Abstract
Numerous sub-cellular through system-level disturbances have been identified in over 1300 articles examining the superoxide dismutase-1 guanine 93 to alanine (SOD1-G93A) transgenic mouse amyotrophic lateral sclerosis (ALS) pathophysiology. Manual assessment of such a broad literature base is daunting. We performed a comprehensive informatics-based systematic review or ‘field analysis’ to agnostically compute and map the current state of the field. Text mining of recaptured articles was used to quantify published data topic breadth and frequency. We constructed a nine-category pathophysiological function-based ontology to systematically organize and quantify the field's primary data. Results demonstrated that the distribution of primary research belonging to each category is: systemic measures an motor function, 59%; inflammation, 46%; cellular energetics, 37%; proteomics, 31%; neural excitability, 22%; apoptosis, 20%; oxidative stress, 18%; aberrant cellular chemistry, 14%; axonal transport, 10%. We constructed a SOD1-G93A field map that visually illustrates and categorizes the 85% most frequently assessed sub-topics. Finally, we present the literature-cited significance of frequently published terms and uncover thinly investigated areas. In conclusion, most articles individually examine at least two categories, which is indicative of the numerous underlying pathophysiological interrelationships. An essential future path is examination of cross-category pathophysiological interrelationships and their co-correspondence to homeostatic regulation and disease progression.
Introduction
Amyotrophic lateral sclerosis (ALS) is characterized by progressive neurodegeneration of the motor neurons, which leads to muscle paralysis, respiratory deficiency, and eventually death. Mutations of the superoxide (copper-zinc) dismutase-1 (SOD1) gene have been identified as contributors to familial ALS, which accounts for approximately 5–10% of all ALS cases (Citation1). The SOD1-G93A (glycine 93 to alanine) mutation is a comparatively rare ALS mutation in humans, but it is the most studied and published mutation within experimental transgenic ALS mouse models (Citation2,Citation3). The SOD1-G93A transgenic ALS model's popularity is largely due to its ALS symptom reproducibility and its widespread availability for purchase from The Jackson Laboratory (jaxmice.jax.org). At the end of the 2014 year, searching for ‘Amyotrophic Lateral Sclerosis’ AND ‘G93A’ in PubMed returned approximately 1300 articles, and the tally was actually greater since not every article using the SOD1-G93A model specifically cites ‘G93A’ in the PubMed-searchable locations (i.e. title, abstract, etc.).
The SOD1-G93A mouse model has been utilized to identify numerous deficits and impairments contributing to or the direct result of the mutation's associated ALS pathophysiology. Briefly, such deficits comprise the following: apoptosis, including changes in pro- and anti-apoptotic signals (Citation4); axonal transport of mitochondria and other key cargoes (Citation5); aberrant cellular chemistry such as reduced enzyme activity and metal mishandling (Citation6); energetics, including disturbances of the physical and functional properties of mitochondria, ATP production and calcium homeostasis (Citation7); genetic damage, including changes in mRNA or DNA; inflammation, including the migration of reactive astrocytes and microglia (Citation8); oxidative stress, resulting from the build-up of free radicals (Citation9); proteomics, characterized by the accumulation of misfolded SOD1 aggregates (Citation10); systemic impairments, including overall system-level neuromuscular function and non-neuromuscular contributors (Citation3). The phenotype severity and disease progression of the SOD1-G93A transgenic mouse is largely dependent upon the transgene copy number (typically denoted as ‘high’ versus ‘low’). The overwhelming majority of SOD1-G93A transgenic mouse studies have used a high copy model, which has an average onset range of 85–100 days and endpoint of 120–160 days (Citation3).
Knowing the distribution and categorization of primary data is a key step towards both consolidating current knowledge and planning new research (Citation11). However, with so many articles covering such an expansive and complex pathophysiology, it is difficult to manually determine what has and has not been examined in the SOD1-G93A transgenic ALS mouse field. Moreover, while traditional literature reviews help in digesting the details of published data, ideas, or mechanisms, their content does not necessarily quantitatively align with what actual primary data exist for a given topic or theorem. Authors of traditional literature reviews must subjectively determine what topics are reported based on the author's exposure to the field. Automated informatics-based systematic reviews or ‘field analyses’ overcome the traditional limitations of manual literature reviews by comprehensively and agnostically searching the primary data of every available article to quantify the breadth and depth of researched topics. The result is an objective map of the overall literature that structurally organizes and numerically identifies topical areas of prevalent data as well as disparate or thinly investigated areas where primary data are sparse.
Consequently, the goals of this informatics-based systematic review of the SOD1-G93A mouse model were to: 1) determine the published breadth and frequency of research topics; 2) systematically organize and categorize primary data articles using a pathophysiological function ontology; and 3) consolidate current knowledge and highlight corresponding future research paths.
Materials and methods
The general method included finding SOD1-G93A articles; recapturing data from the article entities; devising and testing a term-category dictionary for identifying research terms/topics and for ontological categorization; searching the article entities to determine the frequency of primary data terms/research topics; assessment of the publication frequency and distribution within the pathophysiological ontological categories.
Inclusion and exclusion criteria
To obtain the initial primary article selection pool, PubMed searches were conducted in October 2014 to find all published articles with (‘Amyotrophic Lateral Sclerosis’ or ‘ALS’) in the title or abstract and (‘transgenic mouse’ or ‘G93A’) in the title or abstract. Initial primary article selection pool exclusion criteria consisted of: non-English language articles; articles for which full-text pdf downloads were unavailable; and articles labeled as literature reviews. Articles were either downloaded using PubMed Central or from e-journal subscriptions available from the libraries of Georgia Institute of Technology and Emory University. Using these methods, less than 3% of the eligible initial article pool was unavailable for download. Based on these initial criteria, 1997 articles were eligible for inclusion in the initial primary article selection pool.
To obtain the final article pool utilized to conduct this study, within-article keyword searches were performed to find articles that contained ‘G93A’ in at least one of the following locations: article title, abstract, figure caption, or within the figure text (see Data recapture for details). The final article pool consisted of 1339 articles, all of which were included in the field analysis.
It should be noted that the overwhelming majority of studies do not distinguish between strain and transgene copy number in the searchable article entities (and many articles do not mention them at all, even in the full-text methods). Thus, articles were not included or excluded based on transgene copy number (e.g. high, low) or strain (e.g. B6SJL, C57BL/6), i.e. our article pool represents a combined assessment of research topics in the overall SOD1-G93A transgenic mouse field.
