Abstract
In this study, a new amperometric biosensor for the determination of hypoxanthine was developed. To this aim, polypyrrole-polyvinyl sulphonate films were prepared on the platinum electrode by the electropolymerization of pyrrole in the presence of polyvinyl sulphonate. Xanthine oxidase and uricase enzymes were immobilized in polypyrrole-polyvinyl sulphonate via the entrapment method. Optimum conditions of enzyme electrode were determined. Hypoxanthine detection is based on the oxidation of hydrogen peroxide at +400 mV produced by the enzymatic reaction on the enzyme electrode surface. The linear working range of biosensor for hypoxanthine was determined. The effects of pH and temperature on the response of the hypoxanthine biosensor were investigated. Optimum pH and temperature were measured as 8 and 30°C, respectively. Operational and storage stability of the biosensor were determined. After 20 assays, the biosensor sustained 74.5% of its initial performance. After 33 days, the biosensor lost 36% of its initial performance. The performance of the biosensor was tested in real samples.
Introduction
The requirements and regulations in the fields of environmental protection, control of biotechnological processes and certification of food and water quality are becoming more and more urgent. At the same time stricter requirements regarding human and animal health have led to a rising number of clinical and veterinary tests. Therefore, there is a need to develop highly sensitive, fast and economic methods of analysis. The elaboration of biosensors is probably one of the most promising ways of solving some problems concerning sensitive, fast, repetitive and cheap measurements (Arkhypova et al. Citation2008).
Biosensors are analytical devices which tightly combine biorecognition elements and physical transducers for the detection of target compounds. In the enzyme-based biosensors, the biological element is the enzyme which reacts selectively with its substrate. It is well known that the response of a biosensor to the addition of a substrate is determined by the concentration of the product of the enzymatic reaction on the surface of the sensor. The reaction is controlled by the rate of two simultaneous, i.e. the enzymatic conversion of the substrate and the diffusion of the product from the enzyme layer (Amine et al. Citation2006).
Hypoxanthine (C5N4OH4) belongs to a family of heterocycles which in their structures reveal pyrimidinic rings fused to azolic moieties. These are very interesting systems in several research fields (Acevedo-Chavez and Costas Citation1999).
Hypoxanthine is a purine nitrogenous base which is a metabolic intermediate of nucleic acid (Fernandez-Quejon et al. Citation2005). It is also found to be a minor purine base in transfer RNA. In the purine catabolism, hypoxanthine is oxidized to xanthine and then uric acid in human beings (Leszczynski and Shukla Citation2000). In the purines catabolism hypoxanthine is catalytically oxidized by the metalloenzyme xanthine oxidase in the production of uric acid (Acevedo-Chavez and Costas Citation2000). The sooner a fish dies or is caught, then degradation occurs. In fish muscle, nucleotide degradation commences upon death and continues throughout storage. The oxidation of hypoxanthine to xanthine by the catalytic activity of xanthine oxidase together with the production of H2O2 and reaction of O2 was found to be the rate determining step in fish muscle. Therefore, the quantity of hypoxanthine can be used as fish freshness determination. The available quantity of hypoxanthine is frequently used as an indicator of fish freshness of meat in the food industries and some pathological processes in the human body. Thus, the determination of hypoxanthine is of considerable importance for quality control of fish and fish products in food industries (Cui et al. Citation2000). The determination of hypoxanthine can also be achieved via different methods, such as spectrophotometry, chromatography and electrophoresis. But some of these methods are tedious because they require pre-analysis and re-complicated and time-spending procedures (Anık et al. 2007). Electrochemical biosensors have been designed to enable simple and more rapid determination of hypoxanthine (Adeloju and Lawal Citation2010).
