Abstract
Wound is one of the oldest suffering associated with the mankind and its history is as old as humanity. Advances in the field of medical sciences created a pile of knowledge and paved the path for the development of a separate branch specifically devoted for wound healing. The understanding and treatment strategies for wound healing have gone through a great revolution. This article reviews all the aspects of wound healing including the pathway, types and recent advances made in the wound care management in particular moist wound dressings using natural polymers, skin grafts, debridement, growth factor and drug delivery.
Introduction
Wound healing is a complex and dynamic cascade of events initiated by injury (Masre et al. Citation2012). This response to injury is a phylogenetically primitive, yet essential, innate host immune response for the restoration of tissue integrity (Martin Citation1997, Singer and Clark Citation1999). The process of wound healing is divided into sequential phases (Lawrence Citation1998, Phillips Citation2000, Orgill and Demling Citation1988). These phases overlap in terms of time, physiology, and cell type, with each phase not entirely completed before the next begins. Wounds have historically been treated by bizarre methods. Lizard’s dung, pigeon’s blood, cobwebs, boiling oil and hot irons are few of the odd treatments (Orgill and Demling Citation1988). The recent developments and discoveries made in the last decade expanded the knowledge base and technologies related wound sciences. Today, there is an increase in the scientific approach to healing as ‘old’ treatments like larvae therapy, sugar, leeches, tea tree oil, etc. are now rationalized and shown to be effective. The paradigm shift from dry dressings based on woven fabrics towards a moist environment revolutionized developments in this field. Nevertheless, the pathological cases of chronic wounds are still difficult to handle (Reiss et al. Citation2009). Even with the moist concept they require patient and persistent treatment. Continuous advances made in the study of the wound microenvironment, pathophysiology of wounds, and improved techniques in monitoring the response of healing and treatment of chronic wounds offered new hopes for patients suffering from acute and chronic wounds. This article reviews recent advances made in wound care management including stem cell technique, growth factor delivery and modern dressings.
Historical developments
Hippocrates is among pioneers credited with one of the earliest mentions of chronic wounds as he apparently recognized some relationship between leg ulceration and venous disorders (Hippocrates Citation1849). As early as the tenth century, there was widespread belief that curing an ulcer prevented the efflux of dangerous humors which was widely upheld until the eighteenth century (Avicenna et al. 1783). In a recently discovered early fifteenth- century manuscript, chronic wounds, regardless of cause, were divided into seven classes of ulceration based on a Latin nomenclature. The classes include corrosium—a wound that slowly progresses to form a shallow cavity prior to healing followed by virulentum—an old wound in which exudate is liquid and plentiful and difficillis consolidationis—an old wound that is difficult to heal, but not a cancer or an inflamed sore.
In 1775, the physician John Hunter reported that the sores of poor people can be healed in a speedy manner by rest in a horizontal position, fresh provisions and warmth in hospitals (Hunter Citation1837). Another significant contribution was made by John Gay in 1867, when he not only described clot formation and postthrombotic recanalization, but also recorded that ulceration could occur in the absence of varicose veins and introduced the term “venous ulcer”(Gay Citation1868). Perhaps the most significant advances in both the understanding of chronic wound pathophysiology and the introduction of modern wound dressings have taken place within the last 40–50 years. highlights the historical aspects of developments made in wound treatments.
Table I. Historical development in wound treatments.
Types of wounds
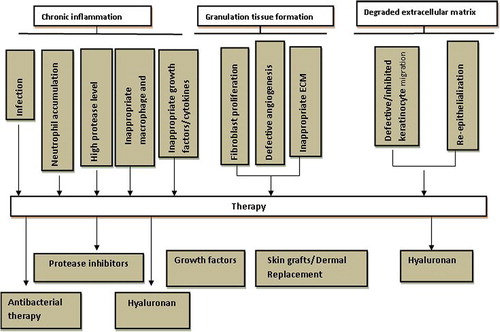
Wounds can be classified as acute or chronic (). Acute wounds can be defined as any interruption in the continuity of any tissue of the body (Wysocki Citation1999). This is in contradistinction to a chronic wound, which can be defined as an intervention in the continuity of any tissue that requires a prolonged time to heal, does not heal, or reoccurs (Wysocki Citation1999). Acute wounds heal as anticipated and proceed through a normal, orderly, and timely reparative process that results in sustained restoration of anatomic and functional integrity. Chronic wounds do not heal as anticipated and fail to proceed through this process or proceed through the process but fail to establish a sustained anatomic and functional result (Lazarus et al. Citation1994). Chronic wounds may get stuck in any of the phases of wound healing for greater than 6 week (Collier Citation2006).
Table II. Types of wounds.
Trajectory wound healing
Stages in wound healing
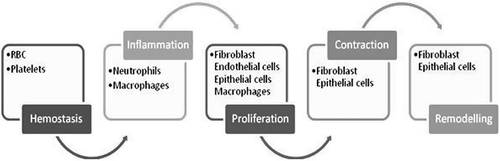
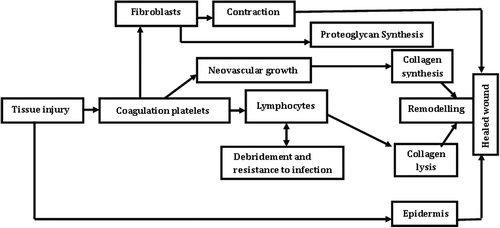
Wound healing involves a highly orchestered sequence of events that is triggered by tissue injury and ends by either partial or complete regeneration or by repair. Healing process can be divided into the five overlapping stages of hemostasis, inflammation, proliferation, contraction, and remodeling involving various effector cells ().