Data recapture
Data were recaptured from the following article locations, referred to as entities: article title, abstract, figure captions, and within figure text. ‘Within figure’ text included any text labeled on or within a figure or table, e.g. the x-y axis labels, bar graph categorical labels, legends, etc. Recaptured data were obtained from downloaded full-text pdf files. Abstract, title, and reference information was exported directly from PubMed. Figure captions and within figure text was manually scraped from the full-text pdf articles using a standard keyboard copy and paste command (Citation12). Any special characters that did copy correctly were manually revised. A quality control team independently assessed all data recapture to ensure complete accuracy. Recaptured data were transcribed into a custom project-specific searchable relational database (www.pathology-dynamics.org). The database is implemented in Filemaker 13 Pro Advanced (Filemaker, Inc.).
Term-category dictionary
A dictionary of corresponding terms and categories (referred to as a term-category dictionary) was constructed that assigned frequent SOD1-G93A pathophysiology article terms and phrases to their most probable ontological category. The term- category dictionary allowed for automated searching of recaptured text and labeling of the articles entity's and the overall article's most probable ontological categories.
The chosen ontological categories were based on a previously published scheme (Citation2) developed from a meta-analysis of SOD1-G93A traditional literature review articles. The ontology was used to categorize primary research data based on underlying pathophysiological function. The ontological categories consisted of: Apoptosis, Axonal Transport, Chemistry, Energetics, Excitability, Genetic Damage, Inflammation, Oxidative Stress, Proteomics and Systemic. The ontological categories are defined in detail in the Results and Discussion section.
To determine the most frequent keywords and phrases in the SOD1-G93A articles, word and phrase frequency analysis was performed using freely available software from WriteWords. Approximately 6500 different terms and phrases were identified and sorted by their number of appearances in each recaptured entity and by their total number of appearances in the articles. Non-scientific words insignificant to the analysis (e.g. of, in, and, etc.) were immediately excluded. Subsequently, a group of trained researchers in SOD1-G93A pathophysiology preliminarily labeled the most frequent 2000 terms by their most likely ontological category.
After performing the first ontological test set search, some individual terms were combined to provide for better specificity (see details in Ontological Test sets). Ultimately, 670 terms and phrases were selected for inclusion of the term-category dictionary (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1047455).
Field searches
Each keyword or phrase in the term-category dictionary was searched in the recaptured data article entities (article title, abstract, figure caption, and within figure text). If the search keyword was a single word, a whole word search was performed. For a phrase, a whole word search was performed for each word but not necessarily in the order of the words, e.g. ‘copper concentration’ and ‘concentration of copper’ were detected upon searching for ‘copper concentration’. If the search was positive, the figure and article was labeled by the corresponding ontological category of that term. The categories identified in the figure caption and within figure text were combined to represent each individual figure's ontological categorization. The categories identified in the article title, figure caption, and within figure text were combined to represent the overall categorization of each article. It should be noted that the abstract was ultimately excluded from the overall article categorization due to the number of false-positive hits it produced (see Test sets). Similarly, the ontological category, Genetic Damage, was individually excluded from the final field analysis results to decrease false-positives; corresponding articles were re-categorized according to the cited location of genetic damage (see Test sets).
Test sets
Test sets of SOD1-G93A figures and corresponding articles were constructed to determine which recaptured article entities should be searched and to evaluate the term-category dictionary. Each test set minimally consisted of 500–600 figures from 100 different SOD1-G93A articles, which included primary data representing each ontological category. For the purpose of evaluating the term-category dictionary, the test set's ontological categorization was separately and manually determined by independent visual inspection of the article's primary data by five trained SOD1-G93A pathophysiology researchers.
Evaluation included measures of sensitivity and specificity. Sensitivity (the ability of a test to identify a condition correctly) and specificity (the ability of a test to exclude a condition correctly) are often used to assess the capability of a search to produce accurate results. Sensitivity is defined as: number of true-positives (TP) divided by the sum of the number of true-positives and false-negatives (FN): TP/ [TP + FN]. Specificity is defined as the number of true- negatives divided by the sum of true-negatives and false-positives: TN/[TN + FP]. While having both a high sensitivity and specificity is ideal, realistically optimization is typically favored towards one or the other depending on the search/test outcome goal, i.e. whether it is more important that the search/test includes or excludes a condition. Given that our protocol searches multiple terms per entity and thus allows multiple categories to be assigned, specificity (the ability to exclude) was given greater priority in the test set design and assessment.
We assessed the article entities’ ability to correctly represent the primary data contained within the article. Searching the abstract text resulted in > 50% false-positives, i.e. over 50% of the abstracts contained key terms or phrases that were either not represented/relevant to the article's primary data or were not present in the article's figure caption or within figure text. If the abstracts were to be used as part of the determination of the articles’ overall categorization, their false-positive terms would result in the addition of non-relevant categories. In contrast, searching the figure captions and within figure text resulted in < 2% false-positives and article titles < 4%. Therefore, as noted in field searches, the abstract search was excluded from the final ontological categorization of an article.
Subsequently, we assessed the ability of each ontological category to represent the corresponding article's primary data. All categories had greater than 95% specificity with the exception of Genetic Damage. The category Genetic Damage consisted of many general terms (see Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1047455), which resulted in low specificity (< 70%). Fortunately, genetic damage is typically measured in a specific location, organelle or pathway. Thus, articles containing primary data that examined genetic damage were labeled with the category(ies) that corresponded to the location or physiology affected by the genetic damage (e.g. mitochondrial mRNA damage → Energetics).
Finally, an ontological test set was also utilized to assess the term-category dictionary itself. An initial test to identify false-positive and negatives resulted in 86.1% sensitivity and 79.0% specificity for articles and 81.5% sensitivity and 87.6% specificity for figures. Care was taken to increase specificity by combining terms that created numerous false-positives into more specific phrases (e.g. aggregation → protein aggregation). After correction, another test set was utilized to evaluate the final dictionary's accuracy: 87.9% sensitivity and 99.9% specificity for papers and 77.9% sensitivity and 97.5% specificity for figures. Given that specificity was the priority, an overall specificity > 98% was considered acceptable for the study goals.
Results and discussion
We first present the field analysis results, including the overall distribution of research articles with primary data belonging to each of the nine ontological categories: Apoptosis, Axonal Transport, Chemistry, Energetics, Excitability, Inflammation, Oxidative Stress, Proteomics and Systemic. Subsequently, the quantitative topical distribution of research articles in each of the individual categories is presented and explained. Finally, we conclude with a discussion on categorical relationships and future directions.
