Abstract
A relative preservation of eye movements is notable in ALS, but saccadic functions have not been studied longitudinally. ALS overlaps with FTD, typically involving executive dysfunction, and eye-tracking offers additional potential for the assessment of extramotor pathology where writing and speaking are both impaired. Eye-tracking measures (including anti-saccade, trail-making and visual search tasks) were assessed at six-monthly intervals for up to two years in a group of ALS (n = 61) and primary lateral sclerosis (n = 7) patients, compared to healthy age-matched controls (n = 39) assessed on a single occasion. Task performance was explored speculatively in relation to resting-state functional MRI (R-FMRI) network connectivity. Results showed that ALS patients were impaired on executive and visual search tasks despite normal basic saccadic function, and impairments in the PLS patients were unexpectedly often more severe. No significant progression was detected longitudinally in either group. No changes in R-FMRI network connectivity were identified in relation to patient performance. In conclusion, eye-tracking offers an objective means to assess extramotor cerebral involvement in ALS. The relative resistance of pure oculomotor function is confirmed, and higher-level executive impairments do not follow the same rate of decline as physical disability. PLS patients may have more cortical dysfunction than has been previously appreciated.
Introduction
Some of the very earliest descriptions of ALS particularly noted the characteristic sparing of oculomotor as well as sphincter and sensory functions (Lockhart-Clarke's descriptions are reviewed in (Citation1)). However, individual case reports, particularly if disease course is prolonged through mechanical ventilation, suggest a resistance rather than complete sparing of oculomotor function, with ophthalmoplegia and nystagmus reported, as well as more subtle abnormalities in saccadic function (comprehensively reviewed in (Citation2)). ALS is recognized to have clinical, pathological and genetic overlap with frontotemporal dementia (FTD). Dementia meeting diagnostic criteria for FTD occurs in up to 15% of ALS patients, and cognitive impairment may be found in over one-third (Citation3). Dysexecutive function is a prominent component of the cognitive profile in ALS, and associated with adverse prognosis (Citation4). Eye-tracking tasks might have particular value in overcoming the interference from combined limb and bulbar disability that may hinder the cognitive assessment of ALS patients (Citation5).
Anti-saccade performance was previously shown to be particularly impaired in ALS patients (Citation6), but longitudinal changes have not been previously explored. Separately, our group developed a novel cognitive application of eye-tracking based upon the Trail Making Test (TMT) of executive function. Pilot studies in healthy volunteers showed a good correlation with performance on the conventional written TMT, particularly on part B (Citation7). We therefore wished to undertake a larger eye-tracking study in ALS to characterize the natural history of saccadic functions, and to explore extramotor involvement through eye-driven cognitive tests. Evidence for memory deficits in ALS extends beyond that explainable by a dysexecutive syndrome (Citation8), and language is another vulnerable cognitive domain (Citation9). Therefore, in addition to the executive anti-saccade and TMT tasks, a third test of visual search skills was planned, involving a (language and pictorial) cued paradigm requiring working memory resources.
As part of a longitudinal multimodal biomarker discovery cohort in ALS, we integrated eye-tracking tasks in a large group of patients and healthy controls to assess their potential as biomarkers of extramotor cerebral involvement. In an exploratory imaging study, we attempted to link performance with cerebral network connectivity derived from resting-state functional MRI (R-FMRI).
Methods
Participants
Prevalent and incident cases of ALS, and primary lateral sclerosis (PLS), were recruited between 2009 and 2012 from a tertiary referral centre as part of the Oxford Study for Biomarkers in Motor Neuron Disease (B0ioMOx). Diagnosis was confirmed according to the revised El Escorial criteria by two experienced neurologists (MRT, KT) (Citation10,Citation11). Patients were offered repeat assessments every six months up to a maximum of two years. Healthy controls similar in age, handedness and level of education were recruited for assessment at a single time-point only.
All physical (MRT), eye-tracking and cognitive (RS,CMB), and neuroimaging (MRT) assessments were performed on the same day. Disability was assessed using the revised ALS Functional Rating Score (ALSFRS-R, range 0–48 with lower scores reflecting greater disability). Cognitive function was assessed using the revised Addenbrooke's Cognitive Examination (ACE-R (Citation12)), plus the standard written Trail Making Test (TMT (Citation13)) for those with adequate upper limb function. Rate of disability progression (δALSFRS-R) was calculated as drop in ALSFRS-R from a presumed baseline score of 48 divided by the disease duration in months from reported symptom onset. A measure of the burden of clinical upper motor neuron (UMN) signs was based upon pathological reflex sum score (Citation14).
Although systematic genotyping was not undertaken, the patient group was considered to be apparently sporadic on the basis that none of the participants reported a positive family history of ALS or FTD apart from one, who was known to carry a C9orf72 hexanucleotide repeat expansion and included in the analysis. All participants provided written informed consent and the study was approved by the South Central Oxford Research Ethics Committee B.
Eye-tracking
Eye-tracking data were acquired using a tower-mounted Eyelink 1000 infra-red eye-tracker (SR Research) focusing on a single eye while stimuli were displayed on a screen 60 cm away. Eyelink calibration and validation was performed prior to each experimental session and data were captured at 1000 Hz. Chin and forehead rests were applied to minimize head movement during recording and spectacles were permitted providing acquisition was not impeded. Stimulus presentation and data capture was programmed using Experiment Builder software (SR Research). The entire eye-tracking session lasted approximately 40 min.
