Abstract
Objective: The objective of the present study was to develop targeted engineered nanoerythrosomes based intravenous formulation of antimalarial drug pyrimethamine. Material and methods: The nanoerythrosomes formulation was developed by sonication method and optimized for effective drug loading at variable drug concentration, surface morphology, viscosity and sedimentation volume. Results: The in vitro drug release of formulated product was found to be delayed after 8 hours, having good stability at 4 ± 1°C and showing controlled in vivo release. Tissue distribution studies showed higher accumulation of drug in the liver (18.71 ± 1.4 μg/ml) (P < 0.05) at 1 hour in case of pyrimethamine-loaded nanoerythrosomes as compared to that in free drug (12.82 ± 0.7 μg/ml). Higher amount of drug, i.e. 14.18 ± 0.9 μg/ml (P < 0.05), was found after 24 hours in the liver in case of pyrimethamine-loaded nanoerythrosomes as compared to free drug concentration of 9.72 ± 0.5 μg/ml). Discussion: Data showed that developed pyrimethamine-loaded nanoerythrosomes hold promise for targeting and controlling the release of drug and for improving treatment of malaria when they are combined with rapid acting antimalarials such as artemisinin. Conclusion: A decrease in the concentration of pyrimethamine in kidneys and lungs after 24 hours was observed as compared to that observed after 1 hour, showing no or little involvement of these organs in the clearance of drug-loaded nanoerythrosomes.
Malaria, a major parasitic disease, is widely spread in tropical and subtropical regions around the world. Of the 300 million people infected annually with malaria, approximately 2 million die of it (Breman et al. Citation2004, Breman Citation2001). The mortality, morbidity and economic burden demand that attention be focused on this area. It has been estimated that malaria kills between 1and 3 million people annually, most of whom are children under the age of 5 and pregnant women (WHO and UNICEF Citation2005). Malaria is caused by protozoal parasites of genous Plasmodium. The four identified species of parasite responsible for inflicting human malaria are Plasmodium falciparium, Plasmodium vivax, Plasmodium ovale and Plasmodium malariae. Of these P. falciparum and P.vivax account for more than 95% of malaria cases in the world. Malaria infections caused by P.falciparum are prevalent in the major parts of Africa, Saharian Africa and East-Asian countries. Whereas P. vivax is the causative species primarily of Indian sub continent (Vangapandu et al. Citation2007). Malaria caused by P vivax, P. ovale and P. malariae is often called benign malaria. In contrast P. falciparum malaria can progress within a few days from uncomplicated malaria to severe malaria with a fatal outcome in 10%–40% of all cases of severe malaria, depending on the time lag between the onset of the symptoms and effective treatment, as well as on the hospital facilities available for the management of complications of the malaria (Wilairatana et al. Citation2002). Various classes of drugs such as the quinolines, antifolates, artemisinins and antibiotics have been shown to exhibit antimalarial activity (Mital Citation2007, Bray et al. Citation2005). Pyrimethamine is an antimalarial drug which acts by inhibiting the conversion of folic acid to folinic acid (Budawai et al. Citation2003). It is widely used in the treatment of P. falciparum infection along with sulfodixine as combination therapy, but successful use of pyrimethamine is limited due to its toxic effect, particularly depression of haematopoisis. Acute overdose causes convulsion, tachycardia, respiratory depression and circulatory collapse, and death may also occur (Fullerton Citation1982). Therefore, it is necessary to target and optimize pyrimethamine formulation directly to liver cells, where its activity is most required, using some nanometric molecular drug-delivery systems. The rationale behind the use of nanoerythrosomes as drug carriers is based on the certainty that senescent (damaged) cells will be removed from circulation by phagocytic reticuloendothelial cells in the liver (Moorjani et al. Citation1996). In the present study, pyrimethamine was bonded onto erythrocyte ghost membrane with the gluteraldehyde as a spacer to prepare drug conjugate and thus avoid leaching problem of drug during circulation (DeLoach and Barton Citation1982).
Materials and methods
Materials
Pyrimethamine was obtained as a benevolent gift from IPCA Laboratories Ltd. (Mumbai, India). Gluteraldehyde was purchased from Spectrochem (Mumbai, India). All other chemicals and reagents used were of analytical grade.