Overall SOD1-G93A Field Analysis
We performed a field analysis based on key word searches of article titles, figure captions, and within figure text to examine the prevalence of the different types of pathophysiological research in the SOD1-G93A ALS transgenic mouse model. The order of overall prevalence of primary data corresponding to the defined ontological categories of the SOD1-G93A ALS published literature is as follows: 1) systemic and functional measures, 59%; 2) inflammation, 46%; 3) cellular energetics, 37%; 4) proteomics, 31%; 5) excitability, 22%; 6) apoptosis, 20%; 7) oxidative stress,18%; 8) aberrant cellular chemistry, 14%; and 9) axonal transport, 10%. shows the distribution of articles across the nine categories as a percentage of the total SOD1-G93A articles along with their absolute article count.
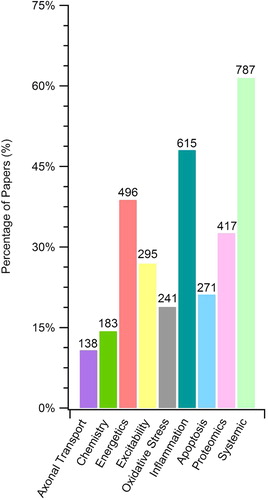
All included primary articles and their figures were labeled with at least one category using the term-category dictionary. However, given the complexity of the SOD1-G93A pathology, most articles actually belonged to more than one category. For example, a primarily proteomic study examining protein aggregation in conjunction with iron sulfur protein (ISP) belongs to both chemistry (due to ‘iron’) and proteomics (due to ‘protein aggregation’). The average number of categories labeled for each article using the term-category dictionary search was 2.6 with a standard deviation of ± 1.6. Multi-category assignment to SOD1-G93A articles is, in large part, due to the numerous cross-category pathophysiological relationships (see Categorical relationships and future directions).
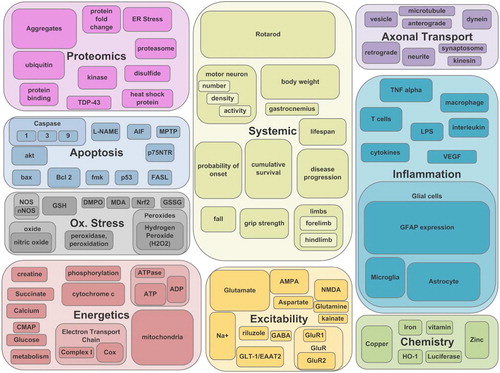
The most common 12 terms of each category (with the variations ‘or’-merged into one search) accounted for the categorization of greater than 85% of the articles in the category. Thus, these most prevalent 12 terms per category were used to develop the field analysis map shown in . The field analysis map visually illustrates the proportion of the SOD1-G93A primary data encompassed by each overall category and also the most prevalent within category terms. The size of the boxes corresponds to the approximate relative size of the categories or the categorical terms. In we reveal the number of articles and figures/tables with primary data corresponding to each of the 12 most prevalent terms per category. The full term-category dictionary and field analysis assessment is shown in Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1047455.
Table I. The 12 most common terms or phrases per category are presented with a description and the resultant number of articles (A) and figures (F). Note that the 12 terms provided more than 85% coverage in each category (see Test sets).
Topical Analysis within Ontological Categories
We present the informatics results of the topical analysis performed within each of the ontological categories and provide brief explanations of the topics’ significance to the SOD1-G93A transgenic mouse pathophysiology. Given the goals of this informatics-based systematic review, the primary purpose of the following text is to quantify and expound upon the preponderance of articles and sub-topics included in each ontological category. For further in-depth discussion of mechanisms and published experimental study results, we refer the reader to the cited references or topic-specific traditional literature reviews.
Apoptosis. Apoptosis, representing 21% of the SOD1-G93A transgenic ALS mouse literature, encompasses all programmed cell death signaling pathways. Apoptosis has multiple relationships with other ontological categories given that the ultimate endpoint of the ALS pathology is cell death.
Caspases, and in particular caspase 1, 3, and 9, are responsible for many of the signaling cascades (Citation13) that initiate apoptosis and, as such, are the most represented term under this category (122 articles, 45% of Apoptosis). Intracerebroventricular administration of zVAD-fmk, a broad caspase inhibitor, has been shown to delay disease onset and mortality in SOD1 ALS mice (Citation13). Other key signals include Bcl-2 (31 articles, 11% of Apoptosis), which has both pro- and anti-apoptotic mechanisms, and Bax (25 articles, 9% of Apoptosis), which is pro-apoptotic (Citation4,Citation14). Many studies have examined the neuroprotective effects of Bcl-2 and how, in abundance, it could be used to abolish the proapoptotic component of Bax in SOD1-G93A mice (Citation4,Citation15).
P53 and p75NTR have been examined equally with 17 articles each, collectively representing 13% of the Apoptosis literature. An increased level of p53 tumor protein is observed in ALS patients (Citation16), but the absence of p53 does not affect the SOD1-G93A mice (Citation17,Citation18). p75NTR is a neurotrophin receptor that regulates signal cascades and functions of cells, and has been implicated in motor neuron degeneration in ALS (Citation19). Reduction of Fas ligands (FASL) was examined in 11 articles as a way to increase survival in ALS mice (Citation20). MPTP, examined in 10 articles, is a neurotoxin known to induce apoptosis that has been shown to increase SOD1 activity when administered to SOD1-G93A mice (Citation21).
Axonal Transport. Comprising just 10% of the SOD1-G93A literature, axonal transport has been the least studied pathophysiological category. Molecular motors carry necessary constituents in the axon from the soma to the neuromuscular junction (i.e. anterograde transport via kinesin) and from the neuromuscular junction to the soma (i.e. retrograde transport via dynein) (Citation22,Citation23). Mutations to the machinery and cargoes can impair their attachment to the motor proteins and their mobility (Citation5). Notably, in SOD1-G93A transgenic ALS mice, axonal transport deficits appear well before cell degeneration occurs (Citation24). In addition to possible transport-specific defects, axonal transport is thought to be further hindered due to inadequate mitochondrial ATP (see Energetics), an over-abundance of misfolded SOD protein aggregates (see Proteomics), and possible excitotoxic burdens (see Excitability).
Retrograde movement was the term that appeared most often in the axonal transport literature (44 articles, 23% of Axonal Transport) with dynein ranking third in frequency (20 articles, 14% of Axonal Transport). A mutation in dynein has been shown to rescue axonal transport defects and overall extend the lifespan of ALS SOD1-G93A mice (Citation25). Comparatively, anterograde transport (16 articles, 12% of Axonal Transport) by kinesin (seven articles, 5% of Axonal Transport) does not appear as frequently in the SOD1-G93A literature despite both anterograde and retrograde transport deficits having been documented in SOD1-G93A mice (Citation26).