Saccadometry. Pro- and anti-saccades were assessed in consecutive separate blocks after standardized verbal instruction. Twenty-four trials of each saccade type were completed per block; one block of each saccade task was undertaken at the start of the eye-tracker session and repeated at the end (96 trials in total). A central blue square cue was presented on a black screen for fixation at the start of each trial. After an interval, jittered between 1000 ms and 1750 ms in 250-ms steps, a yellow square target appeared at a lateral visual angle of 10° (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1054292). The laterality of the target randomly varied. During the pro-saccade block, participants were required to saccade to the location of the target; during the anti-saccade block saccades were made to the opposite side of the screen. If the participants failed to suppress a reflexive pro-saccade then they were encouraged to make a corrective anti-saccade. The trial timed out if no saccade was made within 2000 ms. Saccades were excluded from analysis if made prior to target presentation, with latency under 100 ms or with amplitude less than 1°. Saccadic latency was defined as the time from target appearance to the initiation of a valid saccade.
Eye-tracking Trail Making Test
TMT performance was assessed according to a recently developed eye-tracking paradigm (Citation7). In brief, a screen displayed a horizontally flipped version of the written trail to maintain a similar arrangement of items but accommodating a landscape format. Participants were required to identify items on the trail by fixating on the item for at least 400 ms, whereupon selection by gaze contingency was acknowledged by a change in colour from white to red of a ring surrounding the item. Only a correctly identified item would change colour, the previously selected item was marked as completed by a change from red to dark grey (Supplementary Figure 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1054292). An eight-item practice trial was performed prior to each 25-item test trial as per the written protocol. The number of fixations was recorded in addition to time for test completion within a trial time-out of 200 s. Verbal prompts were provided in the occurrence of perseveration or inattention. Video recordings were reviewed in order to detect and correct for any calibration errors interfering with fixation detection; if due to poor calibration, fixation beyond 400 ms was required for the eye-tracker to acknowledge item selection, this additional time was subtracted from the total task completion time.
Visual search task. A gaze-contingent paradigm was designed to assess the ability to visually identify a primed item from distractors on the basis of a unique combination of two possible forms (disc or ring) with two possible colours (red or green) (Citation15). Following verbal instruction, calibration and validation, the first of 40 trials commenced with a central cue presented on the display. This took the form of either white text describing the target or an image of the target itself. An interval of 800 ms was then followed by presentation of the target accompanied by nine distractors arranged in a circular formation (Supplementary Figure 3 to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1054292). Blocks consisted of either 40 word-cued trials or 40 picture-cued trials; the order of conditions was balanced across participants. Fixation for a minimum of 400 ms was required for gaze-contingent selection. The number of fixations required before finding the correct target was recorded, in addition to the time to completion of trial (search latency). Verbal prompts were provided on request if the participant failed to recall the cue; additional time required in this instance was included in the search latency.
Statistical analysis
Analysis was performed using SPSS (version 21, IBM) and Matlab (MathWorks). Groups were compared using independent or paired t-tests, or Mann-Whitney U if distributions non-normal, one-way ANOVA preceded by Levene test of homogeneity and with Welch correction in the event of uneven variance. Results were confirmed by ANCOVA, using age as covariate. Post hoc testing was corrected for multiple comparisons using Hochberg's GT2 given the discrepancy in group sizes, and mean differences were confirmed using 95% confidence intervals with 1000 bootstrapped parameter estimates. Longitudinal assessment used Friedman's test if non-normal distributed data were detected by Shapiro-Wilk. All tests were two-tailed, with p < 0.05 considered significant. Clinical correlations were partial correlated for age unless otherwise stated; Spearman's rho was used for non-parametric data.
MRI
Acquisition. Scans were performed at the Oxford Centre for Clinical Magnetic Resonance Research using a 3T Siemens Trio scanner (Siemens AG, Erlangen, Germany) with a 12-channel head coil. Whole-brain functional imaging at rest was performed using a gradient echo EPI sequence (TR/TE = 3000/28 ms, flip angle = 89°, 3-mm isotropic resolution, 6 min acquisition time). For consistency, subjects were instructed to close their eyes throughout, but to remain awake. Furthermore, a field map was acquired using a gradient echo imaging sequence (2 × 2 × 2 mm3 voxel size; 65 slices; echo time 1/echo time 2/repetition time = 5.19 ms/7.65 ms/655 ms) to account for distortions in the EPI data caused by field inhomogeneities.
Analysis. Resting-state analysis was performed using probabilistic independent component analysis (ICA; implemented in the FSL tool MELODIC (Citation16) (Details in Supplementary methods to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1054292).
Results
Participants
Sixty-one ALS, seven PLS, and 39 healthy controls were studied. Demographic and clinical data are shown in . Nearly half of the patients (n = 29) had repeated assessment. Eye-tracker tasks were well tolerated by nearly all patients, 97% completed saccadometry, 96% the eye-tracking version of the TMT and 91% the visual search task. In contrast, only 76% of patients were able to complete the ACE-R and 75% the written version of the TMT. ALSFRS-R was lower among ALS patients unable to complete the written tests (28 versus 36, p < 0.001). Resting-state functional MRI data were available for 48 ALS patients and 19 controls that had completed saccadometry, 47 patients who completed the eye-tracking TMT, and 45 patients who completed the visual search task.
Table I. Participant details. There were significantly more males within the ALS group and lower ACE-R in patients (with a trend towards lower ACE-R in the PLS subgroup, although not surviving correction for age). Although there was no difference in mean levels of disability, the PLS group had an expected slower rate of disability progression, longer disease duration, and greater clinical UMN scores.
