Abstract
Clinical trial networks, shared clinical databases, and human biospecimen repositories are examples of infrastructure resources aimed at enhancing and expediting clinical and/or patient oriented research to uncover the etiology and pathogenesis of amyotrophic lateral sclerosis (ALS), a rapidly progressive neurodegenerative disease that leads to the paralysis of voluntary muscles. The current status of such infrastructure resources, as well as opportunities and impediments, were discussed at the second Tarrytown ALS meeting held in September 2011. The discussion focused on resources developed and maintained by ALS clinics and centers in North America and Europe, various clinical trial networks, U.S. government federal agencies including the National Institutes of Health (NIH), the Agency for Toxic Substances and Disease Registry (ATSDR) and the Centers for Disease Control and Prevention (CDC), and several voluntary disease organizations that support ALS research activities. Key recommendations included 1) the establishment of shared databases among individual ALS clinics to enhance the coordination of resources and data analyses; 2) the expansion of quality-controlled human biospecimen banks; and 3) the adoption of uniform data standards, such as the recently developed Common Data Elements (CDEs) for ALS clinical research. The value of clinical trial networks such as the Northeast ALS (NEALS) Consortium and the Western ALS (WALS) Consortium was recognized, and strategies to further enhance and complement these networks and their research resources were discussed.
Introduction
The purpose of this supplement is to provide an overview of available infrastructure resources for clinical and/or patient oriented research aimed at increasing our understanding of the etiology and pathogenesis of ALS, and to discuss current impediments and gaps in this area as well as opportunities. The following sections review a variety of infrastructure resources for ALS research that were discussed at the 2011 second Tarrytown ALS meeting and ensuing correspondence among meeting participants. contains a representative list of such resources developed and/or supported by ALS clinicians and investigators, government agencies, and voluntary disease organizations. Our review is mainly focused on North America.
Table I. Infrastructure resources for ALS clinical research in North America.
Infrastructure resources at ALS clinics in North America
ALS patient care is most effectively provided by the multidisciplinary clinic arrangement adopted by many specialized ALS clinics in North America Citation(1). Most of these ALS clinics have developed site- specific infrastructure resources for patient care and clinical research that rely on a variety of funding sources, including US government agencies, national and local patient support organizations and philanthropic groups. The results of an anonymous survey, undertaken shortly after the second Tarrytown ALS meeting by its organizers, revealed that only a subgroup of ALS clinics in North America has developed or has access to multi-site clinical study databases (). The development of more broadly accessible, multi-site databases − both in terms of data capture and data retrieval − between the ALS clinics is expected to facilitate and enhance clinical research studies that focus on unraveling phenotypic variation or environmental risk factors for ALS. Such multi-site databases would also enable more seamless communication among these clinical research centers. Challenges for the establishment of such multi-site databases include the requirement for dedicated financial support, standardization of collected data elements, consistency in data entry, and the need to overcome possible psychological barriers to sharing de-identified clinical information among individual clinics.
Table II. Clinical research infrastructure at specialized ALS clinics in North America.
The above-mentioned survey of specialized ALS clinics also revealed that only a subgroup of clinics maintains human biospecimen collections, also referred to as biobanks (). These biobanks provide critical infrastructure for ALS research, and efforts to expand them are warranted. For example, human biofluid collections provide critical specimens for the discovery of molecular biomarkers for ALS. The need for such molecular biomarkers, which could be used in combination with electrophysiological or imaging based biomarkers, is urgent and multi-fold, since they could be used to expedite diagnosis, define prognosis, provide insight into pathophysiology, and monitor drug efficacy during clinical trials (Citation2,Citation3). As exemplified by the expeditious creation of a large ALS DNA sample collection within the NINDS Human Genetics Resource Center in 2006, ALS biobanking efforts benefit from collaborations between academic investigators, government agencies and voluntary organizations Citation(4). Future biobanking projects may benefit from similarly collaborative approaches. Another critical aspect of biobanking is the development of standardized methods for collection, long-term storage, retrieval, and sample distribution. Furthermore, the value of biospecimens to scientists and clinicians correlates with the completeness and relevance of the associated phenotypical and clinical information. Skin biopsy samples, if processed properly, can easily be banked as cultured skin fibroblasts and serve as a source for induced pluripotent stem cells (iPSCs), which can be re-derived into disease-relevant cell types (Citation4,Citation5). These iPSC-derived cell lines have considerable potential as model systems for ALS research.