Chemistry. The ontological category of chemistry, accounting for 14% of the SOD1-G93A literature, includes measures of aberrant cellular chemistry, enzymatics, catalytics and metal mishandling present in the SOD1-G93A ALS pathophysiology (Citation2). Copper and zinc collectively represent 39% of the chemistry literature, although their frequency is slightly over-represented due to their appearance in the name of ‘copper zinc superoxide dismutase-1’. Beyond their involvement in SOD1, copper and zinc concentrations have been measured in different locations, with decreases shown in the liver and spinal cord (Citation27). The effect of zinc supplementation in SOD1-G93A mice has been examined, including its increased affect on NMDA-mediated excitotoxicity in SOD1, as well as its negligible impact on survival (Citation28).
Another frequent cellular chemistry assessment is iron homeostasis, which represents 9% of the chemistry literature. Iron homeostasis has been shown to be impaired in both SOD1-G93A transgenic mice and in human ALS patients (Citation29). An increase in iron content and iron genes expression has been observed in G93A-SOD1-transfected neuroblastoma cells compared to wild-type counterparts (Citation6). Iron could also be contributing to the disease via the Fenton reaction, which accelerates hydroxyl radical production that damages cellular DNA (Citation30).
Heme oxygenase-1 (HO-1), representing 9% of the chemistry literature, is an enzyme that assists in degrading heme, and has been observed to increase as ALS progresses (Citation31). Vitamins (Citation32), lithium (Citation33) and valproic acid (VPA) have been explored (Citation34) as treatment options but have shown negligible success.
Energetics. Energetics, which encompasses mitochondrial production of ATP via cellular respiration, is the third most represented ontological category, encompassing 39% of the SOD1-G93A transgenic mouse literature. Understandably, mitochondria and variations of this word are the most represented terms, encompassing 39% of the Energetics category itself. As ALS progresses, mitochondrial ability to produce ATP decreases (Citation35), which leads to axonal transport deficiencies, axonal retraction, denervation, and death of cells via apoptosis (Citation36). SOD1-G93A mice mitochondria change in both physical appearance and chemical functionality as the disease progresses (Citation37).
Calcium, the second most prevalent Energetics term, represents 20% of the Energetics ontological category. Calcium overload, a known issue in SOD1-G93A mice, leads to cell death via increased membrane permeability and loss of ATP production. Overexpression of Ca2+ binding proteins such as parvalbumin (Citation38) and calbindin D28K (Citation38,Citation39) have been shown to improve disease parameters. Interestingly, most articles examining calcium homeostasis utilize in vitro assessments (Citation40), although in vivo examination is increasing (Citation41). Finally, it should be noted that while calcium is listed in Energetics because the majority of articles examining it investigate calcium handling by mitochondria (Citation42), there are also other aspects of calcium that are clearly related to excitability due to its role in neural transmission and axonal transport (Citation41).
Glucose and related cellular pathway machinery encompasses the remainder of the most prevalent keywords in the Energetics category, as shown in . Specifically, glucose utilization rates are impaired in SOD1-G93A mice in as early as 60 days (Citation43). GAPDH, an enzyme important for the breakdown of glucose for energy, as well as creatine, have also been found to decrease by approximately 40% in SOD1-G93A mouse models (Citation44). Creatine treatment has been shown to protect against excitotoxic lesions created by NMDA (Citation45) and MPTP, which interferes with complex I, slowing mitochondrial metabolism. Finally, complex I, pyruvate, and cytochrome C have all have shown some forms of deficiency in SOD1-G93A mice (Citation35).
Excitability. Excitability is ontologically defined as the physiological pathways involved in producing action potentials. Excitability is an integral aspect of the ALS pathophysiology, representing 23% of the SOD1-G93A transgenic mouse literature. More specifically, excitability encompasses excitotoxicity, the pathological toxic over-excitation of neurons that is thought to contribute to the neuronal degeneration seen in SOD1-G93A ALS mice (Citation46). However, there has been recent debate as to whether SOD1-G93A mice experience hyperexcitability (Citation47), hypoexcitability (Citation48) or a combination of both that changes with temporal disease progression (Citation2).
Over-excitation due to glutamate homeostasis is the most frequently cited excitotoxic contributor (160 + articles, 54% of Excitability). Most of the SOD1-G93A specific research has focused on glutamate uptake, concentrations of glutamate in various locations and their effects on the systemic progression of the disease. Since overstimulation is caused by the influx of sodium (56 articles, 18% of Excitability) and calcium ions, various treatments have been tried to inhibit the voltage-gated sodium and calcium channels. Riluzole (12% of Excitability literature), one of the most common treatments for clinical ALS, is thought to work by inactivating the voltage dependent sodium receptors (Citation49,Citation50) on the glutamatergic nerve terminals (Citation46,Citation51).
The two main receptors of glutamate, calcium-permeable AMPA (α-amino-3-hydroxy-5-methyl- 4-isoxazole propionic acid) and NMDA (N-methyl-D-aspartate), are also researched heavily with the SOD1-G93A model and collectively represent 21% of the SOD1-G93A Excitability literature. AMPA receptors lacking GluR2 expression have high calcium permeability and are therefore more susceptible to motor neuron death from excitotoxicity (Citation51). Loss, decrease or immunoreactivity of glutamate transporters (56 articles, 19% of Excitability), including EAAT2 (excitatory amino acid transporter-2) (Citation52), all of which have been shown in SOD1-G93A mice, may also give rise to selective motor neuron degeneration.
GABA, another frequent topic in the excitability literature (7% of Excitability), is a neurotransmitter responsible for regulating excitability. Related topics frequently examined include GABA's current density and amplitude (Citation53), concentration (Citation54), release and transmission upon treatments, by itself (Citation55), or with glycine (Citation56), methionine sulfoximine (MSO) (Citation57), ionomycin (Citation58), and HU210 (Citation59).
Besides glutamate and its related measures, the Compound Muscle Action Potential (CMAP) is the next most frequently investigated measure of excitability (35 articles, 12% of Excitability). While modest at first, it has been observed that CMAP amplitudes drastically decrease in the final weeks before death (Citation60).
In perhaps surprising contrast, other forms of SOD1-G93A traditional electrophysiological properties of motor neurons were not in the top 12 or upper 85% of Excitability terms. However, a sector of research is ongoing with, for example, persistent inward currents or PICs (Citation50,Citation61), frequency-current or F-I gain (Citation48,Citation62), and dendritic processing (Citation63), to name just a few examples.
