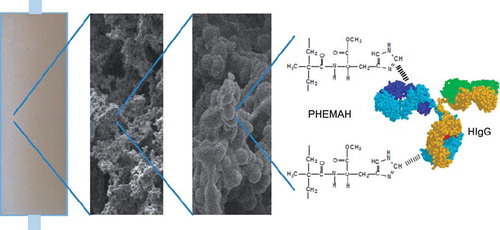
Abstract
In this study a novel composite cryogel column was synthesized for affinity depletion of immunoglobulin G (IgG) from human serum. The microsphere-embedding technique combines the large surface area of microspheres with excellent properties of cryogels. The poly(hydroxyethyl methacrylate- N-methacryloyl-L-histidine methyl ester) microsphere (2 μm size)- embedded composite cryogel (MECC) column was synthesized and characterized by various methods. The specific surface area of the composite cryogel was found as 92 m2/g by using BET measurement. Maximum HIgG adsorption on MECC column was found as 50.5 mg/g. MECC column can be reused many times with no apparent decrease in HIgG adsorption capacity.
Introduction
Human plasma plays an essential role in clinical diagnosis. The characterization of the human plasma is the most difficult protein-containing sample due to the wide dynamic concentration range of its proteins and the tremendous heterogenity of its glycoproteins (Anderson and Anderson Citation2002). Proteins like immunoglobulin G (HIgG), human serum albumin (HSA), transferrin, IgA, haptoglobin represent over 85% of the total protein mass in human plasma. HSA and HIgG constitute nearly the 75% of these proteins. These high-abundance proteins prevent the detection of low-abundance proteins, which are potential biomarkers for various diseases (Bailey et al. Citation2003). Removing of high-abundance proteins like HIgG reduces the hindrance effect and allows the detection of low abundant proteins. Several studies were performed for the removal of the high abundant proteins from human serum (e.g., ultracentrifugal filtration, dye affinity and immunoaffinity chromatography) (Altıntaş and Denizli Citation2006, Zhou et al. Citation2004, Andac et al. Citation2012, Fang and Zhang Citation2008). For the depletion of the several proteins from human serum, different techniques were achieved: protein A/protein G containing affinity colums (Garipcan and Denizli Citation2002), immobilized metal chelate affinty (IMAC) columns with different chelating metals such as Cu2+, Fe2+,Ga2+ (Riggs et al. Citation2001, Hemdan and Porath Citation1985, Erzengin et al. Citation2011) pseudospesific ligand attached molecular imprinted affinity columns (Derazshamshir et al. Citation2010), pseudo-affinity chromatography, e.g. thiophilic ligands (Coffinier et al. Citation2002) or various bio-mimetic ligands (Verdoliva et al. Citation2002, Lowe et al. Citation2001). Amino acids as pesudospecific ligands may hold certain advantages for bioaffinity techniques, as they are not likely to cause an immune response in the case of leakage into the product. These ligands are also much more stable than protein ligands because they do not require a specific tertiary structure for maintaining biological activity (Huang and Carbonell Citation1999). They offer additional advantages over biological ligands in terms of economy, ease of immobilization and high adsorption capacity. Due to all these reasons in this study histidine amino acid used as a pseudospesific ligand. Histidine interacts through its carboxyl, amino and imidazole groups with several proteins at around their isoelectric points and has shown particular efficacy in separating HIgG from human plasma (El-Kak et al. Citation1992).
The aim of this study is to prepare a composite cryogel column embedded with histidine containing microspheres, for the depletion of HIgG from human serum. The high porosity of cryogels makes them appropriate candidates as the basis for such supermacroporous chromatographic materials (Plieva et al. Citation2011, Arvidsson et al. Citation2003). Owing to supermacroporosity and interconnected pore-structure, such a chromatographic matrix has a very low flow resistance (Ünlü et al. Citation2011, Arvidsson et al. Citation2002). Cryogels have large pores, as a consequence of that they have low surface area and also low adsorption capacity. To overcome the drawbacks of the cryogels, microsphere-embedding method would be useful improvement for enhancing the adsorption capacity (Ünlü et al. Citation2011, Koç et al. Citation2011)
In this study, poly(hydroxyethyl methacrylate-N-methacryloyl-L-histidine methyl ester) MECC columns were prepared for selective and efficient depletion of HIgG from human serum. 2-Hydroxyethyl methacrylate was selected as the basic component because of its inertness, mechanical strength, chemical and biological stability and biocompatibility (Çimen and Denizli Citation2012). N-methacryloyl-L-histidine methyl ester (MAH) was synthesized as a functional ligand for the interaction with HIgG via imidazole group prior to polymerization. The MECC column was characterized by scanning electron microscopy (SEM), swelling tests, porosity measurement, FTIR and elemental analysis. HIgG adsorption on MECC column from aqueous solutions and human serum were performed. Desorption of HIgG and reusability of the column was also tested.
