliance requirements that there was no undue commercial influence or control.
The second step was the creation of a Consortium formed between the Steering Committee members, which included six lupus experts with responsibility for the programme content, and three support organisations: the European CME Forum for managing the grant and overseeing the regulatory and CME compliance of the Consortium; Exemplar CE Europe for facilitating the development and implementation of the meeting programme and scientific content; and Adevez as the secretariat also responsible for logistics, travel and on-site management. This meant that, as a Consortium, all parties took equal and collective responsibility for the success of the programme, from content generation and delivery to regulatory compliance, CME adherence and financial management.
The third provision arose from a discussion with the European CME Forum's local tax authority, Her Majesty's Revenue and Customs (HMRC; the UK tax authority), which advised that, as the supporting companies would not gain direct commercial benefit from supporting this educational activity, the accounts should be run ‘outside the scope of value-added tax (VAT)’ at 0%. This proved to be a valuable demonstration to the pharmaceutical company supporters that the structure was robust and independent and satisfied the strict compliance requirements of the financial supporters.
The Consortium submitted a grant request to run a 1.5-day meeting for 300 delegates in Barcelona, Catalonia, Spain. In time, the application was approved and funding was granted subject to the following terms: that there was to be no contact between staff of GSK or HGS and the Steering Committee; transparent distance was to be maintained between the financial supporters and control of programme development and content; the Consortium must abide by CME regulations and national laws and undertake the project in the spirit of the EFPIA code of practice and good accounting practices; and the provision of a summary of the expected duties of all concerned. Neither of the financial supporters requested to see any materials before delivery of the meeting.
CME guidelines and rules
Each member of the Consortium was responsible for its own CME and regulatory compliance, with European CME Forum and Exemplar offering additional guidance where required. Exemplar acted as the coordinating organisation, ensuring that content development schedules were met and liaising with the Steering Committee in their joint responsibility for content development, whilst European CME Forum managed regulatory and CME compliance.
Overall planning for the meeting was compliant with the accreditation standards and requirements of the European Accreditation Council for CME (EACCME), an institution of the European Union of Medical Specialists (UEMS). The Consortium followed the UEMS-EACCME guidelines of the time,Citation4 together with guidance from the Good CME Practice group,Citation1 which advises how providers should develop CME programmes, as well as the revised UEMS-EACCME guidelines, which the UEMS had made public during the consultation process.Citation5 The UEMS-EACCME guidelines were subsequently revised and adopted as the present (launched in January 2013) standards for accreditation.Citation6 The 2012 co-Chairs of the Academy (Professors Roger A. Levy and Ricard Cervera) adopted responsibility for the meeting, one of whom fulfilled the role as Scientific Director, as required under UEMS-EACCME rules, to review and approve all materials and take scientific responsibility for meeting content.
Programme development
The Steering Committee, under the guidance of the co-Chairs, worked through an initial needs identification process to identify what they considered to be the knowledge gaps among the international lupus community of physicians, and scientists. Learning objectives were derived and potential speakers were identified. The meeting faculty was selected based on their expertise and suitability to address the topics in question. As the range of experience of the faculty was wide, there was little risk of selection bias amongst the speakers; however, this was still monitored as signed financial disclosures were completed and returned.
Venue selection
While it was known that Barcelona would be the city of choice for the meeting, selecting an appropriate venue for the Lupus Academy was not a simple task. This was due to the fact that most conference hotels able to host a conference of this size are designated as 5 star hotels, which conflicted with the stipulations of the grant that a venue of no more than 4 stars must be used. After some research, an appropriate hotel with a 4 star rating was identified.
On completion of the planning for the meeting and the agreement of the faculty, the paperwork was submitted to EACCME for CME accreditation. CME accreditation was confirmed within 14 days, representing one of the fastest times for review and accreditation that the provider had experienced. Subsequently, the Lupus Academy website was prepared and launched (www.lupus-academy.org), providing details of the programme and instructions for physicians wishing to secure a delegate place.
