ALSUntangled reviews alternative therapies on behalf of patients with ALS (PALS). Here we review the use of Endotherapia (also known as multivalent nanotherapy or GEMALS) for ALS, for which we have had more than 470 requests (Citation1).
Overview
Endotherapia is advertised by a French group as ‘a new therapeutic approach to chronic conditions, including auto-immune, neurodegenerative and proliferative disease’ that is based on ‘genetic predisposition, and immunological, bacterial and environmental factors’ (Citation2). It consists of an ‘individualized’ combination of several components within three different families: fatty acids (which are said to block the sites of germ anchors in the gut), antioxidants and ‘scavengers’ (Citation3). Each of these components is ‘coupled’ to ‘a non-immunogenic chain of poly-L-Lysine (PLL), which reportedly reduces degradation and improves permeability (Citation4). The proponents of Endotherapia suggest that it is effective for multiple sclerosis, rheumatoid arthritis and ALS (Citation2,Citation4), diseases that most believe to have very different pathophysiologies.
Mechanism(s)
To engage in this treatment for ALS, a patient’s blood sample is sent to a laboratory in France, where ‘ELISA analysis’ is performed (Citation5). The pattern of antibodies detected is used to determine what may have triggered the disease in that patient, usually a ‘bacterium’ according to this laboratory, and what resultant autoimmune and inflammatory processes may be playing a role. An example of the antibody pattern being used is shown in (Citation6). This information in turn is then used to generate a specific treatment cocktail for that particular patient. An example of such a cocktail can be found in . The proponents of Endotherapia report that the cocktail is usually the same in the ‘hundreds’ of PALS they have treated (Citation6,Citation7).
Figure 1. An example of the antibody pattern being used by proponents of Endotherapia to treat a patient with ALS (Citation5).
Legend reads (translated from French): ‘Indirect ELISA technique was used. The ‘immunobilan’ is a biological test that quantifies the number of circulating antibodies in the serum of patients affected with chronic conditions (based on draw of 5 ml of serum)’.
Interpretation:
- Values greater than or equal to +2 indicate elevated levels of circulating antibodies.
- Values less than or equal to –2 indicate the formation of immune-complexes
Immune System Activation:
- M = IgM
- A = IgA
- G = IgG
Composition of ‘Type B Bilan’
- Indicators of hyperproduction of tryptophan and hyperproduction of NO and NO2 (free radical involvement).
- Indicators of mucosal hyperpermeability = IgA.
- Indicators of mucosal dysbiosis = IgA.
- Indicators of mycobacterial activation = IgG and IgA.
Indications of ‘Type B Bilan’:
Parkinsons, Alzheimer’s, MS, ALS, spinocerebellar ataxia, Huntington’s, macrophage-induced ‘myofasciite’, progressive supranuclear palsy, multi-system atrophy, Alexander’s disease, Alpers disease, Creutzfeldt-Jakob, Pick’s disease, lysosomal disorder.
Graph 1: Activation of immune system following activation of ‘IDO’ pathways and NO synthases.
Graph 2: Activation of immune system by bacterial constituents, following mucosal hyperpermeability.
Graph 3: Activation or re-activation of immune system after dysbiosis.
Graph 4: Activation or re-activation of immune system by bacterial constituents linked to mycobacteria.
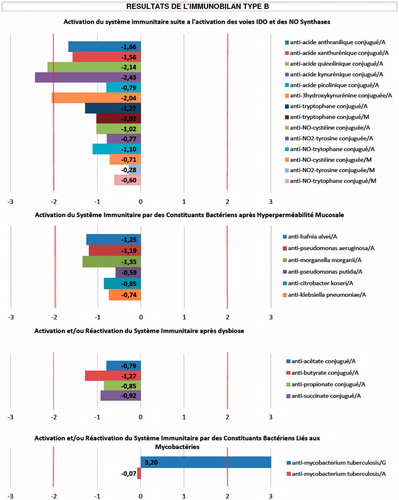
Table I. Components of the Endotherapia cocktail for patients with ALS (Citation4).