Inflammation. One of the fundamental characteristics in progression of ALS is activation of microglia and astrocytes, a process referred to as neuroinflammation. Inflammation is the second-largest SOD1-G93A pathophysiological category as nearly half of the papers were identified as discussing some aspect of neuroinflammation. An in-depth ontological map of this category is shown in . A major goal of inflammation research is to determine which parameters are expediting the disease versus which ones are protecting against it.
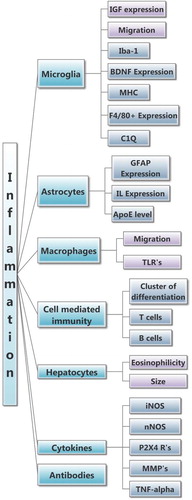
The degree of gliosis, scarring caused by reactive astrocytes, is a major indication of ALS progression in SOD1-G93A mice (Citation64), as such reactive inflammatory cells are thought to contribute to death of the motor neurons (MNs). Not surprisingly, nearly half of the most prevalent inflammation key terms is related to some aspect of gliosis. Gliosis is often assessed using GFAP expression (Citation64), which is also the most prevalent key word in the inflammation literature (240 + articles, 39% of Inflammation).
Microglial activation was the second-most assessed inflammatory topic (28% of Inflammation). In particular, common direct measures encompassed cytokines, including iNOS, TNF-alpha, and various interleukins (IL). An increased level of TNF-alpha (11% of Inflammation) has been observed in human ALS patients, but the absence of TNF-alpha in SOD1-G93A mice did not affect the survival (Citation65). Nonetheless, lowering the activity of other cytokines, such as IL-1beta, has been shown to reduce inflammation and extend survival in SOD1-G93A mice (Citation66). Additionally, macrophage activation (9% of Inflammation), which results in the production of cytokines, has been investigated in SOD1-G93A mice and in clinical patients, where an up-regulation is commonly seen (Citation66). Therefore, unsurprisingly, migration of microglia and macrophages is often used to evaluate the effectiveness of anti-inflammatory treatments (Citation67).
Vascular endothelial growth factor (VEGF), which has been investigated in 44 articles (7% of Inflammation), plays a role in neuronal protection from ischemic and hypoxic damages (Citation68), and, used as a treatment, has shown promising results in both SOD1-G93A mice and in humans (Citation69). Although VEGF has been classified under Inflammation due to its protective effects on neuroinflammation, studies suggest it may also act to reduce excitotoxicity and downstream apoptotic pathways (Citation68).
Oxidative Stress. Oxidative stress, representing 19% of the SOD1-G93A literature, reflects an imbalance between the systemic manifestation of reactive oxygen species and the normal physiological ability to readily detoxify the reactive intermediates or to repair the resulting damage (Citation30). Disturbances in the normal redox state of cells, such as those in SOD1-G93A transgenic mice, can cause toxic effects through the production of peroxides and free radicals. Resulting damage can affect many components of the neural and glial cells, including proteins, lipids, and DNA (Citation9). Furthermore, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can interfere with normal mechanisms of cellular signaling, and so many oxidative stress articles also examine their effects on other ontological categories, including excitability, inflammation and systemic outcomes.
Peroxides, and specifically hydrogen peroxide (H2O2), represent 29% of the SOD1-G93A oxidative stress literature. Peroxides are produced during the electron leakage from mitochondria (Citation9). Thus, most such articles focus on reducing the effects of peroxides produced by damaged mitochondria (Citation70,Citation71).
Other free radicals and oxidants for which the effects of various treatments have been investigated include nitric oxide (NO-) (Citation72) and peroxynitrite (ONOO-) (Citation73). Transcription factor Nrf2 (6% of Oxidative stress) is known to interact with the antioxidant-response element enhancer sequence to increase protein expression involved in antioxidant defense (Citation74). In the SOD1-G93A model, a significant decrease has been cited in the expressions of antioxidant response genes regulated by Nrf2 (Citation75) and the effects of the Nrf2/ARE system activation (Citation76). Other highly cited antioxidants include glutathione and peroxidase, which were found in over 75 + SOD1-G93A articles (33% of Oxidative stress).
Proteomics. The cellular stress caused by aggregates of mutant, misfolded proteins is a hallmark of neurodegenerative diseases including ALS (Citation36). The misfolded and aggregated proteins are considered to play a lead role in the pathophysiology of the SOD1-G93A transgenic ALS mouse. Hence, the majority of papers in proteomics, 168 or 40% of all proteomics-labeled articles, were concerned with aggregation. Mutant SOD1 seems to impair the proteasomal pathway and autophagy of the cell's degradation machinery (Citation36). Thus, instead of undergoing degradation, these misfolded proteins begin to aggregate in the cell. Like the amyloid tangles in Alzheimer's disease, a major question of the proteomics field has been whether these misfolded aggregates are a cause of the ALS pathology, in and of themselves, or if they are simply a pathological side-effect (Citation77).
Misfolded protein degradation is dependent on proteasomes (54 papers, 13% of Proteomics), which have been shown to be impaired in SOD1-G93A mice (Citation78). The two most common proteasomes studied are LMP2 and LMP7, mentioned by eight and six papers, respectively (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1047455). Ubiquitin (89 articles, 21% of Proteomics) labels proteins for degradation, and has also been shown to be inappropriately included in aggregates (Citation10).
Many patients with ALS show inclusions containing ubiquitinated and phosphorylated TAR-DNA binding protein 43 (Citation36). Most of the articles, 23 in total, examined the amount of TDP-43 as a measure of proteomic progression in SOD1-G93A ALS mice. Additional stress factors resulting from protein aggregation, such as overexpression of various heat shock proteins (HSP) and ER stress (54 articles, 13% of Proteomics), are increased in SOD1-G93A mice and are thought to coincide with disease progression (Citation79). The effect of disulfide-linking at cysteine sites has been shown to slow the rate of mutant SOD1 degradation (Citation80) and subsequently increase aggregation (Citation81).
Systemic. The systemic category includes measures that examine the disease on a higher physiological scale including overall tissue death, functional outcomes, and other possible contributors of non-neuromuscular origin. Additionally, disease onset and endpoint measures are contained within the systemic category. Systemic evaluations are important to SOD1-G93A research because they demonstrate the point at which the pathology is impacting overall function and/or health. Thus, it is not surprising that the systemic category is the most frequently assessed SOD1-G93A ontological category (see ). Many articles utilize systemic measures to assess their possible relationship to primary experimental measures from other ontological categories.