Target audience and delegate recruitment
Identifying and inviting an appropriately broad target audience presented a challenge to the Steering Committee. The Committee considered that it would be of value to have as broad a profile of delegates as possible, in terms of experience and geography, to make the interaction fruitful. The identification and recruitment process was conducted by the Consortium, independently of the supporting companies. These companies were unable to supply names of potential delegates, as they were required to maintain sufficient independence from the meeting and to be mindful of data protection laws that forbid the sharing of contact details for non-company purposes.
The Consortium worked closely with national and international lupus and related organisations to deliver invitations to those physicians who it was felt would benefit most from the meeting. The process involved sending invitations by email, followed by review and selection of applicants by seniority, job type and country in order to achieve a broad range of delegates.
Planning and developing an interactive clinically-orientated programme
The clinically orientated educational programme for the Inaugural Meeting of the Lupus Academy was created by the Steering Committee, based on clinical training needs/updates in Europe, South America and Asia.
Programme needs were identified through the Steering Committee's mindful desire to offer a full, translational picture of clinical developments in lupus. The programme centred around a holistic approach that encompassed clinical manifestations, diagnosis, established and novel treatments, prevention and scientific research. The overall meeting learning objectives developed by the Consortium were complemented by individual learning objectives for each of the faculty presentations and workshops (). Full programme details can be found at www.lupus-academy.org.
Table 1. Inaugural meeting programme learning objectives.
Faculty invitations, briefing and content development
Faculty members for the meeting were selected by the Steering Committee on the basis of speciality and geographical location to ensure balanced representation of the broader clinical view of lupus management. Exemplar managed the invitations of the faculty members and provided briefing materials outlining the Lupus Academy's mission statement, global learning objectives, programme structure and educational flow. The faculty were asked to consider only delegate needs and patient outcomes when developing content, and to avoid too much focus on personal research interests.
In line with the faculty briefing materials, the faculty provided specific learning objectives, abstracts, recommended reading and presentations for review by the Steering Committee-led session moderators and the Meeting Course Director (Professor Ricard Cervera, Catalonia, Spain). All presentation-specific learning objectives and content for the meeting were developed and led by the Steering Committee and faculty, independent of potential commercial or clinical bias.
Delegate Profile: multidisciplinary meets multi-skilled learning environment
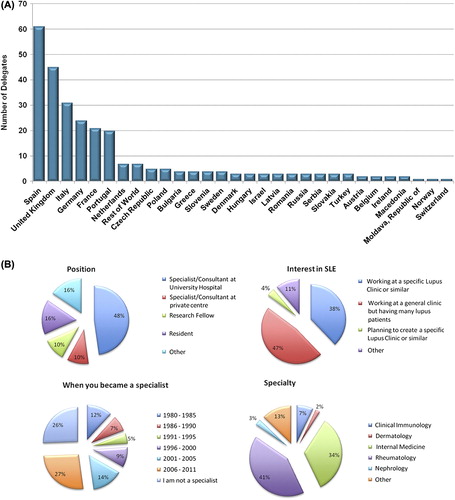
One of the clear benefits of the Lupus Academy is the diversity in specialisms and practice locations of the delegates (/B). Not only does this multifaceted disease area command the interests of rheumatologists, nephrologists, dermatologists, clinical immunologists and internists, but also the educational programme is aimed at specialists with multiple levels of experience, from Resident to Professor. Such a mix of delegates allowed the programme delivery to move away from a didactic one-way channel of learning towards a more interactive sharing of clinical experiences between junior and senior specialists, both during and after the meeting had taken place.
The meeting was open to any specialist with a clinical interest in lupus to attend and full information about the meeting was provided on a public website (www.lupus-academy.org) where delegates could register for the meeting. The meeting was fully booked within two weeks of registration, with more than 50 delegates on a waiting list at the time the meeting took place.