There are multiple problems with this approach. First, there is no evidence that bacteria can trigger ALS through inflammation or autoimmunity. The proponents of Endotherapia claim that E. coli and cyanobacteria are implicated (Citation2,Citation4). The reference they use to support their claim about E. coli (Citation8) is actually a paper on the biochemistry of mutant SOD1 protein expressed within this organism. The study researchers were using E. coli to artificially produce a large amount of the SOD1 protein so they could study it. This type of experiment is commonly performed with different proteins because bacteria can easily be engineered to produce large quantities of abnormal proteins. This paper does not examine the role of E. coli as a cause of human ALS. While cyanobacteria are hypothesized to play a role in ALS, they are thought to do so via a direct neurotoxin they produce called BMAA, not by inducing inflammation and autoimmunity (Citation9). Secondly, the antibody profile supplied () shows no obvious targeting of E. coli or cyanobacteria antibodies, but instead suggests that the proponents of Endotherapia are looking for antibodies to many other bacteria (e.g. Klebsiella, Pseudomonas) that even they have never reported as involved in ALS. Thirdly, it is known that 10% of ALS cases are caused by genetic abnormalities (Citation10); the proponents of Endotherapia do not appear to be carrying out any genetic testing to identify this subset. Fourthly, at least two components of the Endotherapia cocktail have been tested in human ALS and found to have no benefit (Citation11,Citation12); why these would be included in this cocktail is thus unclear. Finally, as of the date of this publication, there are no biomarkers, antibodies or otherwise, that have been demonstrated to confirm the trigger, identify downstream processes that drive progression, or plan successful treatment for patients with sporadic ALS. The proponents of Endotherapia have published papers (Citation13,Citation14) and an abstract (Citation15) on components of their ALS immunoblots, but in our opinion these failed to demonstrate their utility as ALS biomarkers of any kind. ALSUntangled assigns a TOE ‘Mechanism’ grade of D based on this information ().
Table II. TOE Grades for Endotherapia as an ALS treatment.
Pre-clinical data
One published study shows that Endotherapia may delay disease onset and prolong survival in the G93A mutant SOD1 rat model of ALS (Citation16). This study has multiple methodological flaws according to published guidelines (Citation17), including small sample size, start of treatment pre-symptomatically and failure to blind raters. One of the authors has a patent on Endotherapia (Citation18); this creates a potential conflict of interest that is not disclosed. This study has never been independently replicated. ALSUntangled assigns a TOE ‘Mechanism’ grade of C based on this information ().
Data in PALS
No PALS in the online community PatientsLikeMe report taking Endotherapia (Citation19). Google search for Endotherapia in ALS located one PALS who, after seven months of treatment, reports “my speech has become noticeably clearer and picked up speed, my facial muscles feel stronger and my leg strength is improving” (Citation20). This same case has been discussed in multiple chat rooms but no additional clinical details appear (Citation21–23). We do not have medical records to validate these changes. Other PALS report starting Endotherapia (Citation21–23) but we find no other reports of results. ALSUntangled assigns a TOE ‘Cases’ grade of D based on this information ().
There is a published trial of Endotherapia in PALS; details of this appear within the body of two review articles (Citation2,Citation4). Twelve participants reportedly took treatment for periods between three months and 72 months. The ALSAQ40 (Citation24) was used as a measure of their disease severity/progression. Progression in treated participants was compared to ‘the worldwide reference mean speed of ALSAQ40 evolution’ (Citation2). If participants’ ALSAQ40 progressed faster than or equal to the worldwide reference they were said to have had no response to Endotherapia; 17% met this definition. If participants’ ALSAQ40 worsened but at a slower rate than the worldwide reference, they were said to have ‘decreased progression’; 58% met this definition. If participants’ ALSAQ40 did not change over the observation period they were said to have ‘stabilized’; 8% of participants met this definition. If participants’ ALSAQ40 improved they were said to have had a ‘reversal of the evolution of the disease’; 17% met this definition (Citation2). The proponents of Endotherapia state that these results have “sparked huge expectation” (Citation2) and that “no current drug used in ALS treatment has achieved the results found with GEMALS” (Citation4). They extrapolate their results to an increased life expectancy of ‘4.38 years’ for treated participants (Citation2).