Experimental
Materials
L-Histidine methyl ester and methacryloyl chloride were supplied by Sigma (St. Louis, MO). 2-Hydroxyethylmethacrylate (HEMA) was purchased from Sigma (St. Louis, MO) Ethylene glycol dimethacrylate (EGDMA; Merck, Darmstadt, Germany) was used as the crosslinking agent. The polymerization initiator was 2,2-azobisisobutyronitrile (AIBN; BDH, Poole, United Kingdom). Ammonium persulfate (APS), N,N′-methylene-bis(acrylamide) (MBAAm), and N,N,N’, N’-tetramethylene diamine (TEMED) were also obtained from Sigma. Immunoglobulin G (HIgG) purchased from Sigma. All other chemicals were reagent grade and purchased from MerckAG (Darmstadt, Germany). All water used in the experiments was purified using a Barnstead (Dubuque, IA) ROpure LP reverse osmosis unit with a high flow cellulose acetate membrane (Barnstead D2731), followed by exposure to a Barnstead D3804 NANOpure organic/colloid removal and ion Exchange packed bed system. Buffer and sample solutions were prefiltered through a 0.2 mm membrane (Sartorius, Göttingen, Germany). All glasswares were extensively washed with dilute nitric acid before use.
Preparation of MECC column
Synthesis of N-methacryloyl-L-histidine methyl ester (MAH) monomers
The synthesis and characterization of MAH were performed as described previously (Odabaşi et al. 2003). Briefly, 5.0 g of L-histidine methyl ester and 0.2 g of hydroquinone were dissolved in 100 mL of dichloromethane solution. This solution was cooled down to 0°C, 12.7 g triethylamine was added followed by pouring slowly 5.0 mL of methacryloyl chloride and then the mixture was stirred magnetically for 2 h. At the end of this chemical reaction, amino acid methyl ester and triethylamine were extracted with 10% HCl. The product and methacryloyl chloride in dichloromethane were evaporated in a rotary evaporator and separated by column chromatography with chloroform/ethyl acetate mixture.
Preparation of microspheres
The preparation of the microspheres can be described as follows; stabilizing agent (PVAL, 0.3 g) was dissolved in 65 mL deionized water for the preparation of the continuous phase. The monomer phase HEMA (2 mL), EGDMA (4mL), MAH and AIBN (100 mg as initiator) were prepared as dispersion phase. The disperse phase was added to the continuous medium within a two-neck flask with a volume of 500 mL, provided with a blade type stirrer. In order to optimize the amount of the functional monomer (MAH) in microspheres in cryogel, four different kinds of microspheres were synthesized by changing the amount of MAH monomer in polymerization mixture. Polymerization was carried out at 65°C for 4 h and then at 90°C for 1 h with the 700 rpm stirring rate. The adsorbents separated from the polymerization medium by filtration. Then adsorbents washed several times with washing solutions (i.e., a dilute HCl solution, and a water–ethanol mixture). Purity of the adsorbents was followed by observing the change of optical densities of the samples taken from the liquid phase in the recirculation system, and also from the DSC thermograms of the adsorbents obtained by using a differential scanning microcalorimeter (Mettler, Switzerland). Optical density of uncleaned adsorbent was 2.86. But after cleaning operation, this value was reduced to 0.06. In addition, when the thermogram of uncleaned adsorbent was recorded, it had a peak around 60°C. This peak might be originated from AIBN. But after application of this cleaning procedure between 30 and 100°C, the peak was not observed on this thermogram.
Preparation of MECC column
After production of the microspheres, the MECC column was synthesized with the presence of microspheres. MECC column was prepared as follows; the monomer mixture was prepared by dissolving 6 mmol of HEMA and 1mmol of MBAA in 15 mL of deionized water with a monomer concentration of 10%. The final monomer solution was degassed under vacuum for about 5 min to eliminate soluble oxygen. After adding different amounts of microspheres (25 mg, 50 mg, 75 mg, 100 mg) to the monomer mixture, the free radical polymerization was initiated by adding APS and TEMED (1% w/v of the total monomers) in an ice bath at 0°C. Immediately, the reaction mixture was poured into four plastic syringes (5 mL, id. 0.8 cm) with closed outlets at the bottom and was frozen at − 12°C for 24 h. The MECC column was thawed at room temperature. The control cryogel column without microsphere was also prepared in the same manner. After washing with 200 mL of water, the cryogel was stored in a buffer containing 0.02% sodium azide at 4°C until use. Illustration of MECC column for HIgG is represented in .