Preparation of erythrocyte ghost
Hypotonic osmotic lysis method described by Deloach was used for the preparation of ghost suspension (Jain et al. Citation1995). The method involves washing with a series of hypotonic saline solution, each wash being followed by centrifugal concentration of cells. One volume of red blood cells was incubated with an equal volume of hypotonic saline solution (0.7℅). The cells were lysed and washed several times with this solution. After each wash, the content was centrifuged at 1000 rpm for 10 minutes at 4 ± 1°C in a refrigerated centrifuge (model Z206, Hermle, Germany) and the supernatant was aspirated and discarded. The ghost suspension was finally obtained when the supernatant became colourless. The ghost cells were diluted with 0.9% saline to obtain 50% haematocrit and stored at 4 ± 1°C until use (Jain Citation2003).
Preparation of nanoerythrosomes and drug loading
The sonication technique reported earlier (Al-Achi and Greenwood Citation1993) was adopted for the preparation of nanoerythrosomes. The erythrocyte ghost suspension (50% haematocrit in 0.9% saline) was sonicated at 50 W for 5 minutes using a sonicator. During the experiment, the temperature was maintained at 4 ± 1°C and finally nanoerythrosomes were stored at 4 ± 1°C in a refrigerator. Pyrimethamine was conjugated to nanoerythrosomes membrane using gluteraldehyde as a cross-linker. Two thousand five hundred micrograms of drug was added to 2 ml of nanoerythrosomes preparation (50% heamatocrit) in the presence of 0.3% gluteraldehyde (in 0.9% saline solution). The mixture was incubated at 4 ± 1°C for 6 hours and then the reaction was stopped by the addition of 2 ml of 15% glycine solution in isotonic saline. Finally the preparation was stored at 4 ± 1°C in a refrigerator until used.
Optimization of formulation
Pyrimethamine-loaded nanoerythrosomes formulation was optimized on the basis of drug concentration, surface morphology, viscosity and sedimentation volume as follows (Talwar and Jain Citation1992a, Citation1992b, Garín et al. Citation1996, Jain et al. Citation1995, Hamidi et al. Citation2001, Updike and Wakamiya Citation1983, Iher et al. Citation1973).
Optimization of drug concentration
To study the effect of variable drug concentration on conjugation of nanoerythrosomes, the various concentrations representing 1.0, 1.5, 2.0, 2.5 and 3.0 mg/ml of pyrimethamine were used. Other experimental conditions such as sonication voltage (50w), gluteraldehyde concentration (0.03%), incubation period (45 minute) and temperature (37 ± 1°C) were kept constant. Packed loaded nanoerythrosomes were washed with phosphate buffer saline (pH 7.4), deproteinized with acetonitrile (2.0 ml) and subjected to centrifugation at 2500 rpm (Remi, India) for 10 minutes. The clear supernatant was withdrawn and analysed for concentration of drug by HPLC using C-18 column and the mobile phase consisted of phosphate buffer (0.05 M, pH 5):acetonitrile:concentrated perchloric acid (750: 300: 2.5, v/v/v) at a flow rate of 1ml/min (Minzi et al. Citation2005). The percent drug loading was determined as,
Surface morphology
The shapes of normal erythrocytes and erythrocyte ghosts were visualized using optical microscope (Leica, Germany). Scanning electron microscopy (SEM Philips XL30, Netherlands) was performed to evaluate the surface morphology of Pyrimethamine-loaded nanoerythrosomes. A small amount of Pyrimethamine-loaded nanoerythrosomes were stuck on a double-sided tape attached on a metallic sample stand, coated under argon atmosphere with a thin layer of gold, using a POLARON E5100 SEM coating unit, and visualized for surface morphology. The vesicles size was determined by dynamic light-scattering method (DLS) in a multimodal mode using a computerized inspection system (Malvern Zetamaster, ZEM5002, Malvern, UK).
Viscosity and sedimentation volume
A rotatory Brookfield (Synchro Lectric LVT, USA) viscometer was used to determine the viscosity of the formulation. Sedimentation volume of the formulation was measured in a graduated measuring cylinder from the height of sediment using the formula F = Vu/Vo, where F = sedimentation volume, Vu = ultimate volume of sediment and VO = original volume of formulation.