Cross-sectional data: saccadometry
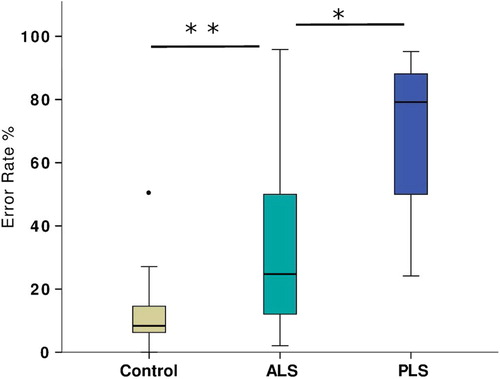
There was no significant group difference in pro-saccade performance. Both ALS and PLS patients were significantly impaired in anti-saccade performance (), demonstrating both increased error rate (Welch's F(2,15) = 30.1, p < 0.001) and increased latency (F(2,101) = 14.5, p < 0.001). A single control subject made an exceptionally high degree of errors (chance level to either laterality), given the resulting z transformed score of 3.97; those data were ‘winsorized’ to the next highest value (50% reduced to 27% errors) prior to further group comparison (). Post hoc testing confirmed significantly more errors in the PLS group than the ALS group (BCa 95% CI of mean difference 14.7–56.3) and slower latencies (BCa 95% CI of mean difference 42.9–166.3). The PLS group was furthermore compared against a subgroup of 25 ALS participants with a UMN score higher than the group median (Citation11), and still was found to make more anti-saccade errors (p = 0.049) with longer latency for correct anti-saccades (p = 0.02). Subgroup comparisons were finally corrected for both UMN score and ACE-R. The difference in anti-saccade latency was preserved. Significant cross-corre1ation between anti-saccade error rate and latency was noted (r2 = 0.44, p < 0.001).
Figure 1. Performance on saccadometry at the initial assessment for all groups demonstrating preservation of pro-saccadic function (including when erroneously made during the anti-saccade task).
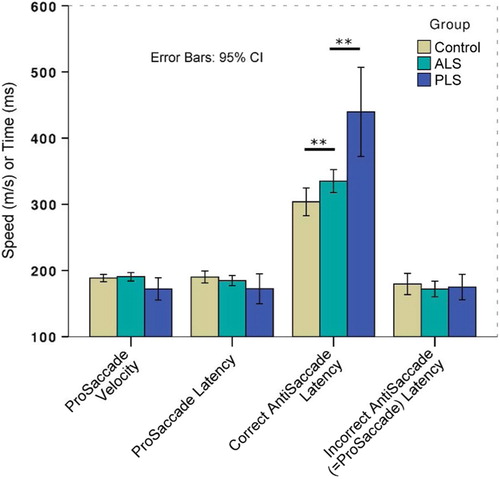
Figure 2. Percentage of anti-saccade errors made during the initial assessment, demonstrating one outlier control subject that was subsequently excluded from analysis.
Within the ALS group, anti-saccade error rates significantly increased with falling ALSFRS-R score (r2 = 0.15, p = 0.02) but without any such correlation in relation to pro-saccade performance. ALS patients with bulbar onset were both slower to initiate anti-saccades (mean difference of 62.8 ms, BCa 95% CI 119.4–12.4, t(Citation54) = 2.87, p = 0.006), and made more anti-saccade errors (mean difference 26.4%, BCa 95% CI 44.9–4.0, t(Citation54) = 3.07, p = 0.003) compared to those with limb onset. There was no significant correlation with disease duration, rate of progression, UMN score or ACE-R performance in the patient group.
Cross-sectional data: Trail Making Tests
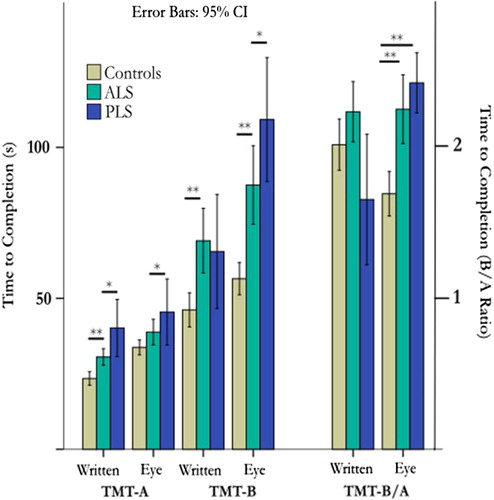
An excess of fixations and saccades were generally noted in patients versus controls during the eye-tracking TMT (). Compared to controls, patients (ALS & PLS combined) were significantly impaired on the eye-tracking TMT-B (Welch's F(2,15) = 25.4, p < 0.001), written TMT-B (Welch's F(2,15) = 9.2, p = 0.002), eye-tracking TMT-A (F(2,100) = 4.0, p = 0.02), and written TMT-A (F(2.87) = 16.0, p < 0.001 (). Post hoc testing revealed that the PLS subgroup performed worse than ALS patients on the written TMT-A (mean difference 9.45 s, BCa 95% CI 17.1–1.5) and eye-tracking TMT-B (mean difference of 27.6 s, BCa 95% CI 46.7–11.0). The cognitive contribution to the TMT performance was further isolated from visuo-motor skills by considering TMT-B completion time relative to TMT-A. Group differences in the B/A ratio (higher ratio denotes cognitive impairment) were more pronounced using the eye-tracking version of the assessment (eye-tracking B/A F(2/100) = 8.95, p <.001, written B/A F(2,86) = 3.60, p = .032). Post hoc testing between groups of the B/A ratio was only significant for the eye-tracking version, not the written, after multiple comparisons correction was applied ().
Figure 3. Fixations and saccades during the eye-tracker TMT-B in an ALS patient (B and D) versus a healthy control subject (A and C).
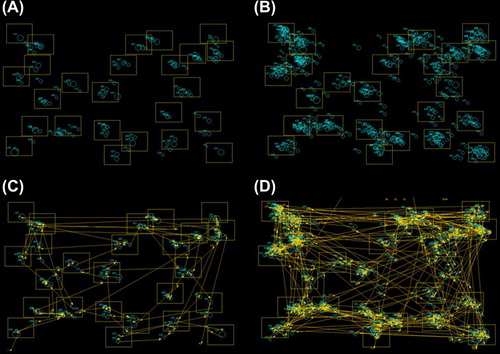
Figure 4. Group performance differences at first time-point on both traditional written TMT and eye-tracker versions, against both time to completion (left panel) and the ratio of time for B/A to better reflect the cognitive component (right panel). Group differences remained pronounced in the eye-tracking version of the task.