Relationship with the pharmaceutical company supporters
The financial supporters were kept informed about developments in delegate registrations (limited to only numbers and country of origin), that the logistical and ground arrangements were on track and also about financial developments relating to release requirements for remaining grant funds. The grant was released in staged payments due to financial procedural reasons rather than any pre-conditions such as reaching specific milestones. The financial supporters approached the European CME Forum for clarification about which of their staff would be allowed to attend the meeting. Pharmaceutical companies are allowed to attend the meetings that they support, as clarified by the Royal College of Physicians,Citation7 but no recognition or guidance about this exists from UEMS-EACCME. In the experience of the European CME Forum, a general rule is that no more than 10% of the total number of delegates should be from supporting companies. An on-site guide was generated for supporting company staff members, which outlined key ‘dos and don'ts’, providing guidance on how to behave so that their presence would not be felt during the meeting. The financial supporters limited their numbers to 27, primarily technical staff (MDs and PhDs) who worked in the Medical Affairs function.
Lupus Academy: timely delivery in Barcelona
With much talk about the recent launch of belimumab, the first treatment licensed for lupus in 50 years, the launch of an independent CME programme in lupus in Barcelona was timely and engendered much interest among the delegates. Before the meeting began, an on-site speaker briefing took place attended by the faculty, Steering Committee co-Chairs and meeting organisers; no presentation content was shared beyond those present in the briefing meeting. This ensured that anyone attending the meeting from a clinical or commercial background would be unaware of the educational content until the meeting was in progress. Likewise, interactive voting questions for the workshops were programmed on-site after the faculty had met to discuss and align the educational content of their presentations.
The Inaugural Meeting was delivered by a faculty of 17 experts in their respective fields. In line with the learning objectives, the core of the programme provided both plenary sessions (including 15 presentations) and four interactive workshops (with keypad voting), where delegates could discuss the challenging case studies presented and relate their own clinical experiences; these workshops were repeated in the morning and afternoon to allow delegates to attend more than one session. The meeting was attended by 296 delegates, from 30 countries across Europe, the Americas and the Asia Pacific region (), and included specialists, residents and research fellows in the fields of rheumatology, immunology, nephrology, dermatology and internal medicine. Each delegate was required to sign in at the start of each session as proof of attendance.
Delegate feedback and evaluation
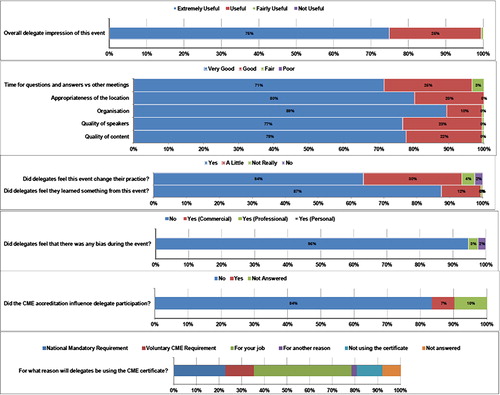
To ensure the future educational value of the Lupus Academy, delegate evaluation forms were created to facilitate the development of future educational programmes based on delegate needs and responses to the educational content. Of the 313 attendees (296 delegates and 17 faculty), 208 were given CME certificates, one for each evaluation form submitted. The majority of delegates rated the educational content, meeting objectives and logistics of the meeting highly (). Almost all delegates stated a preference for independent educational initiatives, like the Lupus Academy, compared to those run and controlled by pharmaceutical companies. Similarly, there were few reports of a perceived perception of commercial bias, which was considered important by the delegates to stimulate open discussion and challenge current thinking in this disease area. Asked whether CME accreditation influenced them to participate, only 14 delegates said yes, the majority from the UK; most respondents commented that the programme and faculty initially attracted them to the meeting.
The final CME-related question asked for what purpose they were going to use their certificate. While many delegates would use the certificate as would be expected (ie, to meet national mandatory or voluntary requirements), the most common answer was to provide evidence to their employer that they were attending an independent educational meeting (ie, to support their application for study leave), which may not be possible when attending a pharmaceutical company meeting.
Many of the delegates responded that they would change their clinical practice as a result of what they had learnt during the meeting. Overall, the key changes delegates would make in clinical practice focused on the management of cardiovascular and renal manifestations and pregnancy in lupus patients, and many delegates reported that they would use more disease activity measures (metrics) in their decision making and specific clinical situations.