In vitro release rate
In vitro release studies were conducted using dialysis membrane molecular weight cut-off 1000 Da (Sigma, USA). Free drug from formulation was removed by centrifugation at 18000 rpm for 20 minutes at 4 ± 1°C. After removal of free drug, 1 ml of formulation was introduced into the dialysis membrane and it was placed in a beaker containing 100 ml of 0.1 N HCl and ethanol mixtures. The beaker was kept over a magnetic stirrer and the temperature of the assembly was maintained at 37 ± 1°C throughout the study. Samples were withdrawn at defined time intervals, replenished with same volume of fresh medium and analysed for drug content by HPLC (Minzi et al. Citation2005). The same procedure was repeated for in vitro release rate of free drug.
Stability studies
Jain and Jain (Citation1998) reported that Pyrimethamine-loaded nanoerythrosomes can be stored without loss of physical integrity when suspended in phosphate buffer saline at 4°C for 4 weeks. Storage stability was accessed at different temperatures viz 4 ± 1°C, room temp and 37 ± 1°C. Pyrimethamine-loaded nanoerythrosomes formulation was kept at variable temperature, and changes in sedimentation volume and relative turbidity were noted periodically.
Centrifugal stress
Effect of centrifugation on sedimentation volume and drug leakage was studied by centrifuging the formulation in centrifuge tubes at different rpm for 15 minutes at 4 ± 1°C. Drug leakage in the supernatant solution was estimated by HPLC.
Turbulence shock
It is the measure of simulating destruction of drug-loaded nanoerythrosomes during injection. The formulation was passed through a 23-gauge hypodermic needle at a flow rate of 10 ml/min, which is comparable to the flow rate of blood. The number of passes was varied as a function of turbulence. The drug leakage in the supernatant was estimated by HPLC after a fixed number of passes.
In vivo evaluation
The formulated products with promising in vitro performance were evaluated for their in vivo performance on albino rats. All the animal studies were conducted in accordance with the protocol approved by the Institutional Animal Ethical Committee of Dr. H.S. Gour University, Sagar.
Experimental animals
The albino rats of either sex (average weight 250 ± 25 g) were maintained under standard laboratory conditions at 22 ± 2°C, relative humidity 50 ± 15% and the period of 12 hours day and 12 hours dark, were used for the experiment. Commercial pellet diet (Hindustan Lever, Mumbai, India) and water were provided ad libitum.
Study protocol
The albino rats were divided into three groups each comprising six rats. The first group was administered a drug solution equivalent to 250 μg of pyrimethamine (calculated at a drug dose level of 1 mg/kg). The second group was administered pyrimethamine-loaded nanoerythrosomes containing an equivalent amount of pyrimethamine through caudal vein. The third group served as a control. The blood samples were collected at different time intervals from retro-orbital plexus using a heparinized syringe and were estimated for drug concentration by HPLC.
Tissue distribution studies of pyrimethamine-loaded nanoerythrosomes were also performed using albino rats of either sex (250 ± 25 g). The rats were divided into three groups each comprising six rats. The animals of the first group were given a drug solution (equivalent to 250 μg) of pyrimethamine through caudal vein. The animals of the second group were administered pyrimethamine-loaded nanoerythrosomes (equivalent to 250 μg) through caudal vein. The third group served as a control. After 1 hour and 24 hours, rats of each group were sacrificed. The body organs (liver, kidneys, lungs and spleen) were removed, washed to get rid of adhering debris and dried with a tissue paper. The organs were homogenized and drug concentration was determined by HPLC.
Statistical analysis
The results were expressed as mean values ± S.E.M (standard error of mean) of five rats. Experimental data were analysed using one-way ANOVA followed by Turkey-Kramer multiple comparison test. P values less than 0.05 were considered statistically significant. Graph Pad Prism Version 3.02 was used for statistical calculations.
Result and discussion
The present investigation was aimed at developing a new family of natural nanoparticulate nanoerythrosomes for delivery of antimalarial drug, pyrimethamine. The formulation was prepared by sonication method and the drug was conjugated to nanoerythrosomes with the help of gluteraldehyde, which served as a cross-linking agent. Glycine was added to stop the reaction of gluteraldehyde. The formulation was optimized for effective drug loading at variable drug concentration. The maximum percentage drug loading (25.20 ± 1.3) was obtained with 2.5 mg/ml drug solution. Percentage loading was found to be nearly the same using 3.0 mg/ml of drug solution, indicating saturation of conjugation sites ().