Hemostasis
The first step in wound healing involves vasoconstriction to decrease blood loss and stimulation of Hageman Factor (XII) to initiate the clotting cascade () (Monaco and Lawrence Citation2003, Ryan and Manjo Citation1987). Wound causes exposure of collagen which is the major protein present in all tissues of the body leading to stimulation of the alternate complement pathway as well as platelet adherence and degranulation (Monaco and Lawrence Citation2003, Wakefield et al. Citation2001). Degranulation causes release of numerous cytokines, such as PDGF (platelet derived growth factor) (Clark et al. Citation1982, Herndon et al. Citation1993). The hemostatic stage prepares for and influences the onset of the next stage of healing- inflammation.
Inflammation
After hemostasis, inflammation is initiated in a few hours after injury. Chemotactic signals attract neutrophils and monocytes to wound sites (Reibman et al. Citation1991). Neutrophils normally begin arriving at the wound site within minutes of injury to provide protection against contaminating bacteria and also activate local fibroblasts and keratinocytes (Hübner et al. Citation1996). The neutrophil infiltration ceases after a few days, and neutrophils are themselves phagocytosed by tissue macrophages. Macrophages continue to accumulate at the wound site by recruitment of blood-borne monocytes and are essential for effective wound healing (Martin Citation1997). Once activated, macrophages also release a series of growth factors and cytokines at the wound site, thus amplifying the earlier wound signals released by degranulating platelets and neutrophils (Martin Citation1997).
Migration and proliferation
The next phase is classified as migration and proliferation phase. This phase of wound healing is characterized mainly by anabolic reactions, i.e., angiogenesis, epithelization, and fibroplasia. The formation of blood vessels is known as angiogenesis, which starts with an endothelial cell bud formed by existing intact vessels (Fisher et al. Citation1995). Soon, endothelial cells migrate through the resulting gap in the direction of the wound following the oxygen gradient. They divide and form tubular structures that connect with other buds. As a result, during the maturation process a new basal membrane develops from the extracellular matrix components. The newly formed vascular loops then connect with intact vessels and differentiate accordingly into capillaries, arterioles, and venules (Martin Citation1997).
For epithelization, proceeding in parallel to angiogenesis, keratinocytes migrate across the wound and as a result reconstitute epidermal covering from the wound margin and hair follicle remnants (Rochat et al. Citation1994). Fibroblasts are the predominant cell type especially at wound edges to form the new loose extracellular matrix consisting of proteoglycans as well as the water-soluble collagen fibers essential for tissue stability () (Garlick and Taichman Citation1994). Collagen is a fibrous protein crucial to the process of wound healing and identified as the most abundant connective tissue protein.
In healthy tissue, the collagen fibers are aligned in basketweave patterns. This organized structure is not achieved in wound healing as the collagen fibers at the wound site fashion themselves in an alignment parallel to the stress lines of the wound. Type I and type III are the collagens most commonly found in healing wounds, although at least 19 different types of collagen have been identified and characterized (Friess Citation1988).
Contraction
In this step wound essentially shrinks by recruiting adjacent tissue and pulling it into the wound. This process is intimate with the phases of proliferation and remodeling, because the key effector cell is the fibroblast (). More specifically, the cell involved is the myofibroblast as first described by Diegelmann and Evans (Citation2004). While motor function is present in all fibroblasts as well as in other cells such as leukocytes, these cells are modified in a way that moves the edges of the wound towards the center, rather than just being motile and moving themselves. Collagen in the extracellular matrix aids in locking the cells in place, thus augmenting the contraction process (Gilbertson et al. Citation2001, Davie et al. Citation1975).
Matrix remodeling
Matrix remodeling, cell maturation, and cell apoptosis create the final phase of wound repair, which overlaps with tissue formation. Once the wound is filled with granulation tissue and covered with a neoepidermis, fibroblasts transform into myofibroblasts, which contract the wound, and epidermal cell differentiate to reestablish the permeability barriers. Endothelial cells appear to be the first cell type to undergo apoptosis, followed by the myofibroblasts, leading gradually to a rather acellular scar (Clark Citation1996). During the proliferation phase of wound healing ( and ), fibroblasts assume a myofibroblast phenotype characterized by large bundles of actin-containing microfilaments disposed along the cytoplasmic face of the plasma membrane of the cells and by cell-cell and cell-matrix linkages (Welch et al. Citation1990, Desmouli re and Gabbiani 1996). The contraction probably requires stimulation by TGF-β1 or TGF-β2 and PDGF, attachment of fibroblasts to the collagen matrix through integrin receptors, and cross-links between individual bundles of collagen (Schiro et al. Citation1991, Clark et al. Citation1989, Montesano and Orci Citation1988, Woodley et al. Citation1991). The overall collagen content of the wound diminishes, while tensile strength increases as a result of structural modification of the newly deposited collagen. The degradation of collagen in the wound is controlled by several proteolytic enzymes termed matrix metalloproteinases (Mignatti et al. Citation1996).
During the granulation tissue formation wounds gain only about 20% of their final strength (Viljanto Citation1964). During this time, fibrillar collagen accumulates relatively rapidly and remodeled by contraction of the wound (Bailey et al. Citation1975). The complete healing can be compared to a synchronized chronological task of various parts of machinery to produce the desired product ().
Table III. Chronological events involved in wound healing.
Factors affecting wound healing
Skin is body’s first point of contact with the outside world. It provides protection for the body and serves many complex functions. These functions can be put at risk if the skin’s integrity is not maintained. The wound healing process is influenced by numerous factors which include intrinsic factors and extrinsic fectors.