Xanthine oxidase (EC 1.1.3.22) is a metalloflavoenzyme composed of two identical subunits, each subunit has a mol.wt. of 145 kDa and contains one each of molybdenum site and FAD site and two Fe–S centers. Xanthine oxidase catalyzes the oxidation of hypoxanthine to xanthine and then xanthine to uric acid with concomitant reduction of molecular oxygen (Bhushan et al. Citation2003). Xanthine oxidase has the unusual property that it can exist in a dehydrogenase (XD) form which uses NAD+ as electron acceptor, and an oxidase form (XO) which uses oxygen as electron acceptor. Both forms have high affinity for hypoxanthine and xanthine as substrates. The conversion of one form to the other may occur under various conditions. The exact function of the enzyme is still unknown, but it seems to be involved in purine catabolism, detoxification of antibiotics and antioxidant capacity by producing urate. The xanthine oxidase form produces reactive oxygen species (Frederiks and Vreeling-Sindelarova Citation2002). The optimum pH for microbial Xanthine oxidase is 7.5–8.0, optimum temperature is 65°C and Km value is 76 μM (White and White).
Uricase (EC 1.7.3.3) is an enzyme which catalyzes the oxidation of uric acid into 5-hydroxy isourate, while reducing dioxygen into hydrogen peroxide. The metastable 5-hydroxy isourate species is latter transformed into 5-allantoine, either naturally or with the help of other enzymes (Altarsha et al. Citation2009). The optimum pH for microbial uricase is 9.0, optimum temperature is 40°C and Km value is 25 μM (White and White).
So far, various enzyme immobilization methods have been reported in existing literature. For instance: enzyme was drop-coated onto a cellulose acetate membrane, bound onto a chitosan membrane by cross-linking, encapsulated by forming polyelectrolyte multiplayer films or sol-gel and immobilized into a polyaniline film by physisorption (Choi et al. Citation2007). Xanthine oxidase and uricase have been immobilized in polypyrrole-polyvinyl sulphonate via the entrapment method. Hypoxanthine determination is based on the oxidation of hydrogen peroxide produced by the enzymatic oxidation from hypoxanthine to allantoine, at +400 mV.
In this study, a new amperometric biosensor was designed for the determination of hypoxanthine. To do this, polypyrrole-polyvinyl sulphonate composite film was prepared on a platinum surface by the electropolymerization of pyrrole out in the presence of polyvinyl sulphonate (Chubey et al. Citation2000).
Materials and methods
Equipments and reagents
Electrochemical polymerization and experiments carried out using a BAS Epsilon-EC Ver1.40.67 N7 electrochemical analyzer with a three electrode cell was equipped with a Pt plate (0.5 cm2) as the working electrode, an Ag/AgCl (3 M KCl) electrode as the reference electrode and a platinum wire (diameter and length; 1 mm, 4 cm, respectively) for the counter electrode. For the pH setting of buffer solutions an ORION Model 720A pH-ion meter was used. Temperature stability of cell was achieved with Grant W14 thermostat. For the liquid transfers a ±0.05 μL precision micro-pipette was used. Xanthine oxidase (100 unit) and uricase (100 unit) were purchased from Sigma. Other chemicals were obtained from Aldrich. Distilled water was provided by a GFL distilled water device.
The preparation of polypyrrole-polyvinyl sulphonate (PPy-PVS) composite film
Before the electropolymerization, the Pt Plate electrode was cleaned mechanically and chemically. The Pt Plate was mechanically polished with zero number sand paper, then washed with distilled water. The electrode was burned using burner flame and cooled at room temperature. The Pt electrode was held within acetone, ethyl alcohol, concentrated hydrochloric acid and nitric acid for 5 minutes, respectively. Then, the Pt plate surface was washed with distilled water and dried (Qiong and Tuzhi Citation1998, İnem et al. Citation2010). The polyvinyl sulphonate was carried to the Pt surface together with polypyrrole. Meanwhile, xanthine oxidase and uricase were entrapped in polymer film. The electrode was immersed in a 5 ml solution of 0.1 M pyrrole, polyvinyl sulphonate, enzyme solutions and distilled water. The solution was purged with argon in order to remove the oxygen (Chubey et al. Citation2000). The electropolymerization was carried out with a six cyclic scan between (−1000 mV) − (+2000) mV potential at 50 mV/s scan rate (Choi et al. Citation2007). The Pt/PPy-PVS-XO-U enzyme electrode was rinsed with buffer solutions and was stored at +4°C in buffer solution (Ghosh et al. Citation1998).