Patients (ALS & PLS) also made significantly more fixations during the TMT-B task compared with controls (Welch's F(2,15) = 19.4, p < 0.001, Supplementary Figure 4 to be found online at http://informahealthcare.com/doi/abs/10.3109/21678421.2015.1054292). There was strong correlation between performance on the written and eye-tracking versions of the TMT, with slightly higher correlation on Part B (r2 = 0.44, p < 0.001) than on Part A (r2 = 0.34, p < 0.001), and preserved when ALS and control groups were analysed separately.
Weak correlations were noted in all patients (ALS & PLS) between the total ACE-R score and eye-tracking TMT-B (r2 = 0.09, p = 0.05), and between ACE-R and TMT-B/A (r2 = 0.11, p = 0.002). There were no significant correlations between eye-tracking TMT performance and ALSFRS-R, rate of progression, disease duration or UMN score. No significant effect of site of symptom onset was noted.
Cross-sectional data: visual search task
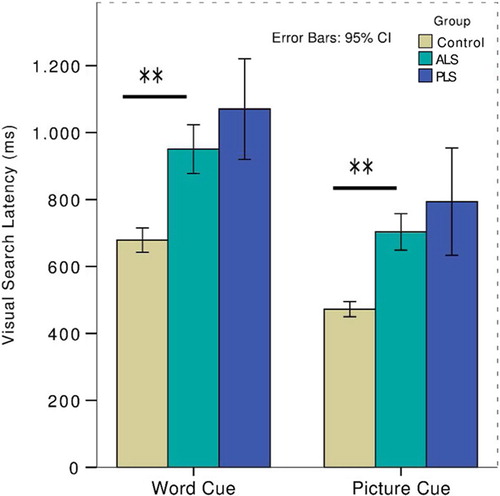
Patients (ALS & PLS combined) were significantly impaired in the visual search task, regardless of the cue modality. Word-cued latency (Welch's F(2,14) = 36.3, p < 0.001) and picture-cued latency (Welch's F(2,13) = 39.2, p < 0.001) were significantly prolonged in both patient groups. Post hoc tests did not detect any differential performance between ALS and PLS patients (). Patient groups required more fixations prior to identifying the target regardless of cue modality and there was a high degree of cross-correlation between latency and number of fixations (r2 = 0.75, p < 0.001) and between performance after different cue types (r2 = 0.79, p < 0.001). There was no two-way interaction between cue type and disease status.
Figure 5. Group performance at the first time-point on the visual search task split by cue type. Patients were significantly impaired regardless of cue type and disease subtype.
Within the ALS group there was a weak correlation between ACE-R performance and that of both word-cued search as measured by latency (r2 = 0.12, p = 0.036) and picture-cued search as measured by fixations (r2 = 0.17, p = 0.011) but this relationship did not withstand correction for age. Otherwise, the visual search tasks did not reveal any significant relationship with clinical markers and there was no significant difference for site of onset.
Longitudinal study
Group comparisons were re-analysed using the last available set of investigations for those participants that had repeat assessments. The group differences were essentially unchanged from those detected at the first assessment. Of note, there was still no effect of group on performance of pro-saccade tasks. A differential effect of patient group (PLS worse than ALS) for the eye-tracking TMT-B was confirmed (Welch's F(2,14.6) = 24.2, p < 0.001).
An analysis of repeated measures of performance within both ALS and PLS revealed, in summary, a high degree of performance stability for all eye-tracking (as well as written) measurements. For the six ALS patients assessed on four occasions, over a mean total interval of 20 months, no effect of time was shown on any eye-tracker performance measures. Direct comparison of first visit data against final visit also did not reveal any effect of time other than a trend towards improved performance on the eye-tracking TMT part A (n = 29, first visit mean completion time 36.7 s, final visit 33.6 s, mean time-interval 11.8 min).
Functional MRI correlates
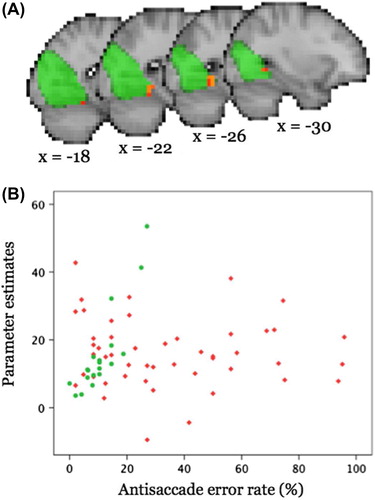
Neither the visual search task nor outcomes from the eye-tracking TMT-B correlated significantly with network coherence for any of the eight RSNs of interest in any of the groups. In healthy controls, anti-saccade error rate was found to correlate positively with coherence within the medial/occipital pole visual network () and negatively with functional connectivity (FC) of the lateral visual network for a very small region in the anterior cingulate gyrus (after full significance correction for pcorr < 0.003). No significant correlation between FC measures and anti-saccade error rate was observed for patients for any of the tested RSNs.
Figure 6. A) Significant (pcorr < 0.0031) positive correlation (red-yellow) between anti-saccade error rate and coherence within the middle/occipital pole visual network in controls shown in sagittal views (x = MNI coordinate). The respective RSN is shown in green colour (thresholded at z > 3). Results are overlaid onto the (down-sampled) T1-weighted MNI template. B) Scatterplot illustrating the relationship between parameter estimates averaged across all voxels in which the coherence with the RSN correlates significantly with anti-saccade error rate in controls (controls, green; patients, red).
Discussion
This study demonstrated that higher-level oculomotor tasks are sensitive to ALS pathology, exemplified by striking sparing of pro-saccade function in the presence of impairment of anti-saccades. Unexpectedly, similar and sometimes more severe abnormalities were noted in those with PLS. There was no significant change in eye-tracking performance over time despite increasing physical disability.