Table I. Effect of Pyrimethamine concentration on drug loading.
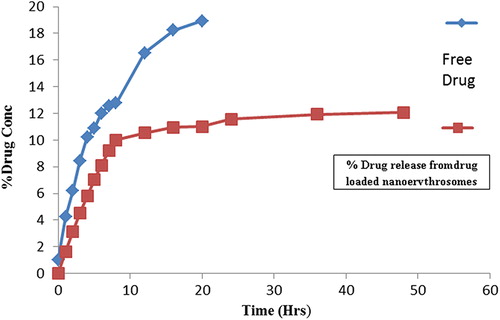
Surface morphological studies displayed spherical shape with a smooth surface and no aggregation was observed (). No difference was observed in the morphological properties of drug-encapsulated erythrocytes. The size of the Pyrimethamine-loaded nanoerythrosomes was found to be 176 ± 30 nm. The viscosity of Pyrimethamine-loaded nanoerythrosomes (34.0 ± 0.2) was slightly higher than that of placebo nanoerythrosomes (29.21 ± 1.2). Sedimentation volume of unity in each case revealed very good uniformity of the formulation. The in vitro release from dialysis membrane was found to be 10.84 ± 0.2 and 53.2 ± 1.1% after 8 hours for pyrimethamine-loaded nanoerythrosomes and free drug, respectively. It was found that the 98.51 ± 2.4% of the free drug was released from the dialysis membrane in 20 hours, while only 22.57 ± 0.3% drug was released in case of pyrimethamine-loaded nanoerythrosomes in 20 hours (). The decreased drug release rate can be ascribed to the cross-linking of drug to nanoerythrosomes by gluteraldehyde.
Figure 1. (A) Photomicrograph of normal erythrocytes. (B) Photomicrograph of erythrocytes ghosts. (C) Photomicrograph of SEM of drug loaded nanoerythrosomes.
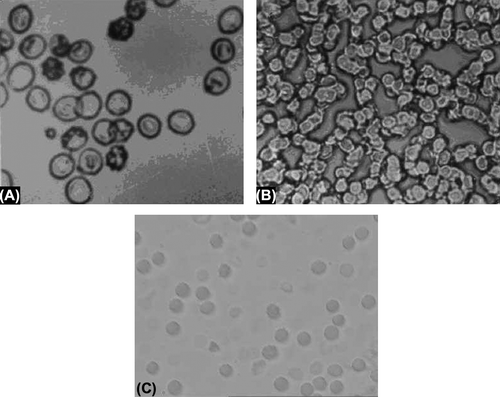
Figure 2. Cumulative percentage of pyrimethamine released from free drug solution and pyrimethamine-loaded nanoerythrosomes.
Stability of formulation was assessed at 4 ± 1°C, room temp and at 37 ± 1°C over time. Formulations exhibited a relatively little change in turbidity at 4 ± 1°C. but more at room temperature and 37 ± 1°C, indicating that the formulation is most stable at 4 ± 1°C. When the values were compared to zero time for each respective temperature, there were significant changes in percentage relative turbidity when the formulations were stored for 48 hours and 72 hours. While storage at different temperatures showed no significant changes up to 48 hours. Hence the formulations must preferable be used within 24 hours in order to avoid changes in turbidity. The effect of temperature on sedimentation volume with ageing was found to be negligible (). Sedimentation volume of formulation after centrifugation at 2500, 5000 and 7500 rpm for 15 minutes remained stable. This indicated stability of formulation upon centrifugal stress. The formulation was stable against centrifugal stress as only 2.72% of drug was released after centrifugation at 7500 rpm for 15 minutes from pyrimethamine-loaded nanoerythrosomes (). The pyrimethamine-loaded nanoerythrosomes formulation was assessed for stability during injection by subjecting to turbulence shock and was also found to withstand turbulence shock as only 0.882 ± 0.08% of drug was found to be released after 15 passes through 23-gauge needle (). These studies suggested that pyrimethamine-loaded nanoerythrosomes are quite stable in nature.
Table II. Effect of aging on turbidity and sedimentation volume of pyrimethamine-loaded nanoerythrosomes.