Procedure
Firstly, a cell was prepared for the amperometric determination. An aqueous buffer solution containing 0.1 M phosphate at pH:8.0 and 0.1 M sodium perchlorate was added to cell. Three electrode systems, which were working electrode, reference electrode and counter electrode, were immersed in the cell. Time elapsed until the steady-state current reached +400 mV. Then, hypoxanthine stock solution was added to the cell by using micro-pipette and stirred for 15 minutes until the reaction completely finished. Then, the resulting current difference was recorded. The current difference was proportional to the hypoxanthine concentration.
Preparation fish extract
Salmo Trutta was chosen for the determination of real samples. A sample of 5 g was taken from the fish and homogenized within 50 mL distilled water. The proteins were precipitated using trichloro acetate (TCA) (Anık et al. 2007). The mixture was filtered through Whatman filter paper. The filtrate was filled to 100 mL with distilled water. A three-pointed calibration curve was drawn by the method of standard addition. The hypoxanthine quantity in the fish sample was determined by using the calibration curve.
Result and discussion
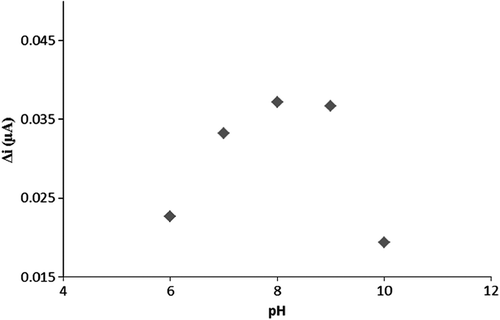
The effect of pH on the activity of Pt/PPy-PVS-XO-U electrode
To determine the optimum pH of the biosensor, the cell in which pH conditions ranging between 6 and 10 were prepared and being added to 0.1 mM hypoxanthine standard solutions. The results are plotted in . According to the pH-plot, the highest current value was obtained at pH:8. Thus, the optimum pH of the enzyme electrode system was determined at pH 8. Searching existing literature, similarly, the optimum pH value was determined as 7.4 for the trienzymatic biosensor (Mallavia et al. Citation2008).
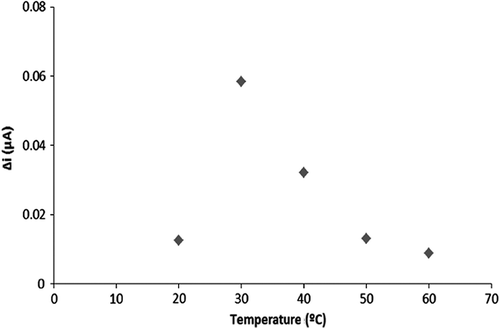
The effect of temperature on the activity of Pt/PPy-PVS-XO-U
To determine the optimum temperature of the biosensor, the cell in which temperature conditions ranging between 20 and 60°C were prepared and being added 0.1 mM hypoxanthine standard solutions. The results were plotted in .
According to the temperature plot, the highest current value was obtained at 30°C. Thus, the optimum temperature of the enzyme electrode system was determined at 30°C. Searching existing literature, the determination of hypoxanthine by using trienzymatic biosensor was measured as 37°C (Mallavia et al. Citation2008).