Characterization of cryogels
Swelling ratio of the MECC column was determined in distilled water. The experiment was conducted as follows: initially dry MECC column was carefully weighed before being placed in a 40 mL vial containing distilled water. The vial was put into an isothermal water bath with a fixed temperature (25°C) for 2 h. The sample was taken out from the water, wiped using a filter paper, and weighed. In different times the weight of MECC column was recorded. After 24 h the final weight of cryogel was recorded. The weight ratio of dry and wet samples was recorded. The water absorption capacity of the MECC column was calculated as [(Ws – Wo)/Wo] × 100, where Wo and Ws are the weights of MECC column before and after uptake of water, respectively. For determination of the gelation yield of the cryogel, first the swollen MECC column sample (1 mL) was put in an oven at 60°C for drying. The constant mass of the dried sample was recorded (mdried). The gelation yield was calculated as (mdried/mt) × 100, mt means the total mass of the monomers in the polymerization mixture. The total pore volume of the swollen MECC column was roughly calculated by weighing the sample (msqueezed gel) after squeezing the water from the macropores, then the porosity was determined as (mswollen gel – msqueezed gel)/mswollen gel × 100. The percent of swollen gel weight is calculated as (mswollen gel–mdried)/mswollen gel × 100. All measurements were done in triplicate and the average values are presented. The flow-rate of water passing through the column was measured at the constant hydrostatic pressure equal to 100 cm water column corresponding to a pressure of ca. 0.01 MPa. At least three measurements were done for each sample (Derazshamshir et al. Citation2010).
Porosity of the polymer sample was measured by the nitrogen sorption technique, performed on Flowsorb II, (Micromeritics Insrument Corporation, Norcross, USA). The specific surface area of particles in dry state was determined by multipoint Brunauer– Emmett–Teller (BET) apparatus (Quantachrome, Nova 2200E, USA). 0.5 g of sample was placed in a sample holder and degassed in a N2-gas stream at 150°C for 1 h. Adsorption of the gas was performed at − 210°C and desorption was performed at room temperature. Values obtained from desorption step were used for the specific surface area calculation.
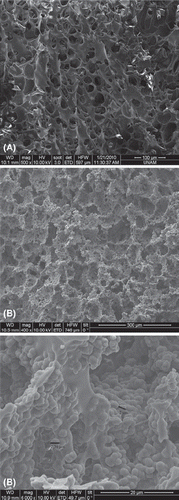
The surface morphology of a cross section of the dried MECC column was investigated by using SEM. The sample was swelled in DI water and then freezed at − 18°C. The freezed sample was then put in to a freezed-dryer for 24 hours at 0.001mbar and − 52°C (Christ Freezed Dryer-Alpha 1-2 LD Maryland, USA). The dried sample's SEM fotographs were examined using a JEOL JSM 5600 SEM (Tokyo, Japan).
To evaluate the amount of MAH in the composite cryogel structure, it was subjected to elemental analysis using a Leco Elemental Analyzer (Model CHNS-932, USA).
HIgG depletion studies from aqueous solution
The HIgG adsorption studies were carried out in a recirculation system at room temperature in 0.1 M phosphate buffer (pH 6.0) for 30 min. Then, the HIgG solution was pumped through the MECC column under recirculation for 2 h. The flow of HIgG solution through the column was enabled using an ALITEA (Sweden) peristaltic pump. The HIgG amount in this solution was determined by Bradford method. HIgG adsorption studies were performed in several stages. In the first group of experiments, the effect of the ligand density on the HIgG adsorption on MECC column was performed. In the second group of experiment the flow rate of the aqueous solution (i.e. 50 mL of the solution with a HIgG content of 2.5 mg/mL) varied between 1.0 and 3.0 mL/min. In the third group of experiments, HIgG adsorption from aqueous solution was studied at different pHs and buffer types (MES, Tris-HCl, phosphate). Adsorption isotherms were also obtained for MECC column. Aqueous solutions containing different amounts of HIgG were used in these experiments. The effects of the amount of the microspheres on HIgG adsorption capacities were also performed in aqueous solution. The alterating amounts of microspheres were embedded into MECC columns and HIgG adsorption behaviors were observed on these columns. The change in the HIgG concentration was followed to obtain the equilibrium adsorption curve.