Previous observations of oculomotor involvement in ALS and PLS
A range of oculomotor impairments are described in ALS (comprehensively reviewed in (Citation17)), sometimes correlated with frontal lobe dysfunction (Citation6,Citation18) or clinical manifestations such as Parkinsonism (Citation19). Previous longitudinal study was limited to two patients (Citation20), while a cross-sectional clinical study failed to demonstrate correlation with disability across 63 subjects (Citation21). Saccadic breakdown of smooth pursuit has been noted in those with PLS (Citation22). Our finding that anti-saccades were impaired in PLS above and beyond typical performance in ALS, has not been previously observed, and would benefit from replication given the limited number of PLS participants who were furthermore imperfectly matched with the ALS group.
ALS patients make anti-saccade errors through failure to suppress reflexive saccadic eye movements in the context of preserved pro-saccade speed and remembered-saccade accuracy (Citation6,Citation23). Additionally, slowed reflexive saccades (Citation24), and an increased proportion of early saccades (although anti-saccades were otherwise preserved) (Citation25) have been reported. Cross-sectionally, we noted increasing anti-saccade error rates with increasing disability. Previous cross-sectional studies did not link oculomotor dysfunction with disease severity (Citation21,Citation25). Our longitudinal data suggest that pure and higher-level oculomotor function remains relatively static throughout the course of the disease in the face of significant progressive disability.
Few histopathological examinations in typical ALS have specifically noted involvement of the oculomotor nuclei (Citation26), in keeping with the functional integrity of pro-saccades, but significant neuronal loss and gliosis were found in an atypical case with ophthalmoplegia (Citation27). The cellular features mirror autopsy findings in anterior horn cells (Citation28), including deposition of phosphorylated TDP-43 (Citation29) and may accumulate through disease progression such that advanced cases of ‘locked-in’ ALS anecdotally demonstrate severe oculomotor dysfunction (Citation30). Relative preservation of extraocular muscle neuromuscular junction integrity is described compared to limb musculature (Citation31). This resistance to the downstream effects of neurodegeneration has been modelled in vivo (Citation32) and may reflect altered susceptibility due to differential expression of genes, with matrix metalloproteinase-9 as one example (Citation33).
Oculomotor tasks in relation to cognition in ALS and PLS
The potential burden of cognitive dysfunction in ALS necessitates its consideration throughout the illness (Citation34), especially very advanced disease when there is a need for assessment of intellectual capacity concerning end-of-life decisions. A cognitive study of severely physically impaired ALS patients in their home environments using binary answer strategies demonstrated surprisingly preserved function (Citation35), which confounded expectations set by previous findings of longitudinal cognitive decline (Citation36). A population based study concluded that those with normal baseline cognitive function tended to remain intact (Citation37), suggesting that there is a variable susceptibility to extramotor involvement in ALS; furthermore, the distribution of affected cognitive domains varies considerably between patients (Citation38). Those patients with bulbar involvement are inconsistently reported to have an over-representation of cognitive impairment (Citation39–41). Our findings support the positive view in demonstrating a more frequent impairment on both anti-saccade and eye-tracking TMT-B within the bulbar-onset sub-group.
Contrary to early descriptions, the cognitive profile of ALS is broadly shared by PLS patients (Citation42,Citation43), although rarely to the extent of overt FTD (Citation44,Citation45). Our data reinforce that executive impairment is a major feature of the PLS phenotype. Cortical atrophy is typically more pronounced in PLS than ALS, and has been shown to extend frontally beyond the pre-central gyrus (Citation46,Citation47). Marked atrophy of the primary motor cortex identifies this region as the neurodegenerative focus responsible for the UMN syndrome. Clinically, the UMN burden score did not account for the worse performance in PLS patients, but the structural correlates of the accompanying cognitive syndrome in PLS have also been identified in white matter abnormalities that include the corpus callosum (Citation48,Citation49).
It was noted that 25% of ALS participants were unable to complete a written TMT, whereas only 5% could not complete the eye-tracking version. In assessment of cognition, eye-tracking tasks may outperform written tasks, particularly when rendered unsuitable by upper limb weakness or bulbar dysfunction. This conclusion is supported by the differential performance on written and eye-tracking versions of the TMT within the patients. The executive function component of TMT-B is to an extent unmasked in the eye-tracking version, as the confounding factor of impaired upper limb motor function is removed. Adjustment for visuo-motor speed is conventionally achieved by calculating the ratio of written TMT-B completion time to an individual's written TMT-A score. The reported eye-tracking TMT typically improved detection of executive function compromise in our patient groups above and beyond this simple transformation (demonstrated in ). However, completion of the eye-tracking TMT-A was still on average prolonged beyond that of healthy controls, indicating that this strategy may not be universally suitable for ALS subjects, in whom a minority may have more deep-seated oculomotor dysfunction (Citation21).
The cortical network basis of oculomotor dysfunction in ALS
Multi-modal imaging studies of healthy subjects demonstrate a widely distributed neural substrate for the control of saccadic eye movements (Citation50), vulnerable to disruption from focal lesions (Citation51) and that overlaps topographically with neuroimaging abnormalities seen in ALS (reviewed in (Citation52)). Saccadic movements show clear-cut abnormalities in those with pure FTD (Citation53,Citation54). The performance of anti-saccades has been particularly linked to activity in the supplementary eyefields and the dorsolateral prefrontal cortex (DLPFC (Citation55)). The DLPFC was among the first regions of abnormal function noted in ALS neuroimaging studies, particularly during tasks of executive function (Citation56), and furthermore was recently shown to be activated insufficiently by ALS participants during anti-saccade preparation (Citation57). Failure to suppress reflexive saccades may reflect widespread motor system hyper-excitability (Citation58) beyond the specific regions such as the DLPFC that are typically implicated in saccadic control by functional neuroimaging (Citation59,Citation60), lesion studies (Citation61) and invasive recordings in primates (Citation62). Cortical hyper- excitability is a well-founded phenomenon in ALS (Citation63,Citation64) and may in part reflect a loss of local inhibitory circuits (Citation65).