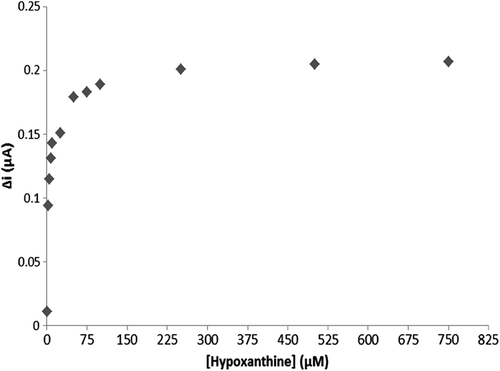
The response of the biosensor to increasing substrate concentration
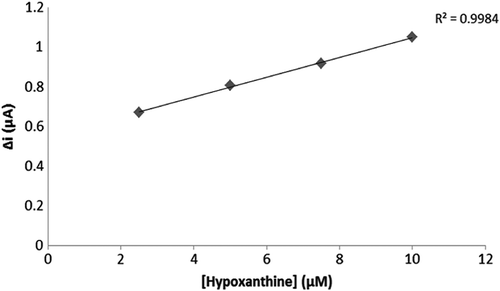
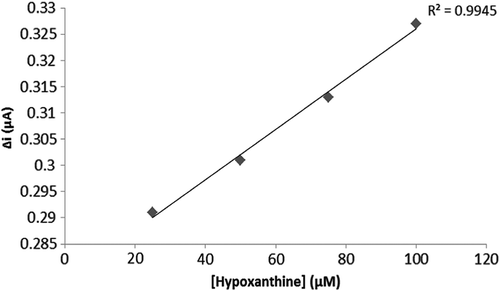
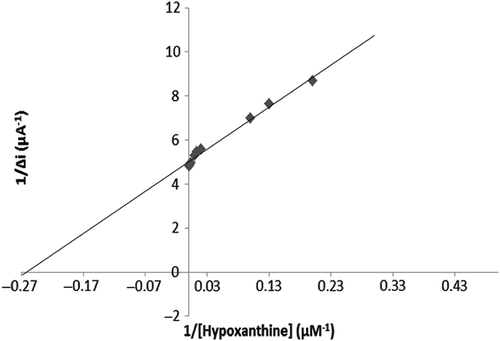
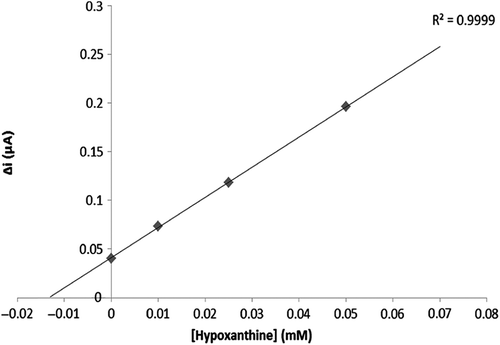
To evaluate the efficacy of the response of the enzyme electrode to increasing substrate concentration, hypoxanthine standard solutions, of which concentrations were 1 μM–0.75 mM, were added to the cell. The results are plotted in . The calibration curve was drawn using the results ( and ). According to this curve, the linear working range and limit of detection were determined. A Lineweaver–Burk plot is drawn in by using this data. and values have been determined for the multienzyme electrode system (Singh et al. Citation2007, Cosnier et al. Citation2008).
Two linear ranges have been reported for the enzyme electrode system: one of them was a 2.5 μM–10 μM plot allowing measurements at low concentrations, and the other one was a 25 μM–10 mM plot allowing measurements at high concentrations.
The and for the multienzyme electrode system was calculated as 3.7 μM and 0.19 μA/min, respectively. Similar results were found in existing literature. For instance: Christine Mousty and coworkers measured the Km value of trienzymatic electrode system as 0.8–2.0 mM, albeit the described Km of free GOD is 33 mM (Cosnier et al. Citation2008).
Operational stability
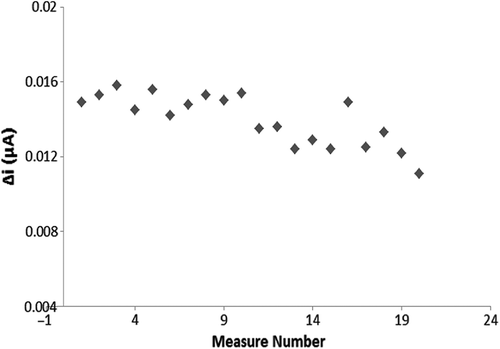
To research the operational stability of a biosensor the experiment was carried out 20 times at constant pH, temperature and substrate concentration (0.1 mM hypoxanthine). The results are plotted in .