Autopsy tissue banks are an equally valuable resource for ALS research but have proven to be challenging to establish and maintain. The United States (U.S.) Department of Veterans Affairs (VA) and a number of academic ALS research centers have established ALS tissue banks, often in conjunction with the Alzheimer's disease research centers sponsored by the National Institute on Aging. However, many of these tissue banks have only limited supplies of samples and most of them do not have the capacity and processes necessary for wide-scale sample distribution and sharing. Therefore, an additional more broadly accessible ALS autopsy tissue bank or a network of virtual ALS center- located banks would be of great value to the ALS research community.
The WALS Consortium
The WALS Consortium is the oldest network for ALS clinical studies and trials in the U.S. Its membership currently includes 30 academic centers. Completed efforts include the undertaking of one of the first natural history studies of ALS, multiple placebo-controlled clinical trials (cyclophosphamide, verapamil, nimodipine, ciliary neurotrophic factor, gabapentin, minocycline, and lithium), and participation in a genome-wide association study that included more than 1200 patients. At present, the Consortium coordinates clinical trials of NP-001 (Neuraltus), rasagiline and zinc. The average recruitment rate of past WALS-led studies and trials was two patients per site per month, which allowed for timely study completion. WALS operates with a central data coordinating center that is supported by a dedicated biostatistician. The achievements of this consortium bear testament to the efficiency of its infrastructure and the expertise of the participating centers. Through data sharing with the NEALS Consortium and other ALS clinical researchers, the WALS Consortium has aggregated data on 616 placebo control ALS subjects from six clinical trials, spanning a nine-year time period (2000 − 2009). At present, the WALS Consortium is working on improving the efficiency of ALS clinical trials while focusing on phase II trials that assess biological effects of tested drugs.
The NEALS Consortium
The goal of the NEALS Consortium is to translate scientific advances into clinical research and new therapies for patients affected by ALS and other motor neuron diseases Citation(7). NEALS currently comprises 103 academic member sites with more than 140 investigators and continues to admit new sites. Consortium members have access to standardized training in clinical trial conduct and a broad range of electronic tools to facilitate clinical trial management. In addition, consortium members can access historical clinical data and biospecimen collections, obtain statistical support for member-initiated studies, and participate in training and certification programs in ALS outcome measures.
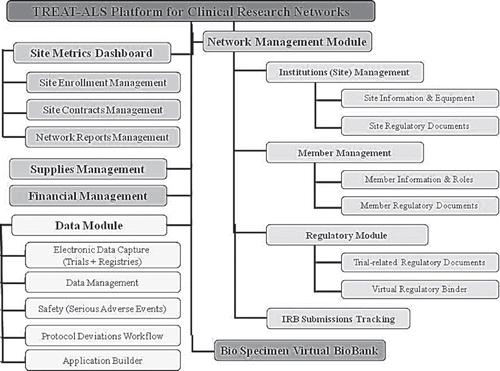
One of the goals of NEALS is to become more actively involved in patient oriented research. In support of this goal, the consortium has worked towards consolidating data from completed clinical studies and trials. Similar efforts have also been initiated by the WALS Consortium, several pharmaceutical companies and the non-profit organization Prize4Life (see below). Currently, NEALS coordinates a variety of clinical studies, including two tissue biomarker studies, and a study assessing motor unit number estimation and electrical impedance myography as surrogate outcome measures for ALS clinical trials. NEALS has also been involved in the development of quantitative strength measurement methods in ALS such as accurate limb isometric muscle strength and hand-held dynamometry. The Consortium coordinates multi-site clinical trials and research studies providing project and data management services and training for new principal investigators and study personnel in ALS clinical research. Its web-based TREAT-ALS platform () allows the Consortium to manage and track information on member sites, their research staff, regulatory documents, site metrics, study supplies and site payments, as well as to manage data and biospecimen repositories.