Desorption of the adsorbed HIgG from MECC column was achieved with pH 7.0, phosphate buffer containing 1M NaCl. Desorption agent was passed through the column containing adsorbed HIgG at 0.5 mL/min flow rate for 1 h. The final HIgG concentration in desorption medium was determined spectrophotometrically. Desorption ratio was calculated from the amount of adsorbed and desorbed HIgG concentration. In order to test the reusability of the composite cryogel, HIgG adsorption-desorption procedure was repeated 10 times by using the same column. After one adsorption-desorption cycle, the MECC column was washed with 50 mM NaOH solution for 30 min, for sterilization. After this procedure, composite cryogel was washed with distilled water. To test the repeated use of MECC column, HIgG adsorption-desorption cycle was repeated 10 times using the same cryogel column.
HIgG depletion studies from human serum
The MECC column was used for HIgG depletion from human serum. The blood is collected from thoroughly controlled voluntary blood donors. Each unit was separately controlled and found negative for hepatit B specific antigen and HIV I, II and hepatitis C antibodies. No preservatives were added to the samples. Blood samples were centrifuged at 3000 g for 3 min at room temperature to separate the serum. The serum samples were filtered using 0.45 μm cellulose acetate microspin filters (Alltech, Deerfield, IL, USA). Equilibration of the cryogel column was performed by passing two column volumes of phosphate buffer saline (PBS pH: 7.4, NaCl: 0.9%) before injection of the serum. Human serum was diluted using 25 mM of PBS with dilution rations of and 1/5. Then, 15 mL of the freshly separated human serum was loaded onto MECC column. The amount of HIgG adsorbed by cryogel was determined by measuring the initial and final concentration of HIgG in serum.
In order to test the binding performance, Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out. Electrophoresis analyses of HIgG depletion in serum samples were performed on 10% separating mini gels (9 cm × 6.5 cm) and 6% stacking gels for 120 min at 100 V. Gels were stained with 0.25% (w/v) Comassie Brillant R 250 in acetic acid-methanol-water mixture (1:3:6, v/v/v) and destained in ethanol-acetic acid-water mixture (1:4:6, v/v/v).
Results and discussions
Characterization of cryogels
represents the swelling properties and linear flow resistance (at hydrostatic pressure, ca. 0.01 mPa) of both control cryogel column and MECC column, respectively. Both columns have macroporous structure for that reason they have great flow rates with a low linear flow resistance. Please keep in mind that control cryogel column has no embedded microsphere in its structure so this control cryogel column has higher swelling ratio than the embedded one. The gaps in the control cryogel column can absorp more water than those of composite cryogel because the microspheres occupied space in the gaps in composite cryogels. All cryogels were produced with high gelation yield (about 90%) and a swelling degree of about 10.
Table I. Swelling properties and flow resistance of cryogels.
The surface area of the control cryogel column and MECC column were found as 54.2 and 92.1 m2/g,respectively, by BET method. Cryogels have low surface area due to the large pores so these results show that particle embedding increases the surface area of the polymer.
The SEM images of the internal structures of the control cryogel column and MECC column are shown in . MECC column has large continuous interconnected pores (10–50 μm in diameter) with thin polymer walls that provide channels for the mobile phase to flow through. As seen in the figure, microspheres are uniformly distributed into the composite cryogel network. Pore size of the matrix is much larger than the size of the protein molecules, allowing them to pass easily. As a result of the convective flow of the solution/human plasma through the pores, the mass transfer resistance is practically negligible.
The FTIR spectra of both MAH monomer and microspheres have the characteristic stretching vibration bands of hydrogen-bonded alcohol, OH, around 3400 cm− 1 The FTIR spectra of microspheres have characteristic amide I and amide II absorption bands at 1645 and 1516 cm− 1, respectively (Odabaşı et al. Citation2007)
To evaluate the amount of the MAH incorporation in to the microspheres, elemental analysis was performed. Note that HEMA and other chemicals in the polymerization formula do not contain nitrogen. This nitrogen amount determined by elemental analysis came from only incorporated MAH groups into the polymeric structure. As mentioned before, to optimize the amount of the functional monomer (MAH) in microspheres in cryogel, four different kinds of microspheres with different MAH content were synthesized.