As seen from , after 20 assays, the biosensor retained 74.5% of its initial performance. Relative standard deviation was calculated as 9.78%. Hirakozu Okuma and Etsuo Watanabe have reported that the residual activity of the multienzyme reactor electrodes were 80% after 500 assays (Okuma and Watanabe Citation2002).
Storage stability
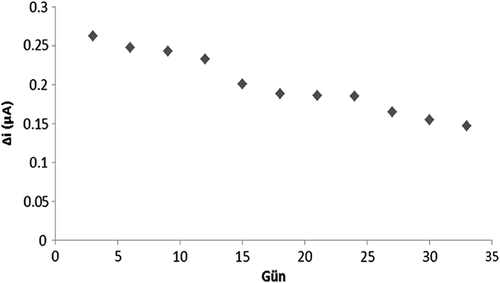
To research the storage stability of a biosensor experiments were conducted at periodic intervals of 33 days at constant pH, temperature and substrate concentration (0.1 mM hypoxanthine stock solution). The results are plotted in .
As seen from , after 33 days, the biosensor lost 36% of its initial performance. The relative standard deviation was calculated as 19.7%. A search of existing literature revealed that A.T. Lawal and S.B. Adeloju have reported a 77% decline in the initial performance of the biosensor after 60 hours (Anık et al. 2007).
Real sample applications
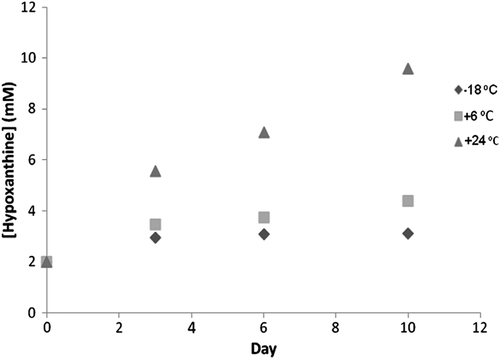
Salmo trutta was chosen for the determination of real samples. The fish was bought while still alive and upon death, the quantity of hypoxanthine in the fish was determined. To monitor the increases of hypoxanthine in fish muscle and to research whether the designed biosensor could determine the increases, fish samples were stored at +24°C, +6°C, and −18°C and were measured every 3 days, respectively. The results were plotted in .
The calibration curve was plotted by using the standard addition method in .
The biosensor was successfully determined the increasing the hypoxanthine of the fish sample.
The increase in the hypoxanthine quantity of the fish sample slowed according to the fish sample stored at +24°C.
The increase in the hypoxanthine quantity of the fish sample almost stopped according to the fish samples stored at +24°C and +6°C.
Conclusion
An amperometric biosensor for the determination of hypoxanthine was fabricated by the immobilization of xanthine oxidase and uricase in a polypyrrole-polyvinyl sulphonate film by electrochemical polymerization. The biosensor had a minimum detectable concentration of 2.5 μM. The two linear concentration ranges for the biosensor were 2.5 μM–10 μM and 25 μM–0.1 mM. After 20 assays, the biosensor retained 74.5% of its initial performance and after 33 days, it lost 44% of its initial performance. Optimum pH and temperature values were determined as 8 and 30°C, respectively. The response of the biosensor in real samples was researched and it has been successfully determined to increase hypoxanthine and hypoxanthine quantity.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Acevedo-Chavez R, Costas ME. 1999. Density functional study of the monocationic hypoxanthine tautomers. J Mol Struct (Theoche). 489:73–85.
- Acevedo-Chavez R, Costas ME. 2000. Density functional study of the anionic hypoxanthine tautomeric forms. J Mol Struct (Theochem). 499:71–84.
- Adeloju SB, Lawal AT. 2010. Comparison of polypyrrole-based xanthine oxidase amperometric and potentiometric biosensors for hypoxanthine. J Mol Catal B Enzymat. 66:270–275.