Intrinsic factors
Drugs
Drugs such as inotropes, steroids, and nonsteroidal anti-inflammatory drugs can affect wound healing. Steroid therapy can produce paper-thin skin which can be easily damaged. Chemotherapy can lower immunological resistance leading to higher rates of infection and kill new cells forming within the body, thereby destroying new cells within the wound bed and delaying healing.
Diseases
Diabetes. Diabetes can delay healing as viscous blood, functional impairment of polymorphonuclear neutrophils, and low delivery of nutrients and oxygen to the wound increases the risk of clinical infection and slows the healing process. Hyperglycaemia impairs leucocyte chemotaxis. Pathological changes in diabetes and nonenzymatic glycation is the mechanism by which proteins, such as collagen, are subject to chronic attack from glucose (Majno and Joris Citation1996).
Rheumatoid arthritis. Rheumatoid arthritis delay healing by affecting the microcirculation within the tissues, lowering the supply of nutrients and oxygen to the wound bed.
Shock
In acute shock, particularly following hemorrhage, the blood supply becomes inadequate to the tissues and leucocytes become sticky and trapped. The number of leucocytes available is greatly reduced and, therefore, the inflammatory response is depressed. Further complications are vasodilation and lowered blood supply, and these factors open the channels for bacterial invasion and potential for septic shock (Majno and Joris Citation1996). Stress causes vasoconstriction and the lowered blood delivery can affect the required nutrient supply to the wound bed (Kiecolt-Glaser et al. Citation1995). Pain causes a stress reaction within the body with resultant vasoconstriction.
Age
The ageing process has a detrimental effect on the skin and immunological system and affects the healing process with age related differences in wound healing. Aging skin recognizes numerous structural changes which include loss of melanocytes and loss of natural protection against ultraviolet rays, decrease in vascularity, decreased innervation with lowered tissue responses, elastic fiber changes, decrease in number and function of sweat glands, sebaceous gland hyperplasia and drying of the tissues, thinning of the epidermis, reduction in production of mast cells, and reduction in fibroblast proliferation leading to a decrease in wound contraction and slower wound healing (Desia Citation1997).
Free radicals
Free radicals are molecules without the full complement of electrons making the molecule unstable and reactive. The instability can cause the molecule to react with other molecules in a chain reaction sometimes causing the radicals to attack DNA, which in turn can make the cell division unstable, with the potential for forming cancer. Free oxygen radicals have also been reported to cause chronic damage to newly formed tissue in chronic injuries (Niwa Citation1987).
Oedema
Human body has a fine fluid balance between potassium and sodium exchange. Serum albumin maintains a pressure within the venous system that balances the fluid between the extracellular and venous systems.
Several conditions produce oedema such as low serum albumin, venous obstruction and lymphatic obstruction (Majno and Joris Citation1996).
Oxygen
Oxygen is vital for the repair of the tissues and, therefore, wound healing is impaired if hypoxia persists (Stadelmann et al. Citation1998). Chronic nonhealing wounds are frequently hypoxic as a consequence of poor blood perfusion, and host and microbial cell metabolism contribute further to lowering the pO2 (Bowler et al. Citation2001). Wound hypoxia predisposes the wound to infection as neutrophils require large amounts of oxygen for phagocytosis and, without oxygen, bacteria could proliferate unchecked. Lack of oxygen reduces mitotic and leucocyte activity, thereby increasing the potential for clinical infection. This condition can be checked by oxygen saturation through administration of fluids to increase blood volume and decrease viscosity. There is also a potential for the use of hyperbaric oxygen although the research undertaken by Winter and Perins demonstrated faster wound epithelialization when patients were placed in a hyperbaric oxygen chamber (Albert Citation2008).
Extrinsic factors
Pressure
Unrelieved pressure should be avoided in pressure ulcers.
Temperature
Cold delays wound healing as any drop in temperature of more than two degrees delay mitotic activity for up to four hours. The reduction in heat also delays entry of macrophages and leucocytes to the wound bed by up to 12 hours. This increases the risk of clinical infection. Wound temperature of greater than 30°C reduces the tensile strength of wounds through decreased perfusion from vasoconstriction.
Application
Poorly applied dressings, too tight compression, no compression when required, dry dressings, dressings that adhere to the wound, and antiseptics all delay wound healing (Leaper Citation1987).
Exudates
Chronic wound exudate delays wound healing by inhibiting the growth of fibroblasts (Phillips et al. Citation1998). Furthermore the presence of microbial antigens perpetuates the inflammatory process leading to further tissue damage (Thompson Citation1998).
Approaches for wound healing
Many older but obsolete methods are still in use in clinical practice. As a first treatment the wound is debrided. After that, under a moist dressing depending on the wound type the healing process is allowed to proceed in moist environment. Infection control treatment is crucial. For further support of the healing process skin substitutes are available as well as vacuum treatment devices. There are numerous wound healing strategies employed (, ).
Debridement
Debridement refers to the removal of devitalized tissue. Accelerating this process makes healing more efficient as devitalized tissue in the wound bed supports bacterial growth and is a physical barrier to healing. Devitalized tissue may also cause excessive amounts of proteases to be released.
The methods of debridement in today’s clinical practice are surgical, enzymatic, autolytic, mechanical, and biologic.
a. Surgical—Sharp surgical debridement is a very fast and efficient way to remove necrotic tissue from the wound bed. It is performed where there is an extensive amount of necrotic tissue or if there is a widespread infection requiring infected material to be removed.
b. Enzymatic debridement means the use of manufactured proteolytic enzymes, i.e., collagenases, papain, and serratiopeptidase. These support naturally occurring enzymes to degrade necrotic tissue (Reiss et al Citation2009). Autolytic debridement is a process performed by phagocytic cells and proteolytic enzymes in the wound site liquefying and separating necrotic tissue from healthy tissue. Wound dressings, which maintain a moist wound bed, can provide an optimal environment for debridement, as they allow migration of the phagocytic cells. Our group has been working on debriding enzyme, particularly serratiopeptidase, which is highly acid labile. We have developed serratiopeptidase loaded lipospheres and PLGA microspheres for safe administration of enzyme to dissolve the necrotic tissue at wound site and to improve the co administration of antimicrobial agents (Singh et al. Citation2009, Singh and Singh Citation2011).