HIgG depletion studies from aqueous solution
Effects of ligand density on HIgG depletion
Affinity matrix design and optimization of the ligand density (i.e. MAH) on the adsorbent are crucial factors on depletion of HIgG. Ligand density has significant influences on the interactions between the ligands and the target protein. When the MAH content of microspheres was increased to 27.5, 35.3, 46.3, 56.5 μmol/g), adsorption of HIgG on MECC column was changed as 43.02, 50.61, 45.7, and 43.1 mg/g, respectively. L-Histidine interacts through its carboxyl, amino and imidazole groups with HIgG protein (Çanak et al. Citation2004) The more the MAH monomer was incorporated in to microsphere embedded in cryogel, the more HIgG depletion capacity of the cryogel became, due to the L-histidine and HIgG interactions. However, above the 35.3 μmol/g MAH loading amounts, this effect becomes less pronounced. This result may be due to the overlapping of MAH monomers on surface, and/or the functional groups of the MAH monomer not being probably in a suitable conformation to interact with HIgG. There is an optimal density at which the MAH monomer has high depletion capacity and an acceptable extent of steric hindrance.
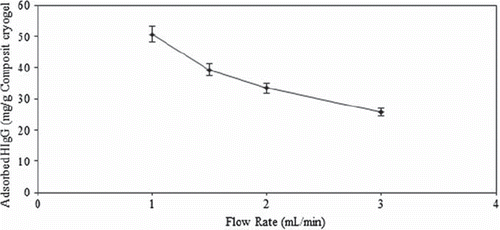
Effects of flow rate on HIgG depletion
The HIgG adsorption of MECC column decreased drastically from 50.5 mg/g to 25.8 mg/g as the flow rate was increased from 1.0 mL/min to 3.0 mL/min, (). Ideally, HIgG molecules would have more time to diffuse properly into the pores of cryogel and bind to binding sites at lower flow rates, and hence a better adsorption amount is achieved at a flow rate of 1.0 mL/min. Cryogels have large pores, probably the limiting stage is not diffusion into pores, binding needs some time. Binding to some poorly exposed sites could be less favorable and hence takes more time, than binding to easily available sites (Derazshamshie et al. 2010)
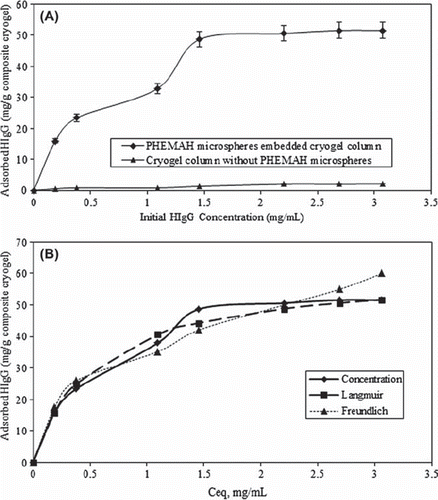
Effects of initial concentration on HIgG depletion
shows the effect of initial HIgG concentration on adsorption. As presented in this figure, with increasing initial HIgG concentration in solution, the amount of HIgG adsorbed per unit mass of the composite cryogel column increases and reaches saturation. The steep slope of the initial part of the adsorption isotherm represents a high affinity between HIgG and incorporated MAH groups. It becomes constant when the protein concentration is greater than 2.5 mg/mL. Maximum adsorption capacity is found to be 50.5 mg/g MECC column. It is of importance for an adsorbent to show little nonspecific adsorption of proteins in bioseparation. The cryogel without microspheres has very low HIgG depletion capacity (2.0 mg/g).
Effect of pH and buffer type on HIgG depletion
To investigate the effect of pH and buffer type on HIgG adsorption onto MECC column, different pHs and buffer systems were studied. For pH experiments, acetate (4.0–5.0) and phosphate (6.0–8.0) buffers at different pH values were studied. Adsorption capacity was observed as 50.5 mg/g at pH 6.0. Adsorption capacity was decreased significantly below and above pH 6.0. To clarify if the HIgG adsorption onto MECC column depended on the buffer systems used, adsorption studies were carried out using MES (5.5–6.5), Tris–HCl (7.0–8.5), phosphate (6.0–8.0) buffers. shows the effects of buffer types on maximum HIgG adsorption onto MECC column.
Table II. Effect of buffer type on HIgG adsorption. MAH content: 35.3 μmol/g; HIgG concentration: 2.5 mg/mL; flow rate: 1 mL/min; T: 25°C.
From the structure of the buffer ions used, it is obvious that Tris-HCl, and phosphate carry one or more charges of the same sign only negative or positive whereas the zwitter ionic buffer MES carries two charges of opposite signs below and very close pH values of its pKa. When the MES buffer was used, MECC column adsorbed higher amounts of HIgG than those adsorbed by the MECC column when the phosphate buffer was used (57.9–50.5 mg/g, respectively). It is obvious from the that, the maximum adsorption capacity obtained when Tris-HCl buffer was used. HIgG molecule is negatively charged at pH 8.0 (isoelectric point: 6.2), specific interactions between HIgG molecule and MAH comonomer at pH 8.0 may result from the conformational state of HIgG molecules (more folded structure) at this pH.