The TMT is a widely used assessment of attentional switching (Citation66) alongside visual search skills (Citation67) and is sensitive to a range of pathology including DLPFC lesions (Citation68). The TMT requires additional cortical activation in prefrontal regions during part B in both younger (Citation69) and older healthy (Citation70) subjects.
Visual searches for word-cued targets are inevitably slower than picture-cued targets (Citation71); we found no group interaction with cue type despite the recent recognition that the burden of cognitive dysfunction in ALS includes significant language impairment (Citation9). Language deficits are independent of dysarthria (Citation72) and are often accompanied by executive dysfunction (Citation3). The visual search task additionally probes visuo-motor cognitive processes involving the prefrontal cortex (Citation73), but also has an obvious dependency on working memory, a domain previously reported abnormal in ALS (Citation74,Citation75). Group differences may not therefore be driven by visuo-spatial deficits that remain infrequent in ALS (Citation76). Impairment in attention demanding visual search tasks is however specifically reported (Citation77), unsurprising given the extensive neural network required to efficiently perform such tasks, particularly when feature binding is involved (Citation78–80). The task failed to distinguish between groups by cue modalities, presumably reflecting an insufficient challenge to language capabilities, given that this is a cognitive domain increasingly recognized as vulnerable in ALS (Citation9).
Limitations
In retrospect, it is conceded that the Addenbrookes Cognitive Examination is not optimized for the specific cognitive syndrome of ALS, and for future studies we would aim to use newer, more tailored tools such as the recently validated Edinburgh Cognitive and Behavioural ALS Screen (ECAS) (Citation81). Few of the ALS participants were taking riluzole, but we did not systematically consider the use of other potentially confounding medication, e.g. amtitriptyline for sialorrhoea. Chronic hypercapnoea due to respiratory insufficiency is another potential confound, although none of the participants was established on assisted ventilation at the time of study, and most were able to tolerate lying flat for up to one hour during MRI. Common to longitudinal studies in ALS is an inherent selection bias such that those subjects followed over repeated visits are most likely to have disease at the milder, less rapidly-progressive end of the spectrum, which necessarily diminishes the power to detect change within individuals (Citation37,Citation82). This factor, alongside the tertiary clinic setting, which may under-represent the population of ALS with cognitive impairment (Citation37), may also contribute to the lack of functional imaging correlates. Increased FC within the ALS-specific cortical network has been linked to higher rates of disease progression (Citation83), and of more specific relevance a correlation of parietal FC with cognitive deficits in ALS (Citation84). Eye-tracking tasks such as those presently described rely on broad neural networks that could conceivably be disrupted at different anatomical locations across individuals with similar performance.
This study involved uniformly apparently sporadic ALS patients, apart from one individual. Studies of patients with ALS associated with the C9orf72 hexanucleotide repeat expansion have suggested consistently increased cognitive impairment in this group (Citation85,Citation86), which would be interesting to explore in relation to oculomotor function.
No prospective studies have yet considered the diagnostic value of oculomotor abnormalities to ALS, although the lower motor neuron disease mimic Kennedy disease may also show impairment (Citation87). The value of anti-saccade error rate as a diagnostic biomarker will be the subject of a prospective study involving those suspected to have ALS, compared with disease mimics.
Supplementary material available online
iafd_a_1054292_sm3841.docx
Download MS Word (164.8 KB)Acknowledgements
We thank all the study participants for their great personal efforts, attending on repeated occasions to gather this unique dataset despite advancing disability in the case of the patients.
We are grateful to Ludovica Griffanti for providing scanner and sequence specific resting-state training data for use in combination with FIX.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Turner MR, Swash M, Ebers GC. Lockhart Clarke’s contribution to the description of amyotrophic lateral sclerosis. Brain: a journal of neurology. 2010;133:3470–9.
- Sharma R, Hicks S, Berna CM, et al. Oculomotor dysfunction in amyotrophic lateral sclerosis: a comprehensive review. Archives of Neurology. 2011;68:857–61.
- Phukan J, Elamin M, Bede P, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population based study. Journal of Neurology, Neurosurgery, and Psychiatry. 2012;83:102–8.
- Elamin M, Phukan J, Bede P, et al. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology. 2011;76:1263–9.
- Cipresso P, Meriggi P, Carelli L, et al. editors. The combined use of Brain Computer Interface and Eye-Tracking technology for cognitive assessment in Amyotrophic Lateral Sclerosis. International Workshop on Pervasive Computing Paradigms for Mental Health; 2012.
- Shaunak S, Orrell RW, O’Sullivan E, et al. Oculomotor function in amyotrophic lateral sclerosis: evidence for frontal impairment. Annals of Neurology. 1995;38:38–44.
- Hicks SL, Sharma R, Khan AN, et al. An eye-tracking version of the trail-making test. PloS One. 2013;8:e84061.
- Machts J, Bittner V, Kasper E, et al. Memory deficits in amyotrophic lateral sclerosis are not exclusively caused by executive dysfunction: a comparative neuropsychological study of amnestic mild cognitive impairment. BMC Neuroscience. 2014;15:83.
- Taylor LJ, Brown RG, Tsermentseli S, et al. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? Journal of Neurology, Neurosurgery, and Psychiatry. 2013;84:494–8.
- Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- Gordon PH, Cheng B, Katz IB, et al. The natural history of primary lateral sclerosis. Neurology. 2006;66:647–53.
- Mioshi E, Dawson K, Mitchell J, et al. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–85.
- Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1:2277–81.