We have also developed multiphase hydrogel of gentamicin (GM) and serratiopeptidase (STP) using PVA-Gelatin for effective and complete wound healing (Singh and Singh Citation2012).
c. Mechanical debridement is a method that physically removes debris from the wound. Examples include conventional dressings causing mechanical separation of necrotic tissue from the wound bed once the dressing is removed and wound irrigation using a pressurized stream of water to remove necrotic tissue.
Biologic larval therapy is an alternative method using sterile maggots that break down, liquefy and remove dead tissue secreting powerful proteolytic enzymes followed by eating of the digested tissue (Saap and Falanga Citation2002).
Infection control in wounds
Bacterial bioburden can cause a delayed or impaired healing. Bacterial burden present in the wound is divided into several degrees depending on the extent of microbial infestation and necessary treatment.
a. Contamination is defined as the presence of nonreplicating bacteria. This is a normal condition in chronic wounds and does not contribute to impair healing.
b. Colonization is defined as the presence of replicating bacteria without a host reaction. Replicating bacteria colonize and contaminate all chronic wounds. Bacterial colonization does not contribute to impair healing.
c. Critical colonization is defined as the presence of replicating microorganisms, which are beginning to cause local tissue damage. There may be subtle local indications that a change in the equilibrium, or increasing bioburden, could be contributing to delay healing (Falanga et al. Citation1994).
d. Infection occurs when bacteria have invaded tissue leading to impaired healing where microbes are multiplying causing a host reaction.
Although bacteria are present in all chronic wounds, generally, only critical colonization and infection indicate an antimicrobial treatment. The most frequently used topical antimicrobials in modern wound care practice include octenidine, iodine, and silver containing products. Chlorhexidine, hydrogen peroxide, and honey as well are in discussion but seem to be used more rarely. In the past, acetic acid, sodium hypochlorite, potassium permanganate, and proflavine have been used. Iodine as element was used in treating wounds mainly in the nineteenth century. Due to its heavy adverse effects it is obsolete today. Therefore, the safer formulations povidone iodine and cadexomer iodine have been developed. Povidone iodine is a polyvinylpyrrolidoneiodine complex; cadexomer iodine is composed of beads of dextrin and epichlorhydrin that carry iodine (Zhou et al. Citation2002). Silver also has a long history as an antimicrobial agent, especially since the late nineteenth century (Klasen Citation2000). Metallic silver is not active, but in aqueous environments silver ions are released and antimicrobial activity depends on the intracellular accumulation of low concentrations of silver ions. These bind to negatively charged components in proteins and nucleic acids, thereby effecting structural changes in bacterial cell walls, membranes, and nucleic acids that affect viability (Lansdown and Silver Citation2002a). Skin discoloration and irritation associated with the use of silver nitrate is responsible for its limited use (Lansdown and Silver Citation2002b). Our group recently demonstrated the safe and controlled delivery of antimicrobial agents through microsphere system of natural and synthetic polymers like Chitosan, Eudragit RS100 and PLGA for effective wound healing (Singh et al. Citation2008, Singh et al. Citation2010a, Singh et al. Citation2010b, Singh et al. Citation2010c, Singh et al. Citation2011).
Skin substitutes for wound healing
Dermal grafts are cellular or acellular, allogenic in nature and, hence, available for immediate use. Tissue engineering has added several skin substitutes to the variety of dressings available for wound treatment (). These products for example consist of fibroblasts and keratinocytes grown on collagen matrices. Bioengineered skin, a bilayered living skin construct has been approved for venous and diabetic ulcers. A living dermal skin equivalent was also approved for diabetic neuropathic ulcers (Cha and Falanga Citation2007). In addition to standard and more advanced treatments, other less commonly used and unusual therapeutic products, not necessarily new, are being used by some clinicians (Lanza Citation2004). For example, cadaver skin is such an alternative; it is a true allograft and is always eventually rejected by the recipient. Skin that lacks a dermis is less able to resist trauma and is prone to contraction, resulting in a poor functional and cosmetic outcome (Cha and Falanga Citation2007). All currently available examples of artificial dermis lack a vascular plexus for the nourishment of the epidermis and require host vasculogenesis into the dermis graft to supply nourishment to the grafted epidermis (Lanza Citation2004). Other strategies for treatment of chronic nonhealing cutaneous wounds include temporary substitutes such as porcine xenografts, synthetic membranes, and autologous and allogeneic epidermal substitutes (Sheridan and Tompkins Citation1990). Recent artificial dermal substitutes are structurally optimized to incorporate the surrounding tissue and to allow cell invasion by fibroblasts and capillaries for subsequent dermal remodeling (Sheridan and Tompkins Citation1990). There are a number of representative skin substitutes and in one such clinical trial the application of Apligraf® has been shown to accelerate wound closure compared to control (Falanga and Sabolinski Citation1999). But, in spite of advances in the treatment of chronic wounds with bioengineered skin, there remain almost 50% of patients who do not heal when their ulcers have been previously resistant to conventional therapy.
Table IV. List of skin substitutes with their composition.