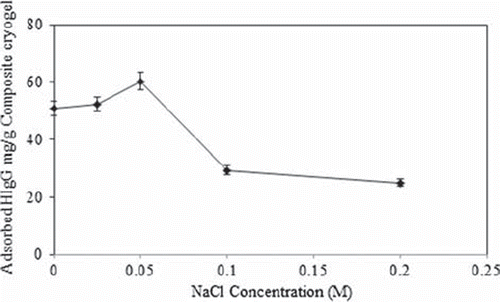
Effect of ionic strenght on HIgG depletion
HIgG adsorption to the MECC column was performed at different NaCl (0–0.2 M) concentrations. The effect of ionic strength on HIgG adsorption is shown in . As seen here, HIgG adsorption capacity decreased with the increasing salt concentration. This result can be attributed to the repulsive electrostatic forces between the microsphere embedded croyogel and HIgG molecules. When the salt concentration increased in the adsorption medium, this can lead to coordination of the deprotonated amino groups of the histidine with cations of the salts, which leads to low protein adsorption.
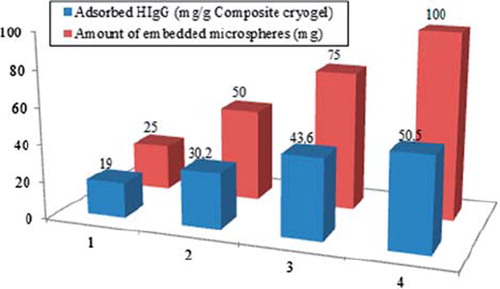
Effect of the amount of embedded microspheres in cryogel on HIgG adsorption
shows the effects of different amounts of embedded microspheres on HIgG adsorption capacities. It is obvious from the figure that HIgG adsorption capacity shows a remarkable increase with increasing amount of the embedded microspheres. It could be concluded that the higher amount of embedded particles increases the surface area of the adsorbent which also increases the amount of specific binding areas on the affinity column.
Adsorption isotherm
To evaluate the adsorption properties, modeling of the equilibrium data has been done using the Langmuir and Freundlich isotherms (Finette et al. Citation1997). According to Langmuir adsorption model, molecules are adsorbed at a fixed number of well-defined sites, each of which is capable of holding only one molecule. These sites are also assumed to be energetically equivalent and distant from each other so that there are no interactions between molecules adsorbed on adjacent sites. The Freundlich isotherm describes reversible adsorption and is not restricted to the formation of the monolayer (Umpleby et al. Citation2001).
The Langmuir adsorption isotherm is shown by Eq. (3)
where, q is the amount of adsorbed HIgG on MECC column (mg/g), Ce is the equilibrium HIgG concentration in solution (mg/mL), b is the Langmuir constant, indicates monolayer adsorption. The other well-known isotherm, which is frequently used to describe adsorption behavior, is the Freundlich isotherm (4):
where KF is the Freundlich adsorption constant (mg/g), Ce is the equilibrium HIgG concentration (mg/mL), and n is the Freundlich exponent which represents the heterogeneity of the system.
The adsorption values such as qmax, b and R2 obtained from Langmuir model were found as 60.9, 1.82 and 0.9903, respectively. On the other hand, KF, n and R2 obtained from Freundlich model were found as 35.23, 0.4376 and 09595, respectively. According to the correlation coefficients of isotherms, Langmuir adsorption model is favorable, and it is better fitted than Freundlich isotherm as shown in .
HIgG depletion studies from human serum and SDS-PAGE studies
The depletion studies of HIgG from serum were performed circulating the human serum through the MECC column. The adsorption amount of MECC column was performed with the different dilution ratios of serum sample (i.e. crude, and 1/5 dilution retios). The maximum adsorption capacity for MECC column was obtained as 214.7 mg/g cryogel for crude serum (for other ratios, 112 and 47 mg/g, respectively). It is obviously seen from these results that the protein adsorption values were decreased with the increasing dilution ratio. The depletion efficiency for HIgG from human serum was obtained 90.8% by using two fold dry cryogel weights. This result indicates that the MECC column bound a large portion of HIgG.