Rotarod performance (a motor function test where the mouse is placed on a rod rotating at either a constant or accelerating speed and time is measured until the animal falls), grip strength, and grip endurance, are all in vivo physical motor function tests that are utilized to assess neuromuscular disease progression in transgenic SOD1-G93A ALS mice (Citation3). Collectively, these functional measures account for approximately 28% of the systemic category.
Neuronal density and overall motor neuron count, which are known to continuously decrease with SOD1-G93A ALS mouse disease progression, are the most prevalent measures of the systemic category, encompassing 41% of the systemic literature. Unlike the functional measures, they can be measured in either in vivo or in vitro experimental settings.
Systemic measures are also used to assess the disease onset and endpoint in in vivo experiments with SOD1-G93A transgenic ALS mice. Measures of onset appear in greater than 98% of articles assigned to the systemic category. Onset is typically defined by the start of a wobbly gait, hindlimb clasping, or a percent drop in rotarod performance (Citation82). The endpoint is typically defined as either full limb paralysis, failure to stand on a rotating rotarod, or death (Citation3,Citation79).
The least represented sector of the systemic category is articles examining potential disease contributors of non-neuromuscular origin. Only a handful of articles were found. Examples include the role of possible liver disease (Citation83) and T-lymphocytes (Citation84). More research is needed in this non-neuromuscular sector given recent clinical findings citing the high relevance and relationships to overall health and/or other antecedent disease (Citation85).
Categorical relationships and future directions
ALS has long been considered a multi-factorial disease (Citation86). This characterization is supported by the many biological and pathological connections between the different presented ontological categories of the SOD1-G93A ALS pathophysiology. Hence, it is not surprising that each primary data article is typically represented by 2–3 different ontological categories. For instance, excitability (electrophysiology, channels, neurotransmitters, etc.) cannot be adequately studied without considering its strong ties to energetics (ATP, mitochondrial calcium homeostasis, etc.) and axonal transport (transport of mitochondria and neurotransmitters, etc.). A further complication of such pathophysiological relationships is that they do not necessarily have the same sign or direction for the entire disease duration. For example, axonal transport appears to initially increase, possibly as a compensatory mechanism, prior to later showing deficits (Citation2,Citation5,Citation87). Experimental measurement of relationships, especially temporal cross-category relationships (e.g. relationship of excitotoxicity to energetics) is difficult due to the required longitudinal and combinatorial experimental design (Citation2,Citation88)
Temporal relationships in the high-copy mouse model have already been shown to drastically impact the overall dynamics of the SOD1-G93A ALS pathophysiology (Citation2,Citation48). Recent clinical ALS evidence has suggested possible neuroprotective effects related to pre-onset homeostatic regulation and possibly even hypervigilant regulation (Citation85). Additionally, theoretical analysis has shown that treatments which address underlying system-level mathematical regulatory instabilities, including homeostatic oscillations, are more promising than traditional single-mechanism strategies (Citation2,Citation23). Collectively, current evidence indicates that future experimental and informatics analysis of within-category and cross-category SOD1-G93A pathophysiological temporal relationships is needed to help solve the many remaining mysteries.
Supplementary material available online
Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1047455.
iafd_a_1047455_sm3784.pdf
Download PDF (248 KB)Acknowledgements
Grants were received from the USA National Institute of Health (NS081426 and NS069616) to CSM.
Declaration of interest: The authors declare no conflict of interest with this manuscript.
References
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–55.
- Mitchell CS, Lee RH. Dynamic meta-analysis as a therapeutic prediction tool for amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis InTech. 2012. InTech. 10.5772/32384. Available from: http://www.intechopen.com/books/amyotrophic-lateral-sclerosis/dynamic-meta-analysis-as-a-therapeutic-prediction-tool-for-amyotrophic-lateral-sclerosis
- Pfohl SR, Halicek MT, Mitchell CS. Characterization of the Contribution of Genetic Background and Gender to Disease Progression in the SOD1-G93A Mouse Model of Amyotrophic Lateral Sclerosis: A Meta-Analysis. J Neuromuscul Dis. 2015;10.3233/JND-140068
- Gonzalez de Aguilar JL, Gordon JW, Rene F, de Tapia M, Lutz-Bucher B, Gaiddon C, et al. Alteration of the Bcl-x/Bax ratio in a transgenic mouse model of amyotrophic lateral sclerosis: evidence for the implication of the p53 signaling pathway. Neurobiol Dis. 2000;7:406–15.
- Mitchell CS, Lee RH. Cargo distributions differentiate pathological axonal transport impairments. J Theor Biol. 2012;300:277–91.
- Hadzhieva M, Kirches E, Wilisch-Neumann A, Pachow D, Wallesch M, Schoenfeld P, et al. Dysregulation of iron protein expression in the G93A model of amyotrophic lateral sclerosis. Neuroscience. 2013;230:94–101.
- Shi P, Gal J, Kwinter DM, Liu X, Zhu H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2010;1802:45–51.
- Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–63.
- Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–41.
- Stieber A, Gonatas JO, Gonatas NK. Aggregation of ubiquitin and a mutant ALS-linked SOD1 protein correlate with disease progression and fragmentation of the Golgi apparatus. J Neurol Sci. 2000;173:53–62.
- Gupta A, Ludascher B, Grethe JS, Martone ME. Towards a formalization of disease-specific ontologies for neuroinformatics. Neural Netw. 2003;16:1277–92.
- Mitchell CS, Halicek M, Kim R, Tilva K, Lee RH. Analysis of ALS transgenic mouse research I: 1622 papers and counting…. Society for Neuroscience; San Diego, CA 2013.
- Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288: 335–9.
- Vukosavic S, Dubois-Dauphin M, Romero N, Przedborski S. Bax and Bcl-2 interaction in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1999; 73:2460–8.
- Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, et al. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–86.
- Barbosa LF, Cerqueira FM, Macedo AF, Garcia CC, Angeli JP, Schumacher RI, et al. Increased SOD1 association with chromatin, DNA damage, p53 activation, and apoptosis in a cellular model of SOD1-linked ALS. Biochim Biophys Acta. 2010;1802:462–71.
- Kuntz Ct, Kinoshita Y, Beal MF, Donehower LA, Morrison RS. Absence of p53: no effect in a transgenic mouse model of familial amyotrophic lateral sclerosis. Exp Neurol. 2000;165:184–90.
- Prudlo J, Koenig J, Graser J, Burckhardt E, Mestres P, Menger M, et al. Motor neuron cell death in a mouse model of FALS is not mediated by the p53 cell survival regulator. Brain Res. 2000;879:183–7.