Growth factors
There are several growth factors that are being evaluated in clinical trials (Gharaee-Kermani and Phan Citation2001). These growth factors play major roles in local inflammation, reepithelialization, granulation, tissue formation, neovascularization, and extracellular matrix production from various cell sources and through diverse mechanisms. There have been extensive investigations into wound healing by the exogenous application. Based on the diverse results, the type of the individual wound is an essential criterion for the choices of growth factors. Therefore, the approval of Regranex® only for diabetic foot ulcers is feasible. Richard et al. conducted a trial with b-FGF on diabetic foot ulcers and observed no advantage of verum over the placebo control (Richard et al. Citation1995). In another trial, EGF was exogenously applied to patients with diabetic foot ulcers and a significant enhancement of healing and a reduction of healing time were reported (Tsang et al. Citation2003). Effect of KGF-2 or repifermin on chronic venous ulcers was evaluated during clinical trial and a significant acceleration of wound closure was observed (Robson et al. Citation2001).
For PDGF-BB (platelet-derived growth factor consisting of BB-homodimer) or becaplermin, several clinical trials finally leading to the approval of Regranex®in 1999 for the treatment of diabetic foot ulcers have also been published. Efficacy and safety in diabetic foot ulcers have been proofed (Embil et al. Citation2000). Similar trials, e.g., concerning pressure ulcers, acute, and open surgical wounds have also been conducted with promising results but not yet leading to an approval (Cohen and Eaglstein Citation2001, Shackelford et al. Citation2002). In Regranex® PDGF is formulated in an aqueous carboxymethyl cellulose hydrogel. Further, the formulation contains an acetate buffer, lysine hydrochloride, and sodium chloride. Another new technology for augmenting levels of growth factors in wounds is by gene transfer. Andree et al. used particle-mediated and micro seeding gene transfer to deliver human EGF to porcine wounds (Andree et al. Citation1994). A high expression of EGF, as well as a significant acceleration of healing, was shown in the transfected wounds.
To overcome this problem and to make allowance to the thought of growth factors acting in concert, methods of autologous growth factor application have been developed (Andree et al. Citation1994).
Moist wound treatment
In 1962, George Winter, from Smith & Nephew Inc. demonstrated that wounds epithelialized more rapidly under occlusive dressings as it maintained a moist wound surface (Winter Citation1962). Numerous studies followed which demonstrated that wound occlusion and moisture increased all phases of healing (Mertz and Eaglstein Citation1984).
Advantages of moist wound treatment (Eaglstein Citation2001):
• During moist treatment the likelihood of scarring is reduced because there is no scab formation.
• Moisture is essentially required for the activity of growth factors and proteolytic enzymes. It is as well necessary for surface oxygen delivery and an efficient nutrient delivery. As a result, moisture improves the processes of the migration and proliferation phase by providing the cells with the ability to migrate across the wound surface leading to increased rate of epithelization and angiogenesis.
• With a moist environment the nerve endings are cushioned and protected which gives relief from pain.
Moist wound treatment is done by various means including moisture-retentive dressings like occlusive films, hydrogels, and hydrocolloids; absorbent dressings like foams and alginates.
• Film dressings—occlusive films are semipermeable polyurethane dressings that are coated with an adhesive. They are used for minor exudating wounds. Their purpose is to prevent bacterial infection by shielding, to absorb low amounts of exudate and to maintain a moist wound environment for fresh epithelial tissue. The dressings insure a gaseous exchange for vaporizing superfluous liquid.
• Hydrogels—Hydrogels are used to treat wounds with low exudate levels. They contain high amounts of water. Most products contain sodium carboxymethyl cellulose or polyacrylates swollen to an amorphous gel in a propylene glycol water mixture. Hydrogel dressings are used to hydrate necrotic tissue, facilitating autolytic debridement, while being able to absorb exudates (Singh and Singh Citation2011).
• Hydrocolloids—used for moderate exudation. They contain a layer of hydrocolloid. This is defined as liquid absorbing particles in an elastic, self-adhesive mass. The particles mostly consist of sodium carboxymethyl cellulose, calcium alginate, pectine, and gelatin. The elastic mass contains different synthetic polymers. The wound exudate binds to the absorbing particles of the hydrocolloid matrix to form a cohesive gel maintaining a moist wound environment. Most products are covered on the upper side by a semipermeable polyurethane film.
• Foams—Double-layer dressings consist of a polyurethane film carrier and polyurethane foam layer on the wound side. They are used for moderate to heavily exuding wounds. Specialty absorbent dressings can be used as secondary dressings.
• Alginates—Alginate dressings are used to cover heavily exuding wounds. They mostly contain a combination of calcium and sodium alginate fibers. Alginates support healing by binding bacteria and debris inside the gel structure and by providing a moist environment (Eaglstein Citation2001).
Gene therapy
This technique involves the introduction of the gene rather than product (growth factor) that is thought to be cheaper and more efficient for treating nonhealing wounds. Technology to introduce genes through physical or biological vectors has existed for some time. The limited success in clinical trials of topical delivery of pluripotent growth factors attracted the notice of scientists toward gene delivery systems. An approach in which genetically modified cells synthesize and deliver the desired growth factor in regulated fashion has been used to overcome the limitations associated with the (topical) application of recombinant growth factor proteins. Preliminary promising results have given hope for treatment of complicated wounds (Sabine et al. Citation2007). This is a new area of research and new trials are underway.