The column reported here has a good depletion capacity (90.8%) for HIgG, which is as good as the kinds of adsorbents reported before in the literature for different proteins in serum: 97.5%, 98.2%, 99.3% for IgG depletion from goat serum using IMAC, with Cu2+-loaded poly(GMA)-IDA, Cu2+ loaded poly (EGDMA-MAH) beads and with PGMA-IDA-Cu2+ beads embedded PHEMA cryogel, respectively (Jain and Gupta Citation2004, Altıntaş et al. Citation2007, Karataş et al. Citation2007), 99.3%, 98.2% and 97.7% for albumin depletion with Cibacron Blue F3GA loaded poly(GMA) beads, Cibacron Blue F3GA-attached mPVALbeads and Cu2+- IDA- attached m(PHEMA) beads, respectively (Altıntaş and Denizli Citation2006, Odabaşı Citation2011, Odabaşı et al. Citation2004) and 94% for immunodepletion of IgG with anti-IgG (Seferovic et al. Citation2008). Following the above-mentioned discussions, we believe that our MECC column offers promising approach with good depletion efficiency of HIgG.
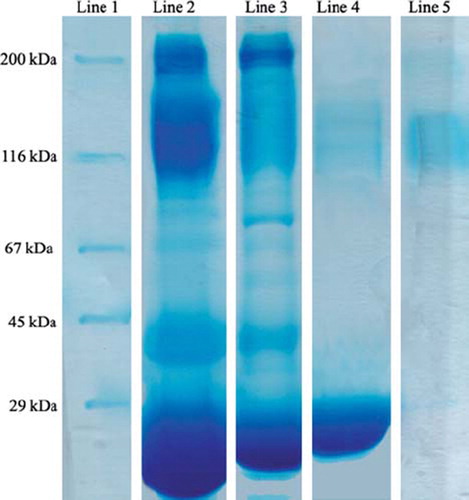
To test the efficiency of HIgG depletion from human serum, crude serum and eluted proteins were analyzed by gel electrophoresis. shows SDS PAGE analysis of human serum before and after treatment with MECC column. Depletion of HIgG can be clearly seen from lane 2 and lane 3. As seen from lane 4 the eluted proteins from MECC column include mainly the HIgG. With the 90.8% of the HIgG depletion amount, it may be concluded that MECC column is sufficient in terms of depletion of HIgG simultaneously from human serum with high efficiency.
Conclusion
Microsphere-embedding process combines the superior features of monolithic cryogel column (e.g. short diffusion path, low pressure drop, and very short residence) with microsphere-based stationary phases (e.g. high surface area). Therefore, the high mass transfer and the high flow rate of embedded monolithic cryogel column provide an intensive interaction of protein in the mobile phase with ligands on embedded particles (Sproß and Sinz Citation2011). As a result, this MECC column having high adsorption capacity may be a useful tool in the protein separation technology.
Acknowledgments
The authors wish to thank the National Nanotechnology Research Center at Bilkent University for providing the SEM images.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Altıntaş EB, Denizli A, 2006. Efficient removal of albumin from human serum by monosize dye affinity beads. J Chromatogr B. 832: 216–223.
- Altıntaş EB, Tüzmen N, Uzun L, Denizli A. 2007. Immobilized metal affinity adsorption for antibody depletion from human serum with monosize beads. Ind Eng Chem Res. 46:7802–7810.
- Andac M. Galaev I, Denizli A. 2012. Dye attached poly(hydroxyethyl methacrylate) cryogel for albumin depletion from human serum. J Sep Sci. 35:1173–1182.
- Anderson NL, Anderson NG. 2002. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 1:845–867.
- Arvidsson P, Plieva FM, Savina IN, Lozinsky VI, Fexby S, Bülow L, et al. 2002. Chromatography of microbial cells using continuous supermacroporous affinity and ion-exchange columns. J Chromatogr A. 977:27–38.
- Arvidsson P, Plieva FM, Lozinsky VI, Galaev IY, Mattiasson B. 2003. Direct chromatographic capture of enzyme from crude homogenate using immobilized metal affinity chromatography on a continuous supermacroporous adsorbent. J Chromatogr A. 986:275–290.
- Bailey J, Zhang K, Zolotarjova N, Nicol G, Szafranski C. 2003. Removing high-abundance proteins from serum. Genet Eng News. 23:32–36.
- Coffinier Y, Legallais C, Vijayalakshmi MA. 2002.. Separation of IgG from human plasma using thiophilic hollow fiber membranes. J Membr Sci. 208:13–22.
- Çanak Y, Ozkara S, Akgol S, Denizli A. 2004. Pseudospecific bioaffinity chromatography of IgG. React Funct Polym. 61:369–377.
- Çimen D, Denizli A. 2012. Immobilized metal affinity monolithic cryogels for cytochrome c purification. Colloids Surf B. 93:29–35.
- Derazshamshir A, Baydemir G, Andac M, Say R, Galaev IY, Denizli A. 2010. Macromol Chem Phys. 211:657–668.