Table III. Effect of centrifugal force and turbulence shock on stability of drug-loaded nanoerythrosomes.
The formulated product with promising in vitro performance was evaluated for in vivo performance on albino rats. The blood serum concentration of drug solution and formulation after IV administration has been shown in . After 24 hours 7.03 ± 1.1 μg/ml of pyrimethamine was found in the blood using drug solution, while it was found to be 4.89 ± 0.5 μg/ml for pyrimethamine-loaded nanoerythrosomes. This shows that the developed formulation has capacity to control drug delivery. The tissue distribution data of pyrimethamine suggested that rapid accumulation of drug occurred in target organ such as liver (18.71 ± 2.20 μg/ml) in 1 hour in case of drug-loaded nanoerythrosomes, which was almost 40% higher than that in free drug (12.82 ± 1.21 μg/ml). A higher amount of drug, i.e. 14.18 ± 1.08 μg/ml, was still found in the liver in case of pyrimethamine-loaded nanoerythrosomes as compared tofree drug concentrations of 9.72 ± 0.82 μg/ml, respectively, after 24 hours (P < 0.05) ( and ). Talwar and Jain (1992) also reported targeted delivery of antimalarial drug (Primaquine) by erythrocytes and found similar higher hepatic localization of drug by erythrocytic carriers. The higher localization of drug in target organ in case of pyrimethamine-loaded nanoerythrosomes might be due to removal of the carrier system by Kupffer cells of the liver. Tissue drug distribution studies in kidneys suggest higher accumulation of drug (7.43 ± 0.84 and 5.63 ± 0.25 μg/ml) in the kidneys at 1 hour and 24 hours, respectively, in case of free drug as compared to 3.74 ± 0.34 and 2.80 ± 0.85 μg/ml in case of pyrimethamine-loaded nanoerythrosomes (P < 0.05–0.01). The carrier system has also retarded the excretion of pyrimethamine from the kidneys.
Figure 3. The blood plasma concentration of free drug solution and pyrimethamine-loaded nanoerythrosomes.
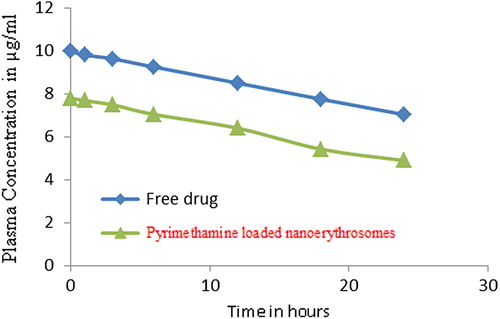
Figure 4. Tissue distribution of pyrimethamine loaded nanoerythrosomes at various time intervals (n = 3).
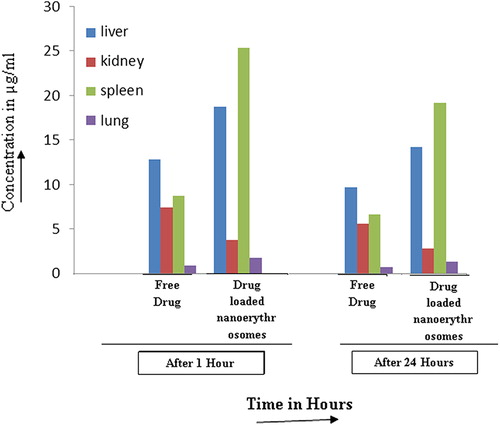
Table IV. Tissue distribution study of pyrimethamine and pyrimethamine-loaded nanoerythrosomes.
Conclusion
Formulation, characterization, in vitro release profile and in vivo targeting ability of the pyrimethamine-loaded nanoerythrosomes formulation were evaluated in the present study. The investigations revealed that the use of nanoerythrosomes for targeting of pyrimethamine is promising. An enhanced controlled release in vitro and in vivo, higher accumulation of the drug in case of drug-loaded nanoerythrosomes as compared to that in free drug in the target organs reveals suitability of formulation for controlled release and drug targeting. In conclusion, pyrimethamine-loaded nanoerythrosomes as a new carrier hold promise for targeting and systemic controlled release. The work can be taken up for further studies and pilot scale study by pharmaceutical industries. The formulation can be designed more in-depth and elaborative studies should be conducted so as to develop more effective, fast acting injectable preparation, which apart from having fast onset of action will have prolonged activity also. Research study requires developed laboratories, highly sensitive instruments and trained researchers apart from maintenance of class 100 room or LAF facilities.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al-Achi A, Greenwood R. 1993. Human insulin binding to erythrocyte membrane. Drug Dev Ind Pharm. 19:673–684.