The NEALS Consortium Biofluid Repository
In addition to its clinical research and trial activities, NEALS has established a human biofluid and DNA repository. The repository currently includes plasma from approximately 200 ALS patients, 50 pure upper motor neuron (UMN)/lower motor neuron (LMN) patients, 100 disease mimics, and 100 healthy volunteers. It also includes DNA samples from approximately 200 ALS patients, 40 pure UMN/LMN patients, 40 disease mimics, and 50 healthy volunteers. The primary goal of the repository is to provide a research resource for biomarker discovery and validation. The biosamples are well-annotated and, when available, linked to longitudinal data. Sample quality is ensured by the adoption of rigorous standard operating procedures for biospecimen acquisition, processing, shipment, and storage. The repository utilizes a web-based platform that allows parametric sample searches and filtering by subject-related data elements Citation(2). Banked samples are available to the broader scientific community following review of the proposed research purpose by a committee of NEALS peer investigators.
The National ALS Research Group (ALSRG)
The ALSRG is a network of approximately 180 healthcare professionals and scientists involved in clinical ALS research, patient care, and/or education that has the goal of optimizing the infrastructure for clinical ALS research in North America Citation(8). The concept was developed at the first Tarrytown ALS meeting in 2003, and the group was founded shortly thereafter. An early initiative of ALSRG included the establishment of a DNA collection of over 1800 ALS patient samples and 1500 control samples with associated clinical data. The collection is part of the NINDS Human Genetics Resource Center and broadly accessible Citation(4). More recently, ALSRG has partnered with ATSDR to optimize the clinical data set that is being gathered by the National ALS Registry. Current efforts include the development of educational material to improve patient enrollment in ALS clinical studies and trials, and to provide objective information on available treatments. This material is posted on the ALSRG website for public consumption. Collaboration between the ALSRG and the World Federation of Neurology's ALS Research Group Citation(9) has resulted in a program called ALSUntangled (Citation10), which uses social networking to investigate alternative and off-label ALS treatments. Furthermore, collaboration between the ALSRG and the NEALS Consortium has led to the formation of the ‘ALS Clinical Research Learning Institute’. Its inaugural meeting was held in the fall of 2011, and brought ALS patients and caregivers together for a ‘crash course’ in research and advocacy. The meeting participants are now acting as ‘research ambassadors’ in the ALS community.
The NINDS Repository
The NINDS Human Genetics Resource Center, also known as the NINDS Repository, is a multi-disease biobank that includes collections for Parkinsonism, stroke, motor neuron disease, Tourette syndrome and several other neurological diseases, as well as control samples (Citation11). The mission of the NINDS Repository, which is a public resource of de-identified human samples with associated clinical data, is to accelerate the discovery of risk factors for complex neurological disorders. The motor neuron disease collection, which has been cofunded by the ALS Association and the Muscular Dystrophy Association, currently offers more than 2000 samples for ALS and 238 samples for other adult-onset motor neuron diseases Citation(4). In many cases, samples from blood relatives and spouses of patients are also available. A recent development of the Repository is the addition of human cell lines for reprogramming purposes. Researchers of the NINDS-funded ALS iPSC Consortium are collaborating to make fibroblasts from ALS patients that have familial and sporadic forms of the disease available. These cells can be de-differentiated into iPSCs and then reprogrammed into motor neurons, astrocytes, or other types of cells that are relevant to a given disease. Characterization of the differentiated cells to identify phenotypes should give researchers new insights into the pathogenetic and pathophysiologic processes of ALS. Researchers can access these resources through the website of the NINDS Repository. The NINDS Repository has also taken on other new initiatives to adapt to changing research priorities, including the emergence of next-generation sequencing approaches and increased interest in biomarker research.
The NINDS Common Data Elements (CDE) Project
The purpose of the NINDS CDE Project is to develop data standards for clinical research within the neurological community (Citation12). Central to this project is the creation of common definitions and data sets so that clinical information is consistently captured and recorded across studies. The NINDS CDE collection includes general CDEs that cross diseases and define standards for broadly applicable data domains such as medical history, scores on neurological assessments, demographic information, and details about medications used by participants throughout a study. In addition to these general elements, NINDS has developed sets of CDEs tailored to research involving specific diseases. These disease-specific sets include core CDEs that should be used in all studies for the disease, supplementary CDEs that are an extended optional set, and exploratory CDEs that have not been validated as yet. To date, disease-specific CDEs have been developed for ALS, congenital muscular dystrophy, epilepsy, Friedreich's ataxia, Parkinson's disease, spinal cord injury, stroke and traumatic brain injury. CDEs for several other neurological disorders are currently in development.