Stem cell therapy
The development in stem cell therapy has opened the path for treatment of wound using this technology. There is potential that stem cells may reconstitute dermal, vascular, and other elements required for optimum wound healing. Though the technique is still in its infancy, Badiavas et al. (Citation2003) have shown that direct application of autologous bone marrow and its cultured cells may accelerate the healing process (Badiavas et al. Citation2003). Because of the relative ease with which they are cultured, bone marrow-derived mesenchymal stem cells have been tested in pilot studies as a promising approach for introducing them into the wound. It might turn out, however, that other types of stem cells will be more effective, including those derived from hair follicles, or perhaps subsets of bone marrow-derived cultured cells (Cha and Falanga Citation2007). Still, proper wound care and adherence to basic principles cannot be bypassed, even by the most sophisticated approaches.
Miscellaneous
Recent approaches to wound healing includes the use of metals like gold and silver for complete wound healing. Hendi, Citation2011 has synthesized and evaluated silver nanoparticle for its wound healing activity and found that rapid healing and improved cosmetic appearance occurred within 15 days (Hendi Citation2011). Leu et al Citation2012 studied wound healing activity of topically applied nanoparticles of gold with epigallocatechin gallate (AuEA) and concluded that AuEA significantly accelerated cutaneous wound healing (Leu et al. Citation2012).
Conclusion
Wounds are one of the major causes of deformity and death in the world. The extensive research in the area of wound healing has greatly enhanced the knowledge regarding treatment strategies for a variety of wounds. It is the major cause of financial burden on the healthcare system in general and on patients in particular. No single treatment is completely successful for wound healing as complete wound healing depends on the multiple molecular and cellular mechanisms. The evolution of wound healing as specialty division of medicine is selfexplanatory to emphasize the importance of wound healing. This has led to revolutionary advances in wound healing knowledge and treatment including tissue engineering, growth factors, animal fetal cell research, stem cell research, gene therapy, human skin substitutes, and dressings. These developments in therapy has opened new hopes for the patients suffering from complicated wounds, but still there is a sturdy requirement for improved methods of therapy that should involve the principle of minimizing harm and augment the basic principles of wound care.
Acknowledgement
The authors are thankful to the Director, University Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur (C.G.), India for providing all necessary facilities for carrying out this work and University Grants Commission (UGC/MRP 39-169/2010 (SR)) and (UGC-RA 70-371/2012) for financial assistance.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Albert M. 2008. The Role of Hyberbaric Oxygen Therapy in Wound Healing. Wound Care Canada. 6:60–62.
- Andree C, Swain WF, Page CP, Macklin MD, Slama J, Hatzis D, et al. 1994. In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci USA. 91:12188–12192.
- Avicenna. 1783. De Ulceribus, Liber IV. In: Underwood M. Ed. A Treatise upon Ulcers of the Legs. London: Matthews, pp. 1736–1820.
- Badiavas E, Abedi M, Butmarc J, Falanga V, Quesenberry PJ. 2003. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 196:245–250.
- Bailey AJ, Bazin S, Sims TJ, Le Lous M, Nicoletis C, Delaunay A. 1975. Collagen polymorphism in experimental granulation tissue. Biochim Biophys Acta. 405:412.
- Baldwin G, Colbourne M. 1999. Puncture wounds. Pediat Rev. 20: 21–23.
- Bowler PG, Duerden BI, Armstrong DG. 2001. Wound microbiology and associated approaches to wound management. Clin Microbio Rev. 14:244–269.
- Bruncardi FC. 2005. Schwartz's Principles of Surgery. 8th ed. Schwartz, New York: McGraw-Hill.
- Cha J, Falanga V. 2007. Stem cells in cutaneous wound healing. Clin Dermat. 25:73–78.
- Clark RA, Folkvord JM, Hart CE, Murray MJ, McPherson JM. 1989. Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest. 84:1036.
- Clark RA, Lanigan JM, DellaPelle P, Manseau E, Dvorak HF, Colvin RB. 1982. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 79:264–269.
- Clark RAF. 1996. Wound repair overview and general considerations. In: Te Molecular and Cellular Biology of Wound Repair. New York: Plenum Press, pp. 3–10.
- Cohen MA, Eaglstein WH. 2001. Recombinant human platelet-derived growth factor gel speeds healing of acute full-thickness punch biopsy wounds. J Am Acad Derm. 45:857–862.
- Collier M. 2006. Understanding the principles of wound management. J Wound Care. 15:S7–S10.
- Davie EW, Fujikawa K, Legaz ME, Kato H. 1975. Role of proteases in blood coagulation. Cold Spring Harbor Confer Cell Proliferation. 2:65–77.
- De Santi L. 2005. Pathophysiology and current management of burn injury. Adv Skin Wound Care. 18:323–332.
- Desia H. 1997. Ageing and wounds part 2. J Wound Care. 6:237–239.
- Desmoulière A, Gabbiani G. 1996. The role of the myofbroblast in wound healing and fibrocontractive diseases. In: Clark RAF. Ed. Te Molecular and Cellular Biology of Wound Repair. New York: Plenum Press, pp. 391–402.
- Diegelmann RF, Evans MC. 2004. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 9:283–289.
- Eaglstein WH. 2001. Moist wound healing with occlusive dressings: a clinical focus. Dermatol Surg. 27:175–181.
- Embil JM, Papp K, Sibbald G, Tousignant J, Smiell JM, Wong B, et al. 2000. Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open-label clinical evaluation of efficacy. Wound Rep Reg. 8:162–168.
- Falanga V, Grinnell F, Gilchrest B, Maddox YT, Moshell A. 1994. Workshop on the pathogenesis of chronic wounds. J Invest Dermat. 102:125–127.
- Falanga V, Sabolinski M. 1999. Bilayered living skin construct (APLIGRAF®) accelerates complete closure of hard-to-heal venous ulcers. Wound Rep Regen. 7:201–207.