- Bray PG, Ward SA, O’Neill PM. 2005. Quinolines and artemisinin: chemistry, biology and history. Curr Top Microbiol Immunol. 295:3–38.
- Breman JG, Alilio MS, Mills A. 2004. Conquering the intolerable burden of malaria: what`s new, what's needed: a summary. Am J Trop Med Hyg. 71:1–5.
- Breman JG. 2001. The ears of hippopotamus: manifestations determinants and estimates of the malaria burden. Am J Trop Med Hyg. 64:1–11.
- Budawai S. 2003. Antimalarials. The merck index, 13th ed. Whitehouse Station NJ: Merck and Co. Inc. 1747.
- DeLoach JR, Barton C. 1982. Circulating carrier erythrocytes: slow-release vehicle for an antileukemic drug, cytosine arabinoside. Am J Vet Res. 43:2210–2212.
- Fullerton DS. 1982. Wilson & Gisvold's text book of organic medical and pharmaceutical chemistry. Philadelphia: Lippincott, pp. 205–206.
- Garín MI, Lopez RM, Sanz S, Pinilla M, Luque J. 1996. Erythrocytes as carriers for recombinant human erythropoietin. Pharm Res. 13:869–874.
- Hamidi M, Tajerzadeh, Dehpour AR, Rouini MR, Ejtemaee-Mehr SH. 2001. In vitro characterization of human intact erythrocytes loaded by enalaprilat. Drug Delivery. 8:223–230.
- Iher GM, Glew RM, Schnure FW. 1973. Enzyme loading of erythrocytes. Proc Natl Acad Sci USA. 70:2663–2666.
- Jain NK. 2003. Advances in controlled and novel drug delivery. New Delhi: CBS Publisher and Distributor, pp. 339–340.
- Jain S, Jain SK, Dixit VK. 1995. Erythrocytes–based delivery of isoniazid: preparation and in vitro characterization. Ind Drugs. 32:471–476.
- Jain S, Jain NK. 1998. Preparation, characterization and pharmaceutical potential of engineered erythrocytes. Die Pharmazie. 53:5–14.
- Jain S, Jain SK, Dixit VK. 1995. Erythrocytes based delivery of isoniazid: preparation and in vitro characterization. Ind Drugs. 32:471–476.
- Mital A. 2007. Recent advances in antimalarial compounds and their patents. Curr Med Chem. 14:759–773.
- Minzi OMS, Massele AY, Gustafsson LL, Ericsson O. 2005. Simple and cost-effective liquid chromatographic method for determination of pyrimethamine in whole blood samples dried on filter paper. Chromat B. 814:179–183.
- Moorjani M, Lejeune A, Gicquaud C, Lacroix J, Poyet P, Gaudereault RC. 1996. Nanoerythrosomes, a new derivative of erythrocyteghost ii, identification of the mechanism of action. Anticancer Res. 16:2831–2836.
- Talwar N, Jain NK. 1992a. Erythrocytes as carriers of primaquine preparation: characterization and evaluation. J Control Rel. 20: 133–142.
- Talwar N, Jain NK. 1992b. Erythrocytes as carriers of metronidazole: in-vitro characterization. Drug Dev Ind Pharm. 18:1799–1812.
- Updike SJ, Wakamiya RT. 1983. Infusion of red blood cell-loaded asparaginase in monkey. J Lab Clin Med. 101:679–691.
- Vangapandu S, Jain M, Kaur K, Patil P, Patel SR, Jain R. 2007. Recent advances in antimalarials drug development. Med Res Rev. 27: 65–107.
- WHO and UNICEF. 2005. World malaria report. Geneva: World Health Organization. Available at: http://www.rbm.who.int/wmr2005.
- Wilairatana P, Krudsood S, Reeprasertsuk S, Chalermrut K, Looare-suwan S. 2002. The future outlook of antimalarial drugs and recent work on the treatment of malaria. Arch Med Res. 33:416–421.