The ALS CDE effort was launched in the fall of 2010 when several working groups of ALS experts were formed to identify and define CDEs for four data domains in the context of ALS research: 1) biomarkers and imaging; 2) clinical research and quality of life; 3) cognitive assessment; and 4) genetics. Preliminary CDE recommendations from the working groups were posted for public review and comment in the fall of 2011. The final CDEs were made publicly available in March 2012 (Citation12). It is anticipated that the ALS CDEs will be reviewed and updated on a regular basis.
NeuroNEXT and other NINDS clinical research initiatives
Over the past several years, NINDS has developed multiple initiatives aimed at providing infrastructure resources to enhance clinical research in neurological disease. Through its ‘NeuroNEXT’ initiative (Citation13), the NINDS hopes to help researchers to develop and rapidly execute scientifically rigorous biomarker-supported phase II clinical trials, and to implement studies that aim at validating biomarkers or outcome measures in preparation for trials. This network allows for private and public partnerships with foundations or industry. Another NINDS-supported resource is ‘Neuro-QOL’, a patient-reported outcomes tool aimed at measuring quality of life in neurological clinical research Citation(14). The instrument is based on computerized adaptive testing so that patients only need to respond to questions that are relevant to their particular situation. NINDS and its umbrella organization, the NIH, are also involved in the ‘PROMIS’ project, which is a web-based initiative that collects patient-reported outcomes that can be used across diseases Citation(15). These instruments are expected to enhance clinical research in neurological disorders and facilitate data comparisons across studies.
The National ALS Registry
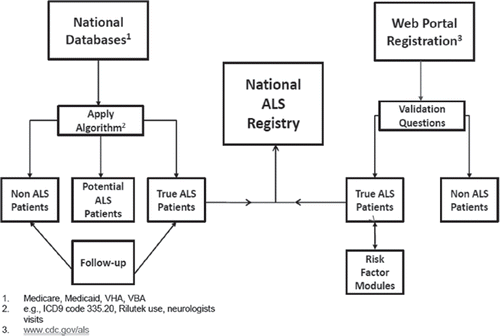
In most states of the U.S., ALS is not a reportable disease. As a result, creating a database that captures all individuals living with ALS is challenging. The ALS Registry Act was signed in 2008 by George W. Bush and allowed the ATSDR/CDC to develop an ALS registry to determine the prevalence and incidence of ALS within the U.S. and to collect data that may provide insights into possible risk factors for the disease. Launched in October 2010, the National ALS Registry uses two approaches to identify ALS cases in the U.S. First, an algorithm assigns people from Medicare, Medicaid, and VA databases into three different groups: a potential ALS patient, an ALS patient, or a non-ALS patient. This algorithm can identify patients even if they are not specifically coded for ALS (). For example, ICD codes, medications and frequency of neurology visits are factored into the algorithm. Secondly, the National ALS Registry uses a secure web portal where patients can self-report while also filling out surveys pertaining to risk factors and quality of life throughout the progression of their disease. Currently, the National ALS Registry project team is working to improve its accessibility to both clinicians and researchers, mapping out the development of a national biobank, and assisting clinicians and researchers in using the Registry to recruit participants for clinical trials and research studies.
New York Brain Bank (NYBB) at Columbia University
NYBB currently holds 200,000 autopsy samples that are electronically tracked and dispersed on a monthly basis. The collection focuses on autopsy samples for neurodegenerative and psychiatric diseases. The number of samples banked for a given disease is determined by demand and available funding. The biobank currently includes approximately 5000 ALS samples and only 2−3 samples are sent to researchers every year. This is a very small number compared to the 400–500 samples that are dispersed for Alzheimer's and Parkinson's diseases per month. Although the ALS sample group is small, careful consideration is taken when labeling and categorizing this group. Three diagnoses (familial ALS, sporadic ALS, and ALS with frontotemporal dementia) are taken into account to assign the distribution of samples, and the neuropathologic diagnosis is combined with the clinical diagnosis to assign a final diagnosis. The use of standard operating procedures and electronic tracking safeguard the identity and quality of the biospecimens, and also ensure that they can be dispersed to researchers in a timely manner.