- Fisher C, Gilbertson-Beadling S, Powers EA, Petzold G, Poorman R, Mitchell MA. 1995. Interstitial collagenase is required for angiogenesis in vitro. Develop Biol. 162:499–510.
- Friess W. 1988. Biomaterial for drug delivery. Eur J Pharm Biopharm. 45:113–136.
- Galen C. 1976. Ad scripti libri. Venice: Vincentium Valgressium. In: Dodd H. Ed. Te Pathology and Surgery of the Veins of the Lower Limb. London: Churchill Livingstone, pp. 4–14.
- Garlick JA, Taichman LB. 1994. Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab invest;J Tech Meth Path. 70:916–924.
- Gay J. 1868. On Varicose Disease of the Lower Extremities and Its Allied Disorders. Lettsomian Lectures of 1867. London: Churchill.
- Gharaee-Kermani M, Phan SH. 2001. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des. 7:1083–103.
- Gilbertson DG, Duf ME, West JW, Kelly JD, Sheppard PO, Hofstrand PD, et al. 2001. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF-a and b receptor. J Biol Chem. 276:27406–27414.
- Hendi A. 2011. Silver nanoparticles mediate differential responses in some of liver and kidney functions during skin wound healing. J King Saud Univ (Science). 23:47–52.
- Herndon DN, Nguten TT, Gilphin DA. 1993. Growth Factors Local and Systemic. Arch Surg. 128:1227–1233.
- Hippocrates. 1849. De Ulcerbis, De Carnabus. In: Adams F. Ed. Te Genuine Works of Hippocrates. London: Sydenham Society. pp. 391–423.
- Hübner G, Brauchle M, Smola H, Madlener M, Fässler R, Werner S. 1996. Differential regulation of pro-inflammatory cytokines during wound healing. Cytokine. 8:548–566.
- Hunter J. 1837. Observations on the infammation of the internal coats of veins. In: Hunter J, Palmer JI. Eds. Te Works of John Hunter. London: Longman, Rees, Orme & Longman, pp. 581–586.
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. 1995. Slowing of wound healing by psychological stress. Lancet. 346:1194–1196.
- Klasen HJ. 2000. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. J Int Soc Burn Inj. 26: 131–138.
- Lansdown AB, Silver G. 2002a. Silver I: Its antibacterial properties and mechanism of action. J Wound Care11:173–177.
- Lansdown AB, Silver G. 2002b. Silver II: Toxicity in mammals and how its products aid wound repair. J Wound Care. 11:125–130.
- Lanza R. 2004. Handbook of Stem Cells. Boston, MA: Academic Press, pp. 731–736.
- Lawrence WT. 1998. Physiology of the acute wounds. Clin Plast Surg. 25:321–328.
- Lazarus GS, Cooper DM, Knighton DR. 1994. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 130:489–493.
- Leaper D. 1987. Antiseptic solutions ples. J Wound Care. 1:27–30.
- Leu JG, Chen SA, Chen HM, Wu WM, Hung CF, Yao YD, et al. 2012. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine. 8: 767–775.
- Majno G, Joris I. 1996. Cells, tissues, and disease. In: Majno G, Joris I. Eds. Principles of General Pathology. Cambridge, MA: Blackwell Science Inc., pp. 230–238.
- Margolis DJ. 1995. Definition of a pressure ulcer. Adv Wound Care. 8:8–10.
- Martin P. 1997. Wound healing-aiming for perfect skin regeneration. Science. 276:75–81.
- Masre SF, Yip GW, Sirajudeen KNS, Ghazali FC. 2012. Quantitative analysis of sulphated glycosaminoglycans content of Malaysian sea cucumber Stichopus hermanni and Stichopus vastus. Nat Prod Res. 26:684–689.
- McCarthy JG. 2006. Current Therapy in Plastic Surgery. 1st ed. Philadelphia, PA: Saunders.
- Mertz PM, Eaglstein WH. 1984. The effect of a semiocclusive dressing on the microbial population in superficial wounds. Arch Surg. 119:287–289.
- Mignatti P, Rifkin DB, Welgus HG, Parks WC. 1996. Proteinases and tissue remodeling. In: Clark RAF. Ed. The Molecular and Cellular Biology of Wound Repair. Plenum Press: New York, pp. 427–474.
- Monaco JL, Lawrence WT. 2003. Acute wound healing: An overview. Clin Plast Surg. 30:1–12.
- Montesano R, Orci L. 1988. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 85:4894–4897.
- Niwa Y. 1987. The ratio of lipidperoxides to superoxide dismutase activity in the skin lesions of patients with severe skin diseases: an acute prognostic indicator. Life Sci. 40:921–927.
- Orgill D, Demling RH. 1988. Current concepts and approaches to wound healing. Crit Care Med. 16:899–908.
- Phillips SJ. 2000. Physiology of wound healing and surgical wound. ASAIO J. 46:S2–S5.
- Phillips TJ, Al-Amoudi HO, Leverkus M, Park HY. 1998. Effect of chronic wound fluid on fibroblasts. J Wound Care. 7:527–532.
- Provencher MT, Allen LR, Gladden MJ. 2006. The underestimation of a glass injury to the hand. Am J Orthop. 35:91–94.
- Reed BR, Clark RAF. 1985. Cutaneous tissue repair: practical implications of current knowledge-II. J Am Acad Dermatol. 13:919–941.
- Reibman J, Meixler S, Lee TC, Gold LI, Cronstein BN, Haines KA, et al. 1991. Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proc Natl Acad Sci. 88:6805–6809.
- Reiss MJ, Han YP, Garner WL. 2009. Alpha1-antichymotrypsin activity correlates with and may modulate matrix metalloproteinase-9 in human acute wounds. Wound Rep Reg. 17:418–426.