- Turner BJ, Murray SS, Piccenna LG, Lopes EC, Kilpatrick TJ, Cheema SS. Effect of p75 neurotrophin receptor antagonist on disease progression in transgenic amyotrophic lateral sclerosis mice. J Neurosci Res. 2004;78:193–9.
- Petri S, Kiaei M, Wille E, Calingasan NY, Flint Beal M. Loss of Fas ligand-function improves survival in G93A-transgenic ALS mice. J Neurol Sci. 2006;251:44–9.
- Fornai F, Carri MT, Ferri A, Paolucci E, Prisco S, Bernardi G, et al. Resistance to striatal dopamine depletion induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice expressing human mutant Cu/Zn superoxide dismutase. Neurosci Lett. 2002;325:124–8.
- Lee RH, Mitchell CS. Axonal transport cargo motor count versus average transport velocity: is fast versus slow transport really single versus multiple motor transport? J Theor Biol. 2015;370:39–44.
- Mitchell CS, Lee RH. A quantitative examination of the role of cargo-exerted forces in axonal transport. J Theor Biol. 2009;257:430–7.
- Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50–6.
- Kieran D, Hafezparast M, Bohnert S, Dick JR, Martin J, Schiavo G, et al. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J Cell Biol. 2005;169:561–7.
- Kuzma-Kozakiewicz M, Kazmierczak B, Usarek E, Baranczyk-Kuzma A. Changes in kinesin expression in the CNS of mice with dynein heavy chain 1 mutation. Acta Biochim Pol. 2013;60:51–5.
- Lelie HL, Liba A, Bourassa MW, Chattopadhyay M, Chan PK, Gralla EB, et al. Copper and zinc metallation status of copper-zinc superoxide dismutase from amyotrophic lateral sclerosis transgenic mice. J Biol Chem. 2011; 286:2795–806.
- Nutini M, Frazzini V, Marini C, Spalloni A, Sensi SL, Longone P. Zinc pre-treatment enhances NMDAR-mediated excitotoxicity in cultured cortical neurons from SOD1-G93A mouse, a model of amyotrophic lateral sclerosis. Neuropharmacology. 2011;60:1200–8.
- Capitanio D, Vasso M, Ratti A, Grignaschi G, Volta M, Moriggi M, et al. Molecular signatures of amyotrophic lateral sclerosis disease progression in hind and forelimb muscles of an SOD1-G93A mouse model. Antioxid Redox Signal. 2012;17:1333–50.
- Barber SC, Mead RJ, Shaw PJ. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta. 2006;1762:1051–67.
- Guo Y, Duan W, Li Z, Huang J, Yin Y, Zhang K, et al. Decreased GLT-1 and increased SOD1 and HO-1 expression in astrocytes contribute to lumbar spinal cord vulnerability of SOD1-G93A transgenic mice. FEBS Lett. 2010;584: 1615–22.
- Gianforcaro A, Solomon JA, Hamadeh MJ. Vitamin D(3) at 50x AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in females. PLoS One. 2013;8:e30243.
- Ferrucci M, Spalloni A, Bartalucci A, Cantafora E, Fulceri F, Nutini M, et al. A systematic study of brainstem motor nuclei in a mouse model of ALS, the effects of lithium. Neurobiol Dis. 2010;37:370–83.
- Sugai F, Yamamoto Y, Miyaguchi K, Zhou Z, Sumi H, Hamasaki T, et al. Benefit of valproic acid in suppressing disease progression of ALS model mice. Eur J Neurosci. 2004;20:3179–83.
- Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, et al. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25:164–72.
- Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14:248–64.
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–16.
- Sasaki S, Warita H, Komori T, Murakami T, Abe K, Iwata M. Parvalbumin and calbindin D-28k immunoreactivity in transgenic mice with a G93A mutant SOD1 gene. Brain Res. 2006;1083:196–203.
- Roy J, Minotti S, Dong L, Figlewicz DA, Durham HD. Glutamate potentiates the toxicity of mutant Cu/Zn superoxide dismutase in motor neurons by postsynaptic calcium-dependent mechanisms. J Neurosci. 1998;18:9673–84.
- Jaiswal MK, Keller BU. Cu/Zn superoxide dismutase typical for familial amyotrophic lateral sclerosis increases the vulnerability of mitochondria and perturbs Ca2+ homeostasis in SOD1-G93A mice. Mol Pharmacol. 2009;75: 478–89.
- Grosskreutz J, van den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–74.
- Kawamata H, Manfredi G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech Ageing Dev. 2010;131:517–26.
- Browne SE, Yang L, DiMauro JP, Fuller SW, Licata SC, Beal MF. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A-SOD1 mouse model of ALS. Neurobiol Dis. 2006; 22:599–610.
- Pierce A, Mirzaei H, Muller F, De Waal E, Taylor AB, Leonard S, et al. GAPDH is conformationally and functionally altered in association with oxidative stress in mouse models of amyotrophic lateral sclerosis. J Mol Biol. 2008; 382:1195–210.
- Beal MF. Neuroprotective effects of creatine. Amino Acids. 2011;40:1305–13.
- van den Bosch L, van Damme P, Bogaert E, Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762: 1068–82.
- Pambo-Pambo A, Durand J, Gueritaud JP. Early excitability changes in lumbar motoneurons of transgenic SOD1-G85R and SOD1-G(93A-Low) mice. J Neurophysiol. 2009;102: 3627–42.
- Delestree N, Manuel M, Iglesias C, Elbasiouny SM, Heckman CJ, Zytnicki D. Adult spinal motor neurons are not hyperexcitable in a mouse model of inherited amyotrophic lateral sclerosis. J Physiol. 2014;592:1687–703.
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurons. J Physiol. 2006;574:819–34.
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na(+) current and its effect on excitability in motor neurons cultured from mutant SOD1 mice. J Physiol. 2005;563: 843–54.
- van Damme P, Dewil M, Robberecht W, van den Bosch L. Excitotoxicity and amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:147–59.
- Warita H, Manabe Y, Murakami T, Shiote M, Shiro Y, Hayashi T, et al. Tardive decrease of astrocytic glutamate transporter protein in transgenic mice with ALS-linked mutant SOD1. Neurol Res. 2002;24:577–81.
- Carunchio I, Mollinari C, Pieri M, Merlo D, Zona C. GAB(A) receptors present higher affinity and modified subunit composition in spinal motor neurons from a genetic model of amyotrophic lateral sclerosis. Eur J Neurosci. 2008;28:1275–85.
- Niessen HG, Debska-Vielhaber G, Sander K, Angenstein F, Ludolph AC, Hilfert L, et al. Metabolic progression markers of neurodegeneration in the transgenic G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:1669–77.