- Menke RA, Korner S, Filippini N, et al. Widespread grey matter pathology dominates the longitudinal cerebral MRI and clinical landscape of amyotrophic lateral sclerosis. Brain : a journal of neurology. 2014.
- Kristjansson A, Driver J. Priming in visual search: separating the effects of target repetition, distractor repetition and role-reversal. Vision Research. 2008;48:1217–32.
- Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. NeuroImage. 2012;62:782–90.
- Donaghy C, Thurtell MJ, Pioro EP, et al. Eye movements in amyotrophic lateral sclerosis and its mimics: a review with illustrative cases. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82:110–6.
- Donaghy C, Pinnock R, Abrahams S, et al. Ocular fixation instabilities in motor neuron disease. A marker of frontal lobe dysfunction? Journal of Neurology. 2009;256:420–6.
- Gizzi M, DiRocco A, Sivak M, et al. Ocular motor function in motor neuron disease. Neurology. 1992;42:1037–46.
- Palmowski A, Jost WH, Prudlo J, et al. Eye movement in amyotrophic lateral sclerosis: a longitudinal study. Ger J Ophthalmol. 1995;4:355–62.
- Moss HE, McCluskey L, Elman L, et al. Cross-sectional evaluation of clinical neuro-ophthalmic abnormalities in an amyotrophic lateral sclerosis population. Journal of the Neurological Sciences. 2012;314:97–101.
- Pringle CE, Hudson AJ, Munoz DG, et al. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain : a journal of neurology. 1992;115:495–520.
- Evdokimidis I, Constantinidis TS, Gourtzelidis P, et al. Frontal lobe dysfunction in amyotrophic lateral sclerosis. J Neurol Sci. 2002;195:25–33.
- Donaghy C, Pinnock R, Abrahams S, et al. Slow saccades in bulbar-onset motor neuron disease. Journal of Neurology. 2010.
- Burrell JR, Carpenter RH, Hodges JR, et al. Early saccades in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:294–301.
- Lawyer T Jr, Netsky MG. Amyotrophic lateral sclerosis: a clinico-anatomic study of 53 cases. ArchNeurol. 1953;69:171–92.
- Harvey DG, Torack RM, Rosenbaum HE. Amyotrophic lateral sclerosis with ophthalmoplegia: a clinicopathologic study. Archives of Neurology. 1979;36:615–7.
- Okamoto K, Hirai S, Amari M, et al. Oculomotor nuclear pathology in amyotrophic lateral sclerosis. Acta Neuropathologica. 1993;85:458–62.
- Mizuno Y, Fujita Y, Takatama M, et al. Comparison of phosphorylated TDP-43-positive inclusions in oculomotor neurons in patients with non-ALS and ALS disorders. Journal of the Neurological Sciences. 2012;315:20–5.
- Mizutani T, Aki M, Shiozawa R, et al. Development of ophthalmoplegia in amyotrophic lateral sclerosis during long-term use of respirators. Journal of the Neurological Sciences. 1990;99:311–9.
- Liu JX, Brannstrom T, Andersen PM, et al. Distinct changes in synaptic protein composition at neuromuscular junctions of extraocular muscles versus limb muscles of ALS donors. PloS One. 2013;8:e57473.
- Mosier DR, Siklos L, Appel SH. Resistance of extraocular motor neuron terminals to effects of amyotrophic lateral sclerosis sera. Neurology. 2000;54:252–5.
- Kaplan A, Spiller KJ, Towne C, et al. Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron. 2014;81:333–48.
- Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurology. 2013;12:368–80.
- Lakerveld J, Kotchoubey B, Kubler A. Cognitive function in patients with late stage amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:25–9.
- Robinson KM, Lacey SC, Grugan P, et al. Cognitive functioning in sporadic amyotrophic lateral sclerosis: a six-month longitudinal study. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:668–70.
- Elamin M, Bede P, Byrne S, et al. Cognitive changes predict functional decline in ALS: a population based longitudinal study. Neurology. 2013;80:1590–7.
- Meier SL, Charleston AJ, Tippett LJ. Cognitive and behavioural deficits associated with the orbitomedial prefrontal cortex in amyotrophic lateral sclerosis. Brain : a journal of neurology. 2010;133:3444–57.
- Abrahams S, Goldstein LH, Al Chalabi A, et al. Relation between cognitive dysfunction and pseudobulbar palsy in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1997;62:464–72.
- Schreiber H, Gaigalat T, Wiedemuth-Catrinescu U, et al. Cognitive function in bulbar- and spinal-onset amyotrophic lateral sclerosis A longitudinal study in 52 patients. Journal of Neurology. 2005.
- Zalonis I, Christidi F, Paraskevas G, et al. Can executive cognitive measures differentiate between patients with spinal- and bulbar-onset amyotrophic lateral sclerosis? Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2012;27: 348–54.
- Caselli RJ, Smith BE, Osborne D. Primary lateral sclerosis: a neuropsychological study. Neurology. 1995;45:2005–9.
- Piquard A, Le Forestier N, Baudoin-Madec V, et al. Neuropsychological changes in patients with primary lateral sclerosis. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2006;7:150–60.
- Grace GM, Orange JB, Rowe A, et al. Neuropsychological functioning in PLS: a comparison with ALS. Can J Neurol Sci. 2011;38:88–97.
- Tan CF, Kakita A, Piao YS, et al. Primary lateral sclerosis: a rare upper-motor-predominant form of amyotrophic lateral sclerosis often accompanied by frontotemporal lobar degeneration with ubiquitinated neuronal inclusions? Report of an autopsy case and a review of the literature. Acta Neuropathologica. 2003;105:615–20.
- Kiernan JA, Hudson AJ. Frontal lobe atrophy in motor neuron diseases. Brain : a journal of neurology. 1994;117: 747–57.
- Kuipers-Upmeijer J, de Jager AE, Hew JM, et al. Primary lateral sclerosis: clinical, neurophysiological, and magnetic resonance findings. J Neurol Neurosurg Psychiatry. 2001; 71:615–20.