- Richard JL, Parer-Richard C, Daures JP, Clouet S, Vannereau D, Bringer J, et al. 1995. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot randomized, doubleblind, placebo-controlled study. Diabetes Care. 18:64–69.
- Robson MC, Phillips TJ, Falanga V, Odenheimer DJ, Parish LC, Jensen JL, et al. 2001. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Rep Reg. 9:347–352.
- Rochat A, Kobayashi K, Barrandon Y. 1994. Location of stem cells of human hair follicles by clonal analysis. Cell. 76:1063–1073.
- Ruszczak Z, Friess W. 2003. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Del Rev. 55:1679–1698.
- Ryan GB, Manjo G. 1987. Acute inflammation — a review. Am J Pathol. 86:183–276.
- Saap LJ, Falanga V. 2002. Debridement performance index and its correlation with complete closure of diabetic foot ulcers. Wound Rep Reg. 10:354–359.
- Sabine AE, Tomas K, Jefrey MD. 2007. Gene therapy and wound healing. Clin Dermatol. 25:79–92.
- Schiro JA, Chan BM, Roswit WT, Kassner PD, Pentland AP. 1991. Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell67:403–410.
- Scholz A. 1993. Historical aspects. In: Westerhof W. Ed. Leg Ulcer:Diagnosis and Treatment. Amsterdam: Elsevier, pp. 5–15.
- Seymour DE, Da Costa N, Hodgson M, Dow J. 1972. Adhesive materials, UK patent #12800631.
- Shackelford DP, Fackler E, Hofman MK, Atkinson S. 2002. Use of topical recombinant human platelet-derived growth factor BB in abdominal wound separation. Am J Obs Gyn. 186:701–704.
- Sheridan RI, Tompkins RG. 1990. Skin substitutes in burns. Burns. 25:97–103.
- Singer AJ, Clark RA. 1999. Cutaneous wound healing. N Engl J Med. 341:738–746.
- Singh D, Dixit VK, Saraf S, Saraf S. 2009. Formulation optimization of serratiopeptidase-loaded PLGA microspheres using selected variables. PDA J Pharm Sci Technol. 63:103–112.
- Singh D, Saraf S, Dixit VK, Saraf S. 2008. Formulation optimization of gentamicin loaded Eudragit RS100 microspheres using factorial design study. Biol Pharm Bull31:662–667.
- Singh D, Singh M, Dixit VK, Saraf S, Saraf S. 2010a. Optimization and characterization of gentamicin loaded chitosan microspheres for effective wound healing. Ind J Pharm Educ Res. 44:171–182.
- Singh D, Singh M, Dixit VK, Saraf S, Saraf S. 2010b. Effect of formulation variables on preparation of GM loaded PLGA microspheres. Indian Drugs47:35–44.
- Singh D, Singh M, Dixit VK, Saraf S, Saraf S. 2010c. Formulation optimization of metronidazole loaded chitosan microspheres for wound management by 3-Factor, 3-level box-behnken design. Micro Nanosystems. 2:70–77.
- Singh D, Singh M. 2011. Development of delivery cargoes for debriding enzymes effective in wound healing. Trend Appl Sci Res. 6:863–876.
- Singh D, Singh MR. 2012. Development of antibiotic and debriding enzyme loaded PLGA microspheres entrapped in PVA-Gelatin hydrogel for complete wound management. Artif Cells Blood Substitutes Biotechnol. Epub ahead, 1–9.
- Singh M, Singh D, Saraf S, Saraf S. 2011. Influence of selected formulation variables on the preparation of peptide loaded lipospheres. Trend Med Res. 6:101–115.
- Stadelmann WK, Digenis AG, Tobin GR. 1998. Impediments to wound healing. Am J Surg. 176:39–44.
- Stefanopoulos PK, Tarantzopoulou AD. 2005. Facial bite wounds: management update. J Oral Maxillofac Surg. 34:464–472.
- Theden JCA. 1782. Neue Bermerkungen und Erfahrungen zue Beriecherung der Wundarzenykunst und Arzenygelahrteit, vol. 2. Berlin: Nicolai.
- Thompson P. 1998. The microbiology of wounds. J Wound Care. 7: 477–478.
- Trott A. 1997. Wounds and Lacerations: Emergency Care and Closure. 2nd ed. St. Louis, MO: Mosby.
- Tsang MW, Wong WK, Hung CS, Lai KM, Tang W, Cheung EY, et al. 2003. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care. 26:1856–1861.
- Viljanto J. 1964. A sponge implantation method for testing connective tissue regeneration in surgical patients. Acta Chir Scand. 333:1–101.
- Wakefield TW, Greenfeld LJ, Mulholland MW, Oldham KT, Zelenock GB, Lillemoe KD. 2001. Hemostasis in Surgery: Scientifc Principals and Practice. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, pp. 275–281.
- Welch MP, Odland GF, Clark RA. 1990. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 110:133–145.
- Winter GD. 1962. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 193:293–294.
- Wiseman R. 1676. Several Chirurgical Treatises. London: Wilthoe & Knapton.
- Woodley DT, Yamauchi M, Wynn KC, Mechanic G, Briggaman RA. 1991. Collagen telopeptides (cross-linking sites) play a role in collagen gel lattice contraction. J Invest Dermatol. 97:580–585.
- Wysocki AB. 1999. Skin anatomy, physiology, and pathophysiology. Nurs Clin North Am. 34:777–797.
- Zhou LH, Nahm WK, Badiavas E, Yuft T, Falanga V. 2002. Slow release iodine preparation and wound healing: in vitro effects consistent with lack of in vivo toxicity in human chronic wounds. Brit J Dermat. 146:365–374.