Delegates were also asked to provide suggestions for the areas of lupus about which they would like to learn more in future meetings. This information, together with the detailed CME and presentation/workshop feedback, was collated and presented to the meeting sponsor and the Steering Committee to help better understand clinicians’ needs when shaping future educational programmes. Coupled with continued clinician feedback, these responses will help to guide educational content towards improving long-term patient outcomes.
Conclusions
Overall, delegate feedback from the Inaugural Meeting of the Lupus Academy was very positive, with many delegates highlighting the positive value of the cutting edge content in facilitating improved clinical practice, together with the absence of commercial bias. In addition, the interactive workshop sessions (with keypad voting) exceeded expectations and will form a greater part of future programmes, allowing delegates more readily to share, discuss and learn from one another's clinical experience in a well-moderated learning environment.
Following the outcomes and feedback from the Inaugural Meeting, the supporting companies agreed to continue sponsoring the Lupus Academy, with a second Annual Meeting that took place in Buenos Aires, Argentina in 2013, and a third meeting that is being planned to take place in Berlin, Germany in March 2014. On reviewing the management and outcomes of the Inaugural Meeting, the Consortium agreed that the second meeting would include a more formal needs assessment, in light of the detailed feedback from the first meeting.
CME accreditation is a positive endorsement for a meeting that satisfies both financial supporters and delegates. However, for the Lupus Academy, it was the approach in its totality that was important to achieve success: the structure of the Consortium; the independence of the programme; the financial management; and the CME accreditation. Together, these elements ensured that the meeting provided an independent and high-quality educational activity for the attending physicians.
Declaration of Interest
The development of this manuscript and Open Access fee was funded by GlaxoSmithKline and Human Genome Sciences, neither company had any input into, or control over, the content development. Since the date of this meeting, GSK has acquired HGS.
Ricard Cervera has provided consultancy/advice to GlaxoSmithKline, Human Genome Sciences, Immunomedics and UCB. Roger A. Levy has provided consultancy/advice to Abbott, GlaxoSmithKline, Human Genome Sciences and Roche. Julian Ball, Nicole Elzebroek and Eugene Pozniak have no relevant financial relationships to disclose.
References
- Farrow S, Gillgrass D, Pearlstone A, Torr J, Pozniak E. Setting CME standards in Europe: guiding principles for medical education. Curr Med Res Opin 2012;28:1861–1871.
- EFPIA Code on the promotion of prescription-only medicines to, and interactions with, healthcare professionals (ratified 2008, amended 2011). Available at: http://www.efpia.eu/sites/efpiaweb.voxteneo.com/files/EFPIA%20Code_Promotion_HCP_-_11.06.14_FINAL_EDITING_07-08-11-mcp-20110630-002-EN-v1_0.pdf. (Accessed on 12 March 2013).
- International Federation of Pharmaceutical Manufacturers (Associations (IFPMA) Code of Practice (2012). Available at: http://www.ifpma.org/ethics/ifpma-code-of-practice/ifpma-code-of-practice.html. (Accessed on 12 March 2013).
- European Union of Medical Specialists (UEMS). Criteria for international accreditation of CME (D9908 rev 2007). Available at: www.uems.net/fileadmin/user_upload/uems_documents/contentogram_doc_client_20120530/D9908rev2007.pdf. (Accessed on 12 March 2013).
- European Union of Medical Specialists (UEMS). The accreditation of live educational events by the EACCME (UEMS 2011/30). Available at: http://admin.uems.net/uploadedfiles/1497.pdf. (Accessed on 12 March 2013).
- European Union of Medical Specialists (UEMS). The accreditation of live educational events by the EACCME (UEMS 2012/30). Available at: http://www.uems.net/uploads/media/UEMS_2012.30_Accreditation_of_Live_Educational_Events_by_EACCME_ADOPTED_02.pdf. (Accessed on 12 March 2013).
- Royal College of Physicians. Continuing Professional Development Application for approval of live events (V8, 2012). Available at: http://www.rcplondon.ac.uk/sites/default/files/documents/cpd_live_event_approval_guidelines_0712.pdf. (Accessed on 12 March 2013).