- Canu E, Agosta F, Galantucci S, et al. Extramotor damage is associated with cognition in primary lateral sclerosis patients. PloS One. 2013;8:e82017.
- Meoded A, Kwan JY, Peters TL, et al. Imaging findings associated with cognitive performance in primary lateral sclerosis and amyotrophic lateral sclerosis. Dementia and Geriatric Cognitive Disorders extra. 2013;3:233–50.
- Anderson EJ, Jones DK, O’Gorman RL, et al. Cortical network for gaze control in humans revealed using multimodal MRI. Cerebral cortex (New York, NY: 1991). 2012;22: 765–75.
- Kennard C, Mannan SK, Nachev P, et al. Cognitive processes in saccade generation. Annals of the New York Academy of Sciences. 2005;1039:176–83.
- Turner MR, Agosta F, Bede P, et al. Neuroimaging in amyotrophic lateral sclerosis. Biomark Med. 2012;6:319–37.
- Boxer AL, Garbutt S, Seeley WW, et al. Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer’s disease. Archives of Neurology. 2012; 69:509–17.
- Burrell JR, Hornberger M, Carpenter RH, et al. Saccadic abnormalities in frontotemporal dementia. Neurology. 2012;78:1816–23.
- Jamadar SD, Fielding J, Egan GF. Quantitative meta- analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Frontiers in Psychology. 2013;4:749.
- Abrahams S, Goldstein LH, Kew JJ, et al. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain : a journal of neurology. 1996;119:2105–20.
- Witiuk K, Fernandez-Ruiz J, McKee R, et al. Cognitive Deterioration and Functional Compensation in ALS Measured with fMRI Using an Inhibitory Task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14260–71.
- Wessel JR, Reynoso HS, Aron AR. Saccade suppression exerts global effects on the motor system. Journal of Neurophysiology. 2013;110:883–90.
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. Journal of Neurophysiology. 2008;99:133–45.
- Pa J, Dutt S, Mirsky JB, et al. The functional oculomotor network and saccadic cognitive control in healthy elders. NeuroImage. 2014;95:61–8.
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, et al. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain : a journal of neurology. 2003;126: 1460–73.
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nature neuroscience. 2006;9:925–31.
- Ziemann U, Winter M, Reimers CD, et al. Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis: evidence from paired transcranial magnetic stimulation. Neurology. 1997;49:1292–8.
- Vucic S, Cheah BC, Yiannikas C, et al. Cortical excitability distinguishes ALS from mimic disorders. Clin Neurophysiol. 2011;122:1860–6.
- Turner MR, Kiernan MC. Does interneuronal dysfunction contribute to neurodegeneration in amyotrophic lateral sclerosis? Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2012;13:245–50.
- Arbuthnott K, Frank J. Trail Making Test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22:518–28.
- Gaudino EA, Geisler MW, Squires NK. Construct validity in the Trail Making Test: what makes part B harder? J Clin Exp Neuropsychol. 1995;17:529–35.
- Stuss DT, Bisschop SM, Alexander MP, et al. The Trail Making Test: a study in focal lesion patients. Psychological Assessment. 2001;13:230–9.
- Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43:1878–86.
- Hagen K, Ehlis AC, Haeussinger FB, et al. Activation during the Trail Making Test measured with functional near-infrared spectroscopy in healthy elderly subjects. NeuroImage. 2014;85:583–91.
- Wolfe JM, Horowitz TS, Kenner N, et al. How fast can you change your mind? The speed of top-down guidance in visual search. Vision Research. 2004;44:1411–26.
- Rippon GA, Scarmeas N, Gordon PH, et al. An observational study of cognitive impairment in amyotrophic lateral sclerosis. Archives of Neurology. 2006;63:345–52.
- Anderson EJ, Mannan SK, Husain M, et al. Involvement of prefrontal cortex in visual search. Experimental Brain Research. 2007;180:289–302.
- Volpato C, Piccione F, Silvoni S, et al. Working memory in amyotrophic lateral sclerosis: auditory event-related potentials and neuropsychological evidence. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2010;27:198–206.
- Hammer A, Vielhaber S, Rodriguez-Fornells A, et al. A neurophysiological analysis of working memory in amyotrophic lateral sclerosis. Brain Research. 2011;1421: 90–9.
- Abrahams S, Newton J, Niven E, et al. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2013.
- Munte TF, Troger MC, Nusser I, et al. Abnormalities of visual search behaviour in ALS patients detected with event-related brain potentials. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1:21–7.
- Nobre AC, Coull JT, Walsh V, et al. Brain activations during visual search: contributions of search efficiency versus feature binding. NeuroImage. 2003;18:91–103.
- Shulman GL, McAvoy MP, Cowan MC, et al. Quantitative analysis of attention and detection signals during visual search. Journal of Neurophysiology. 2003;90:3384–97.
- Monosov IE, Thompson KG. Frontal eye field activity enhances object identification during covert visual search. Journal of Neurophysiology. 2009;102:3656–72.
- Abrahams S, Newton J, Niven E, et al. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:9–14.
- Turner MR, Benatar M. Ensuring continued progress in biomarkers for amyotrophic lateral sclerosis. Muscle and Nerve. 2014.
- Douaud G, Filippini N, Knight S, et al. Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain : a journal of neurology. 2011;134:3470–9.
- Agosta F, Canu E, Valsasina P, et al. Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiology of Aging. 2013;34:419–27.
- Byrne S, Elamin M, Bede P, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population based cohort study. Lancet Neurology. 2012.
- Irwin DJ, McMillan CT, Brettschneider J, et al. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2013;84:163–9.
- Thurtell MJ, Pioro EP, Leigh RJ. Abnormal eye movements in Kennedy disease. Neurology. 2009;72: 1528–30.