UK National DNA Biobank
The UK National Motor Neurone Disease (MND) DNA Biobank was established in 2003 and involves 30 centers countrywide with three hub-centers in Sheffield, Birmingham, and London Citation(16). It contains DNA samples from adults with symptom onset on or after January 2002 − approximately 1600 sporadic ALS cases, 160 familial ALS cases, and 1600 controls. Associated clinical data include age of onset, age at collection date, gender, diagnosis, El Escorial category, ethnicity, date of birth, date of death, collection center, and presentation pattern. A subset of samples has detailed epidemiological data available from a questionnaire designed to be compatible with similar questionnaires used in other studies internationally Citation(17). The samples are pseudonymized and screened for known familial ALS genes. The majority has also been screened on DNA microarrays, and summary statistics and genotypes are deposited at the European Bioinformatics Institute.
Controls were recruited from unaffected family members, partners, and caregivers. Samples from either two parents, or one parent and a sibling, of people with sporadic ALS were collected to form genetic trios. Samples from as many family members as possible of participants with familial ALS were also collected. The acquisition of control samples has been challenging. The MND Association acts as the custodian of the collection.
European Registries
The European ALS Consortium, EURALS, was established in October 2004 at a consensus meeting in Amsterdam. It is a consortium of population based registries and several clinic based cohorts. The main aims of EURALS are to coordinate the scientific activities of ALS population based registries (Ireland, Italy, France, Serbia, Spain, the Netherlands and the United Kingdom) and tertiary centers, and to conduct epidemiological, genetic studies and randomized clinical trials Citation(18). The data are available to the principal investigators of each participating country. The steering committee manages all the principal collaborative research projects. The ongoing European Consortium of ALS Registries allows for an efficient and standardized population based collection of patient samples and lifestyle questionnaires. Well defined and harmonized guidelines on sampling have been implemented across the different registries. This large ALS Registry is believed to provide crucial epidemiological data and has become a key infrastructure of ALS clinical research Citation(19).
Use of this European patient registry remains one of the main objectives within the European Community’s Seventh Framework Programme (FP7) Euro-MOTOR project. The objective of Euro-MOTOR is to discover new causative and disease-modifying pathways to pave the way for novel therapies for ALS. The project also aims to detect key genetic drivers of disease susceptibility and progression, while parametric modeling of the causal connections in identified molecular networks is expected to generate a model of the disease.
In Italy, several prospective epidemiological regional registries contribute to the EURALS database. Among them is the Piemonte and Valle d’Aosta Registry for ALS (PRALS) managed by the University of Torino. During the first 10-year period of observation (1995 − 2004), 1347 residents in the study area were diagnosed with ALS Citation(20). Several other regional Italian registries should also be mentioned; including the Prospective ALS registry in Lombardia (SLALOM group) coordinated by the Neurological Clinic, University of Biccoca, Monza Citation(21); the Tuscany Registry for ALS (TRALS) coordinated by the Neurorehabilitation Unit, Department of Neuroscience, Azienda Ospedaliero-Universitaria Pisana (AOUP); the Sclerosi Laterale Amiotrofica–Puglia (SLAP Registry) established in southern Italy and coordinated by Department of Neurological Sciences, University of Bari Citation(22); and the multicenter-multisource prospective population based registry called LIGALS (Liguria Amyotrophic Lateral Sclerosis Registry) run in Liguria Citation(23). These registries allowed for population based analyses, results of which were published in recent years Citation(24–26).
The Irish MND Research Group has set up and maintained for 15 years the longest running population based register of ALS in the world. It dates back to 1994 and currently has clinical information from over 1400 patients. A DNA bank was added in 1998. This bank contains samples from over 300 patients, and is recognized as one of the best in the world. The Irish MND Register is maintained at the National MND Clinic at Beaumont Hospital Citation(27). The Scottish Motor Neuron Disease Register, perhaps one of the earliest ALS registries, was established in the late 1980s Citation(28). The South-East England ALS (SEALS) Registry is a clinical database that has recorded all ALS cases in a defined population of 3.5 million people in the south-east region of England since 2002 Citation(29). The SEALS Registry has been used to derive an estimate of the lifetime risk of ALS incidence, prevalence, and patterns of geographical clustering, as well as detailed phenotypic patterns. The most important aspect of these registries is that they have contributed key epidemiological data. ALS registries have expanded to other European countries, particularly to Holland, where a large population based registry was the source for many studies Citation(30). In Germany, there are several well-organized ALS registries, including the ALS Registry Swabia managed by the Klinik für Neurologie of the University of Ulm, the Rheinland-Pfalz ALS Registry and the Nordrhein-Westfalen ALS Registry. There is also a network of brain tissue banks, Brain-Net, run from the Institut für Pathologie at Würzburg University and the Institut für Neuropathologie at the University of Aachen. France has several ALS registries as well, in several clinical centers, including Languedoc-Roussillon ALS Registry in Montpellier, several ALS registries in Paris and one at the Neurology Department of the University Hospital of Limoges (Citation31,Citation32).
The Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) Database
Large sample sets are critical for identifying statistically significant and biologically relevant observations, particularly for diseases resulting from the intricate interplay of genetic and environmental factors such as ALS. Pooled clinical trial datasets have proven invaluable for researchers seeking to unravel complex diseases such as multiple sclerosis Citation(33) and Alzheimer's disease.
There have been a number of large phase II and phase III ALS clinical trials conducted over the past 20 + years. While the vast majority of these trials, with the exception of the riluzole trials, have not resulted in the identification of new therapies for ALS, there is still great clinical value in the patient data collected during the course of these studies, particularly in aggregate. With funding from the ALS Therapy Alliance, the ALS non-profit organization Prize4Life Citation(34) partnered with NEALS and NCRI to design and build a comprehensive ALS clinical trials record database and make it freely available as a resource, exclusively for research purposes, to members of the research community Citation(35). The PRO-ACT platform and database houses the largest harmonized dataset from completed clinical trials in ALS. The initial goal of PRO-ACT was to establish a common research support infrastructure to merge and integrate placebo patient data from completed ALS clinical trials to create a powerful new open-access research tool for researchers, statisticians, clinicians, and anyone interested in ‘Big Data’, both in academia and industry.
The PRO-ACT platform makes possible the merging of heterogeneous data from diverse internationally conducted clinical trials, generating an invaluable resource for both the design of future clinical trials and the identification of unique observations, novel correlations, and biomarkers of disease. There are currently over 900 Common Data Elements (CDEs) in the PRO-ACT Common Data Structure that are being used to map data from 13 ALS clinical trials provided by four pharmaceutical companies, along with 5 academic trials.
Discussion
Over the past 10 years, significant progress has been made in developing and maintaining infrastructure resources for clinical and/or patient oriented ALS research. The state of these resources, as well as present challenges, gaps and opportunities, were discussed at the second Tarrytown ALS meeting in September 2011.
At present, over 100 specialized ALS clinics, alone or as members of clinical trial networks or consortia, provide critical infrastructure to expedite the pace of clinical and patient oriented research in ALS and to facilitate interventional trials. Frequently, private foundations contribute ideas and resources to such developments. As an example of such a successful partnership, it is worth mentioning the ALS Association's collaboration with NEALS on the Translational Research Advancing Therapy for ALS (TREAT ALS) network to develop the infrastructure necessary to rapidly complete clinical trials of new therapeutic agents, and to provide partial funding of individual trials. More recently, some of these groups have begun turning their attention to building biobanks. One of the concepts recently introduced to the ALS research community was the creation of ALSBank™, a virtual biobank that pools inventories and associated data from individual biorepositories into a single virtual network Citation(2). However, despite the unquestionable value of these resources, several infrastructure gaps for ALS clinical research still exist. These include a need for establishing more broadly accessible and coordinated autopsy tissue banking efforts, as well as web-based solutions to facilitate the sharing of de-identified clinical data across clinics, regions and countries, and to enhance data mining and meta-analysis.
Currently, multiple ALS autopsy tissue banks already exist at individual research sites. However, many of them experience technical (accessibility, ‘transparency’ of samples held, capacity to disperse samples) and financial challenges. The augmentation of these programs is warranted since, if successfully managed, they have the potential to advance the pace of ALS research. This is evident in light of recent discoveries such as the identification of new causative ALS genes and associated pathologies. In this context, it is important to note that tissue banking programs have to consider multi-fold needs of several groups, including the educational needs of physicians who are being trained in the field, the clinical needs for an accurate diagnosis, and the research needs of the ALS scientific community at large. Tissue examination allows us to correlate clinical and pathological findings, assess the effects of experimental therapeutics, discover new genes and molecules, and to put pathological processes in the context of tissues and cells. Limits in targeted funding have decreased the number of autopsies conducted around the world. In the U.S., there are very few existing ALS autopsy programs, with Columbia University and the National VA ALS Brain Bank being two examples. There are many challenges that must be overcome to ensure the success of these ALS neuropathology programs, mostly involving funding, logistics of getting ALS patients to autopsy in a timely manner, pathologists’ interest in participating, and infrastructure. Development of an ALS-specific nomenclature for tissue samples and an agreement upon standard operating procedures and associated data elements to be collected across all participating sites are necessary for any meaningful collaborative effort. An organized and managed review of these factors is necessary in order to move the field forward and to build a successful resource for the ALS research community. NEALS, the Muscular Dystrophy Association, Prize4Life, and the ALS Association, in collaboration with government agencies, have begun to evaluate how to organize and structure ALS biofluid and tissue repositories with the aim of improving future ALS research.