- Altarsha M, Castro B, Monard G. 2009. Intrinsic reactivity of uric acid with dioxygen: Towards to elucidation of the catalytic mechanism of urate oxidase. Bioorg Chem. 37:111–125.
- Amine A, Bourais I, Mohammadi H, Palleschi G. 2006. Enzyme inhibition based biosensor for food safety and environmental monitoring. Biosens Bioelectron. 21:1405–1423.
- Anık Ü, Çubukçu M, Timur S. 2007. Examination of performance of glassy carbon paste electrode modified with gold nanoparticle and xanthine oxidase for xanthine and hypoxanthine detection. Talanta. 74:434–439.
- Arkhypova VN, Dzyadevych SV, El'skaya AV, Jaffrezic-Renault N, Martelet C, Soldatkin AP. 2008. Amperometric enzyme biosensors: past, present and future. ITBM-RBM. 29:171–180.
- Bhushan B, Halasz A, Hawari J, Paquet L, Spain JC. 2003. Mechanism of xanthine oxidase catalyzed biotransformation of HMX under anaerobic condition. Biochem Biophys Res Commun. 306: 509–515.
- Choi MF, Dong C, Zhang Y, Shuang S, Wen G, Zhou Y. 2007. Development and analytical application of an uric acid biosensor using an uricase-immobilized eggshell membrane. Biosens Bioelectron. 22:1791–1797.
- Chubey A, Gerard M, Malhotra BD, Singhal R, Singh VS. 2000. Immobilization of lactate dehydrogenase on electrochemically prepared polypyrrole-polyvinyl sulphonate composite films for application to lactate biosensors. Sens Actuators. 46:723–729.
- Cosnier S, Mousty C, Mu S, Shan D. 2008. Trienzymatic biosensor for the determination of inorganic phosphate. Biosens Bioelectron. 24:1053–1056.
- Cui D, Hu S, Luo J, Xu C. 2000. Biosensor for detection hypoxanthine based on xanthine oxidase immobilized on chemically modified carbon paste electrode. Anal Chim Acta. 412:55–61.
- Fernandez-Quejon M, De la Fuente M, Navarro R. 2005. Theoretical calculations and vibrational study of hypoxanthine in aqueous solution. J Mol Struct. 744-747:749–757.
- Frederiks WM, Vreeling-Sindelarova H. 2002. Ultrastructural localization of xanthine oxidoreductase activity in isolated rat liver cells. Acta Histochem. 104:29–37.
- Ghosh S, Misra TN, Sarker D. 1998. Development of an amperometric enzyme electrode biosensor for fish freshness detection. Sens Actuators B. 53:58–62.
- İnem B, Karacif K, Kıyak T. 2010. Coating of aluminum with conducting polymer and investigation of the effect of corrosion on coating microstructure. J Fac Eng Arch Gazi Univ. 25:235–241.
- Leszczynski J, Shukla MK. 2000. A DFT investigation on effects of hydration on the tautomeric equilibria of hypoxanthine. J Mol Struct (Theochem). 529:99–112.
- Mallavia R, Mateo CR, Pastor I, Salinas-Castillo A. 2008. Immobilization of a trienzymatic system in a sol-gel matrix: A new fluorescent biosensor for xanthine. Biosens Bioelectron. 24: 1053–1056.
- Okuma H, Watanabe E. 2002. Flow system for fish freshness determination based on double multi-enzyme reactor electrodes. Biosens Bioelectron. 17:367–372.
- Qiong C, Tuzhi P. 1998. Silk fibroin/cellulose acetate membrane electrodes incorporating xanthine oxidase for the determination or fish freshness. Anal Chim Acta. 369:245–251.
- Singh S, Singhal R, Malhotra BD. 2007. İmmobilization of cholesterol esterase and cholesterol oxidase onto sol-gel films for application to cholesterol biosensor. Anal Chim Acta. 582:335–343.
- White DC, White JS. 1997. Source Book Enzyme. Boca Raton: CRC Press, 85–82, 129–132.