There are many serious problems with the above described trial. The ALSAQ40 is a measure of quality of life in ALS (Citation24). While quality of life is undoubtedly important, it is not tightly linked with physical disability in ALS (Citation25) and can even be influenced by psychiatric interventions (Citation26); thus the ALSAQ40 is not an acceptable primary measure of ALS disease progression. Changes in this quality of life scale cannot be extrapolated to changes in life expectancy. The course of ALS is known to be highly variable between patients (Citation27). Thus, with any trial using historical controls, it is critical to match participants to controls on variables that are known to influence progression (Citation28); it does not appear that this occurred here. We are not aware of the ‘worldwide reference’ they are referring to. The sample size is very small sample and the duration of treatment is very short (as little as three months in some patients). The authors never validated participant diagnoses or ALSAQ40 scores; instead they relied on records from treating neurologists (Citation6,Citation7) and how accurate these are is unknown. The authors claim to have treated ‘hundreds’ of patients with Endotherapia (Citation6,Citation7); it is not clear why or how they selected this small subset to report on. Finally, one of the authors has a patent on Endotherapia (Citation18); this creates a potential conflict of interest that is not disclosed in the papers describing the trial (Citation2,Citation4). ALSUntangled assigns a TOE ‘Trials’ grade of D based on this information ().
The proponents of Endotherapia claim to have recently added participants to the above described trial so that their sample size is now 31 (Citation6,Citation21). While they have not published this additional data anywhere the conclusions they are reporting to PALS are unchanged (Citation21). This is still a small sample size and the many other flaws described above remain unchanged, as does our “Trials” grade.
Risks and costs
The proponents of Endotherapia report no side-effects in any of the PALS they have treated (Citation6). However, since they are not following patients themselves it is unclear that there is any kind of rigorous surveillance for adverse events occurring. ALSUntangled assigns a TOE ‘Safety’ grade of U based on this information ().
The blood test and initial reading reportedly costs 90 euros plus shipping (Citation21) and the treatment reportedly costs 1400 euros for six months (Citation6).
Conclusions
Endotherapia has a proposed mechanism that hinges on the ability of an isolated laboratory’s immunoblots to identify the cause and specific pathways that are driving ALS progression. In our opinion this ability has never been convincingly demonstrated. While there is a flawed animal study supporting the utility of Endotherapia in rats, such studies rarely translate into useful human treatments (Citation29). The data on Endotherapia in PALS have so many problems that we believe they are uninterpretable. ALSUntangled does not recommend the use of Endotherapia for ALS at this time. A reasonable next step would be a study to validate the utility of the above-described immunoblots, ideally by a group without a potential conflict of interest.
Declaration of interest
ALSUntangled is sponsored by the ALS Association and the Motor Neurone Disease Association.
References
- http://www.alsuntangled.com/open.php. Accessed December 2, 2015.
- Geffard M, Bisschop L, Duleu S, Pouns O, Ferran G, Bessede A, et al. Endotherapia. Anti-inflammatory and Anti-aging Agents in Medicinal Chemistry. 2010;9:197–211.
- Geffard M, Duleu S, Bessede A, Vigier V, Bodet D, Mangas A, et al. GEMSP: A New Therapeutic Approach to Multiple Sclerosis. Central Nervous System Agents in Medicinal Chemistry. 2012;12:1730–81.
- Geffard M, DeBisschop L, Duleu S, Hassaine N, Mangas A, Covenas R. Endotherapia: a new frontier in the treatment of multiple sclerosis and other chronic diseases. Discovery Medicine. 2010;10:443–51.
- http://www.idrpht.info/Immuno-Bilan#/Immuno-Bilan/. Accessed December 3, 2015.
- Email between ALSUntangled and Dr. M Geffard, November 6, 2015.