Another critical missing component in the field of ALS clinical research is the development of an electronic platform to facilitate broader data sharing. At present, there is a lack of integrative data sharing among ALS clinics and researchers, and there is no systematic approach towards utilization of existing resources, gap analyses, and new resource funding. As the majority of ALS patients do not participate in research studies, their clinical data are not utilized for research purposes. An international web-based clinical data repository platform, NeuroBANK™, in which clinicians and researchers could collect and share their patients’ clinical data, was implemented under the leadership of NEALS, ALSRG and several foundations, including WALS, the ALS Hope Foundation, and the ALS Therapy Alliance. This platform allows ALS clinicians to enter patient data, either manually via a web-based interface or by exporting the data from the electronic medical record systems of their hospitals. One of the objectives of this platform is to allow clinicians to compare patient and/or site outcomes against the averages calculated from the entire dataset, enabling comparative effectiveness research. The data captured by the platform are standardized and harmonized based on a set of agreed-upon forms. The platform supports implementation of non-English language case report forms, functional scales, and questionnaires.
Data sharing is also facilitated by the adoption of data standards such as the CDE set for ALS. CDE adoption will not only allow researchers to use a universal data repository platform for their own clinics’ needs, but will also enhance collaboration and sharing of de-identified information between the clinics and researchers in general. Another emerging resource for ALS clinical research is the PRO-ACT platform developed by NCRI and Prize4Life, which provides open access to merged datasets from past (and hopefully future) clinical trials in ALS. This platform allows researchers worldwide to mine this rich data source in order to address important questions currently confronting the ALS field. One of the initial informatics efforts using these data is an open competition, sponsored by Prize4Life, to develop algorithms capable of predicting fast and slow ALS disease progression with greater power and earlier in disease than ALSFRS. The PRO-ACT database is also invaluable in addressing other important questions in the ALS field regarding natural history, future clinical trial design, other types of patient stratification, and biomarkers.
While significant progress has been made in developing resources that facilitate clinical research, a critical need for standardization and harmonization of these resources as well as increased collaboration among ALS clinicians and researchers, remains. Understanding the current resource ‘landscape’, including the obstacles and impediments that need to be overcome, is imperative if we are to improve ALS clinical research infrastructure and move ALS research forward. ALS clinics, dedicated clinical trial networks, and other investigator-initiated groups are instrumental in this process, along with support from government agencies and voluntary disease organizations. Further efforts to promote efficiencies and avoid duplication of standards and platforms are warranted to optimize the utility of already existing resources and the development of future resources for ALS clinical research.
Acknowledgement
The NINDS and ATSDR have given permission to their officers (Conwit, Gubitz, and Kaufmann, NINDS, and Horton, ATSDR) to submit this manuscript for review.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update. The care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1218–26.
- Sherman A, Bowser R, Grasso D, Power B, Milligan C, Jaffa M, et al. Proposed BioRepository platform solution for the ALS research community. Amyotroph Lateral Scler. 2011;12:11–6.
- Otto M, Bowser R, Turner M, Berry J, Brettschneider J, Connor J, et al. Roadmap and standard operating procedures for biobanking and discovery of neurochemical markers in ALS. Amyotroph Lateral Scler. 2012;13:1–10.
- Gwinn K, Corriveau RA, Mitsumoto H, Bednarz K, Brown RH, Cudkowicz M, et al. Amyotrophic Lateral Sclerosis: An Emerging Era of Collaborative Gene Discovery. PloS One. 2007;1:1254.
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–21.