- El-Kak A, Manjini S, Vijayalakshmi MA. 1992. Interaction of immunoglobulin G with immobilized histidine: mechanistic and kinetic aspects. J Chromatogr A. 604:29–37.
- Erzengin M, Ünlü N, Odabaşı M. 2011. A novel adsorbent for protein chromatography: Supermacroporous monolithic cryogel embedded with Cu2+-attached sporopollenin particles. J Chromatogr A. 1218:484–490.
- Fang X, Zhang W. 2008. Affinity separation and enrichment methods in proteomic analysis. J Proteomics. 71:284–303.
- Finette GMS, Qui-Ming M, Hearn MTW. 1997. Comparative studies on the isothermal characteristics of proteins adsorbed under batch equilibrium conditions to ion-exchange, immobilised metal ion affinity and dye affinity matrices with different ionic strength and temperature conditions. J Chromatogr A. 763:71–90.
- Garipcan B, Denizli A. 2002. A novel affinity support material for the separation of immunoglobulin G from human plasma. Macromol Biosci. 2:135–144.
- Hemdan ES, Porath J. 1985. Development of immobilized metal affinity chromatography II: interaction of amino acids with immobilized nickelimionodiacetat. J Chromatogr. 323:255–264.
- Huang PY, Carbonell RG. 1999. Affinity chromatography screening of soluble combinatorial peptide libraries. Biotechnol Bioeng. 63:633–641.
- Jain S, Gupta MN. 2004. Purification of goat immunoglobulin G by immobilized metal-ion affinity using cross-linked alginate beads. Biotechnol Appl Biochem. 39:319–322.
- Karataş M, Akgöl S, Yavuz H, Say R, Denizli A. 2007. Immunoglobulin G depletion from human serum with metal-chelated beads under magnetic fieldInt. J Biol Macromol. 40:254–270.
- Koç I, Baydemir G, Bayram E, Yavuz H, Denizli A. 2011. Selective removal of 17β-estradiol with molecularly imprinted particle- embedded cryogel systems. J Hazard Mater. 192:1819–1826.
- Lowe CR, Lowe AR, Gupta G. 2001. New developments in affinity chromatography with potential application in the production of biopharmaceuticals. J Biochem Biophys Methods. 49:561–574.
- Odabaşı M, Garipcan B, Denizli A. 2003. Preparation of a novel metal-chelate affinity beads for albumin isolation from human plasma. J Appl Poly Sci. 90:2840–2847.
- Odabaşı M, Uzun L, Denizli A. 2004. Porous magnetic chelator support for albumin adsorption by immobilized metal affinity separation. J Appl Poly Sci. 93:2501–2510.
- Odabaşı M, Say R, Denizli A. 2007. Molecular imprinted particles for lysozyme purification. Mat Sci Eng C. 27:90–99.
- Odabaşı M. 2011. Magnetic dye-affinity beads for human serum albumın purification. Prep Biochem Biotechnol. 41:287–304.
- Plieva FM, Kirsebom H, Mattiasson B. 2011. Preparation of macroporous cryostructurated gel monoliths, their characterization and main applications. J Sep Sci. 34:2164–2172.
- Riggs L, Sioma C, Regnier F. 2001. Automated signature peptide approach for proteomics. J Chromatogr A. 924:359–368.
- Seferovic MD, Krughkov V, Pinto D, Han VKM, Gupta MB. 2008. Quantitative 2-D gel electrophoresis-based expression proteomics of albumin and IgG immunodepleted plasma. J Chromatogr B. 865:147–152.
- Sproß J, Sinz A. 2011. Monolithic media for applications in affinity chromatography. J Sep Sci. 34:1958–1973.
- Umpleby RJ, Baxter SC, Chen Y, Shah RN, Shimizu KD. 2001. Characterization of molecularly imprinted polymers with the Langmuir-Freundlch Isotherm. Anal Chem. 73:4584–4591.
- Ünlü N, Ceylan Ş, Erzengin M, Odabaşı M. 2011. Investigation of protein adsorption performance of Ni2+-attached diatomite particles embedded in composite monolithic cryogels. J Sep Sci. 34:2173–2180.
- Verdoliva A, Pannone F, Rossi M, Catello S, Manfredi V. 2002. Affinity purification of polyclonal antibodies using a new all-D synthetic peptide ligand: comparison with protein A and protein G. J Immunol Methods. 271:77–88.
- Zhou M, David A, Lucas DA, Chan KC, Issaq JJ, Petricoin EF III, et al. 2004. An investigation into the human serum “interactome”. Electrophoresis. 25:289–1298.