- Telephone conversation between ALSUntangled and ‘Pierre’, a researcher working with Dr. M Geffard, November 9, 2015.
- Leinweber B, Barofsy E, Barofsky D, Emilov V, Nylin K, Beckman J. Aggregation of ALS mutant superoxide dismutase expressed in Escherichia coli. Free Radical Biology and Medicine. 2004;36:911–8.
- Bradley W, Mash D. Beyond Guam: the cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotroph Lateral Scler. 2009;S2:7–20.
- Chen S, Sayana P, Zhang X, Le W. Genetics of amyotrophic lateral sclerosis: an update. Mol Neurodegener. 2013;9:28.
- Kaufmann P, Thompson J, Buchsbaum R, Shefner J, Krivickas L, Katz J, et al. Phase II trial of coq10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66:234–44.
- Graf M, Ecker D, Horowski R, Kramer B, Riederer P, Gerlach M, et al. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind study. J Neural Transm. 2005;112:646–60.
- Duleu S, van der Velden C, Poulletier de Gannes F, Tranchant M, Geffard M. Circulating antibodies to NO- and ONOO-modified antigens in amyotrophic lateral sclerosis, Alzheimer’s disease and multiple sclerosis. Immuno-anlayse et biologie specialisee. 2007:22:273–81.
- Duleu S, Mangas A, Poulletier de Gannes F, Tranchant M, Geffard M. Circulating antibodies to conjugated tryptophan derivatives of the IDO pathway in amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s and multiple sclerosis patients. Immuno-anlayse et biologie specialisee. 2008:23:27–34.
- Duleu S, Sevin F, Bessede A, Ferrand G, Poullettier De Gannes F, et al. The indirect involvement of radical processes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;S1:158.
- Nicase C, Coupier J, Dabadie M, de Decker R, Mangas A, Boudet D, et al. Gemals, a new drug candidate, extends lifespan and improves electromyographic parameters in a rat model of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:85–90.
- Ludolph A, Bendotti C, Blaugrund E, Chio A, Greensmith L, Loeffler J, et al. Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph Lateral Scler. 2010;11:38–45.
- http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=6114388.PN.&OS=PN/6114388&RS=PN/6114388. Accessed November 27, 2015.
- https://www.patientslikeme.com/search?utf8=✓&cx=016260609975327438954%3Amf0ormpzdl4&ie=UTF-8&q=endotherapia. Accessed November 27, 2015.
- http://www.illawarramercury.com.au/story/3219048/new-treatment-hope-for-bulli-mnd-sufferer/. Accessed November 27, 2015.
- http://www.alstdi.org/forum/yaf_postst54957p3_endotherapia-treatment-for-mnd-real-progress-or-placebo.aspx. Accessed November 29, 2015.
- https://www.inspire.com/groups/als-association/discussion/endotherapia/. Accessed December 2, 2015.
- http://forum.mndassociation.org/showthread.php?6540-Endotherapia. Accessed December 2, 2015.
- Jenkinson C, Fitzpatrick R, Brennan C, Bromberg M, Swash M. Development and validation of a short measure of health status for individuals with amyotrophic lateral sclerosis/motor neuron disease: the ALSAQ40. J Neurol. 1999;246s3:16–21.
- Lule D, Hacker S, Ludolph A, Birbaumer N, Kubler A. Depression and quality of life in patients with amyotrophic lateral sclerosis. Dtsch Arztebl Int. 2008;105:397–403.
- van Groenestijn A, Schroeder C, Visser-Meuly V, Reenen ET, Veldink J, van Den Berg L. Cognitive behavioural therapy and quality of life in psychologically distressed patients with amyotrophic lateral sclerosis and their caregivers: results of a prematurely stopped randomized controlled trial. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:309–15.
- Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014;10:661–70.
- Donofrio P, Bedlack R. Historical controls in ALS trials: a high seas rescue? Neurology. 2011;6:936–7.
- Benatar M. Lost in translation: treatment trials in the SOD1 mouse and human ALS. Neurobiol Dis. 2007;26:1–13.