- Chaddah MR, Dickie BG, Lyall D, Marshall CJ, Sykes JB, Bruijn LI.Meeting report of the International Consortium of Stem Cell Networks’ Workshop Towards Clinical Trials Using Stem Cells for Amyotrophic Lateral Sclerosis/Motor Neuron Disease. Amyotroph Lateral Scler. 2011;12:315–7.
- http://www.alsconsortium.org
- http://www.alsrg.org
- http://www.wfnals.org
- http://www.alsuntangled.org
- http://www.coriell.org
- http://www.commondataelements.ninds.nih.gov
- http://www.ninds.nih.gov/news_and_events/proceedings/20101217-NEXT.htm
- http://www.neuroqol.org/default.aspx
- http://www.nihpromis.org
- http://www.biobankingsolutions.ac.uk/sample-data-access/collections/motor-neurone-disease/
- Shatunov A, Mok K, Newhouse S, Weale ME, Smith B, Vance C, et al. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol. 2010;9:986–94.
- Beghi E. 127th ENMC International Workshop: implementation of a European registry of ALS. Neuromuscul Disord. 2006;16:46–53.
- Beghi E. Logroscino G, Chio A, Hardiman O, Mitchell D, Swingler R, et al. ;EURALS Consortium. The epidemiology of ALS and the role of population based registries. Biochimica et Biophysica Acta. 2006;176:1150–7.
- Chiò A, Mora G, Calvo A, Mazzini L, Bottacchi E, Mutani R; PARALS. Epidemiology of ALS in Italy: a 10-year prospective population based study. Neurology. 2009;72: 725–31.
- Beghi E, Millul A, Micheli A, Vitelli E, Logroscino G; SLALOM Group. Incidence of ALS in Lombardy, Italy. Neurology. 2007;68:141–5.
- Zoccolella S, Beghi E, Palagano G, Fraddosio A, Guerra V, Samarelli , et al. Riluzole and amyotrophic lateral sclerosis survival: a population based study in southern Italy. European Journal of Neurology. 2007;14:262–8.
- Bandettini di Poggio M, Sormani MP, Truffelli R, Mandich P, Origone P, Verdiani S, et al. ;LIGALS. Clinical epidemiology of ALS in Liguria, Italy. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:52–7.
- Beghi E, Logroscino G, Chio A, Hardiman O, Mitchell D, Swingler R, et al. The epidemiology of ALS and the role of population based registries. Mol Basis Dis. 2006; 1762: 1150–7.
- Beghi E, Logroscino G, Micheli A, Millul A, Perini M, Riva R, et al. Validity of hospital discharge diagnoses for the assessment of prevalence and incidence of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2001;2: 99–104.
- Chio A, Mora G. Early symptom progression rate is related to ALS outcome: a prospective population based study − reply. Neurology. 2002;59:2013.
- Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Amyotrophic lateral sclerosis mimic syndromes: a population based study. Arch Neurol. 2000;57: 109–13.
- Chancellor AM, Swingler RJ, Fraser H, Clarke JA, Warlow CP. Utility of Scottish Morbidity and Mortality Data for Epidemiologic Studies of Motor Neuron Disease. J Epidemiol Commun. 1993;47:116–20.
- Abhinav K, Stanton B, Johnston C, Hardstaff J, Orrell RW, Howard R, et al. Amyotrophic lateral sclerosis in south-east England: a population based study. The South-East England register for amyotrophic lateral sclerosis (SEALS Registry). Neuroepidemiology. 2007;29:44–8.
- Huisman MHB, de Jong SW, van Doormaal PTC, Weinreich SS, Schelhaas HJ, van der Kooi AJ, et al. Population based epidemiology of amyotrophic lateral sclerosis using capture-recapture methodology. J Neurol Neurosur. 2011;82:1165–70.
- Preux PM, Druet-Cabanac M, Couratier P, Debrock C, Truong T, Marcharia W, et al. Estimation of the amyotrophic lateral sclerosis incidence by capture-recapture method in the Limousin region of France. J Clin Epidemiol. 2000; 53:1025–9.
- Gil J, Funalot B, Verschueren A, Danel-Brunaud V, Camu W, Vandenberghe N, et al. Causes of death among French patients with amyotrophic lateral sclerosis: a prospective study. European Journal of Neurology: the official journal of the European Federation of Neurological Societies. 2008;15:1245–51.
- Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5:244–50.
- http://www.prize4life.org
- http://www.alsdatabase.org