- Milanese M, Zappettini S, Jacchetti E, Bonifacino T, Cervetto C, Usai C, et al. In vitro activation of GAT1 transporters expressed in spinal cord gliosomes stimulates glutamate release that is abnormally elevated in the SOD1-G93A(+) mouse model of amyotrophic lateral sclerosis. J Neurochem. 2010;113:489–501.
- Raiteri L, Stigliani S, Zappettini S, Mercuri NB, Raiteri M, Bonanno G. Excessive and precocious glutamate release in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology. 2004;46:782–92.
- Ghoddoussi F, Galloway MP, Jambekar A, Bame M, Needleman R, Brusilow WS. Methionine sulfoximine, an inhibitor of glutamine synthetase, lowers brain glutamine and glutamate in a mouse model of ALS. J Neurol Sci. 2010;290:41–7.
- Milanese M, Zappettini S, Onofri F, Musazzi L, Tardito D, Bonifacino T, et al. Abnormal exocytotic release of glutamate in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2011;116:1028–42.
- Rossi S, de Chiara V, Musella A, Cozzolino M, Bernardi G, Maccarrone M, et al. Abnormal sensitivity of cannabinoid CB1 receptors in the striatum of mice with experimental amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11:83–90.
- Llado J, Haenggeli C, Pardo A, Wong V, Benson L, Coccia C, et al. Degeneration of respiratory motor neurons in the SOD1-G93A transgenic rat model of ALS. Neurobiol Dis. 2006;21:110–8.
- Quinlan KA, Schuster JE, Fu R, Siddique T, Heckman CJ. Altered postnatal maturation of electrical properties in spinal motor neurons in a mouse model of amyotrophic lateral sclerosis. J Physiol. 2011;589:2245–60.
- Mitchell CS, Lee RH. The dynamics of somatic input processing in spinal motor neurons in vivo. J Neurophysiol. 2011;105:1170–8.
- Elbasiouny SM, Amendola J, Durand J, Heckman CJ. Evidence from computer simulations for alterations in the membrane biophysical properties and dendritic processing of synaptic inputs in mutant superoxide dismutase-1 motor neurons. J Neurosci. 2010;30:5544–58.
- Papadimitriou D, Le Verche V, Jacquier A, Ikiz B, Przedborski S, Re DB. Inflammation in ALS and SMA: sorting out the good from the evil. Neurobiol Dis. 2010;37: 493–502.
- Gowing G, Dequen F, Soucy G, Julien JP. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase-1 mutations. J Neurosci. 2006;26:11397–402.
- Meissner F, Molawi K, Zychlinsky A. Mutant superoxide dismutase-1-induced IL-1beta accelerates ALS pathogenesis. Proc Natl Acad Sci U S A. 2010;107:13046–50.
- Yamasaki R, Tanaka M, Fukunaga M, Tateishi T, Kikuchi H, Motomura K, et al. Restoration of microglial function by granulocyte-colony stimulating factor in ALS model mice. J Neuroimmunol. 2010;229:51–62.
- Greenberg DA, Jin K. VEGF and ALS: the luckiest growth factor? Trends Mol Med. 2004;10:1–3.
- Sathasivam S. VEGF and ALS. Neurosci Res. 2008; 62:71–7.
- Danzeisen R, Schwalenstoecker B, Gillardon F, Buerger E, Krzykalla V, Klinder K, et al. Targeted antioxidative and neuroprotective properties of the dopamine agonist pramipexole and its non-dopaminergic enantiomer SND919CL2x [(+)2-amino-4,5,6,7-tetrahydro-6-Lpropylamino-benzathiazole dihydrochloride]. J Pharmacol Exp Ther. 2006;316:189–99.
- Li B, Xu W, Luo C, Gozal D, Liu R. VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res Mol Brain Res. 2003;111:155–64.
- Cookson MR, Menzies FM, Manning P, Eggett CJ, Figlewicz DA, McNeil CJ, et al. Cu/Zn superoxide dismutase (SOD1) mutations associated with familial amyotrophic lateral sclerosis (ALS) affect cellular free radical release in the presence of oxidative stress. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3:75–85.
- Cassina P, Pehar M, Vargas MR, Castellanos R, Barbeito AG, Estevez AG, et al. Astrocyte activation by fibroblast growth factor-1 and motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2005;93: 38–46.
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5.
- Kirby J, Halligan E, Baptista MJ, Allen S, Heath PR, Holden H, et al. Mutant SOD1 alters the motor neuronal transcriptome: implications for familial ALS. Brain. 2005; 128:1686–706.
- Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M, et al. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic Biol Med. 2011;51:88–96.
- Foley AM, Ammar ZM, Lee RH, Mitchell CS. Systematic review of the relationship between amyloid-beta levels and measures of transgenic mouse cognitive deficit in Alzheimer’s disease. J Alzheimers Dis. 2015;44:787–95.
- Aquilano K, Rotilio G, Ciriolo MR. Proteasome activation and nNOS down-regulation in neuroblastoma cells expressing a Cu/Zn superoxide dismutase mutant involved in familial ALS. J Neurochem. 2003;85:1324–35.
- Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–5.
- Niwa J, Yamada S, Ishigaki S, Sone J, Takahashi M, Katsuno M, et al. Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1. J Biol Chem. 2007;282: 28087–95.
- Karch CM, Borchelt DR. A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283: 13528–37.
- Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1-G93A mice. Muscle Nerve. 2005;32:43–50.
- Finkelstein A, Kunis G, Seksenyan A, Ronen A, Berkutzki T, Azoulay D, et al. Abnormal changes in NKT cells, the IGF-1 axis, and liver pathology in an animal model of ALS. PLoS One. 2011;6:e22374.
- Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, et al. T-lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105:17913–8.
- Mitchell CS, Hollinger SK, Goswami SD, Polak MA, Lee RH, Glass JD. Antecedent Disease Is Less Prevalent in Amyotrophic Lateral Sclerosis. Neurodegener Dis. 2015;15:109–13. 10.1159/000369812.
- Eisen A. Amyotrophic lateral sclerosis is a multifactorial disease. Muscle Nerve. 1995;18:741–52.
- de Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720–8.
- Mitchell CS, Lee RH. Pathology dynamics predict spinal cord injury therapeutic success. Journal of Neurotrauma. 2008;25:1483–97.
- Oh YK, Shin KS, Yuan J, Kang SJ. Superoxide dismutase-1 mutants related to amyotrophic lateral sclerosis induce endoplasmic stress in neuro2a cells. J Neurochem. 2008; 104:993–1005.
