Abstract
Objectives: To reduce the mean molecular weight ![]() and to increase the effective polymerization ratio (REff) of polymerized human placenta hemoglobin (PolyPHb). Methods: Three factors of GDA-PolyPHb process such as the approach of feeding GDA (FGDA), hemoglobin concentration ([Hb]) and the molar ratio of GDA, and hemoglobin(RGDA:Hb) were investigated. Finally, the expansion experiments were conducted with optimal conditions. Results: The data showed that the HBOCs with the REff of 67.35% and lower
and to increase the effective polymerization ratio (REff) of polymerized human placenta hemoglobin (PolyPHb). Methods: Three factors of GDA-PolyPHb process such as the approach of feeding GDA (FGDA), hemoglobin concentration ([Hb]) and the molar ratio of GDA, and hemoglobin(RGDA:Hb) were investigated. Finally, the expansion experiments were conducted with optimal conditions. Results: The data showed that the HBOCs with the REff of 67.35% and lower ![]() of 162.70 kDa were prepared by optimal conditions. Conclusion: Compared to original process, the optimal process greatly decreased the
of 162.70 kDa were prepared by optimal conditions. Conclusion: Compared to original process, the optimal process greatly decreased the ![]() and increased the REff.
and increased the REff.
Introduction
Blood transfusion plays an increasingly important part in emergencies. But this therapy is with high risks, including the transmission of infectious disease and immunologic reactions (Estep et al. Citation2008). When compared with traditional transfusion, HBOCs have many advantages, for example, they do not require blood typing or cross-matching, can be stored for long periods of time, and are essentially free from infectious agents. Natanson et al. (Citation2008) had reported 3711 cases of clinical trial and the survival rate of HBOCs group was 91.7%. Professor T.M.S. Chang's (Citation2000) report also pointed out that HBOCs were used in patients of blood loss 2/3 and greater in prehospital first aid, and the control group had no patient survived, while the HBOCs group had no one case of death. Moreover, for the past several years, a HBOCs product had been approved and marketed for veterinary use in the United States and a similar product is approved for human use in South Africa (Kocian and Spahn Citation2008).
Side effects such as hypertension, myocardial infarction, and acute kidney failure were observed, which inhibited further development and approval for clinical use of these products (Estep et al. Citation2008, Phibin et al. Citation2005). The exact mechanism for these side effects was not clear, but we believe molecular weight range might be the one of the main factors. For example, the early polymerization hemoglobin products of Biopure and Northfield were above 400 kDa in mean molecular weight (![]() ); while the Hemopure (HBOC-201) of Biopure's third-generation product had reduced the
); while the Hemopure (HBOC-201) of Biopure's third-generation product had reduced the ![]() of 250 kDa, and the HBOC-301's
of 250 kDa, and the HBOC-301's ![]() (200 kDa) was lower. And it was demonstrated markedly by the failure in clinical trials of diaspirin-crosslinked Hb (64 kDa) (Estep et al. Citation2008). But one potential answer is to decrease the
(200 kDa) was lower. And it was demonstrated markedly by the failure in clinical trials of diaspirin-crosslinked Hb (64 kDa) (Estep et al. Citation2008). But one potential answer is to decrease the ![]() of HBOCs products; for example, the
of HBOCs products; for example, the ![]() of hydroxyethyl starch products had gradually reduced from 480 kDa to 200 kDa (HES) and 130 kDa (Voluven) (American Dupont HESSPan), that is, the trend was the same as the
of hydroxyethyl starch products had gradually reduced from 480 kDa to 200 kDa (HES) and 130 kDa (Voluven) (American Dupont HESSPan), that is, the trend was the same as the ![]() of HBOCs which gradually reduced from high to medial in the development of the practice.
of HBOCs which gradually reduced from high to medial in the development of the practice.
The main goal of this study was to reduce ![]() and to increase effective polymerization ratio (REff) of GDA-PolyPHb.
and to increase effective polymerization ratio (REff) of GDA-PolyPHb.
Materials and methods
Reagents and equipments
Reagents. Human placental blood was obtained from Sichuan New Life Stem Cell Biotech Inc. (Chengdu, Sichuan) and Tianjin Union Stem Cell Gene Engineering Co. LTD (Tianjin). GDA was purchased from Sigma (Sigma, AR) and all other reagents were analytical reagents (AR).
Main instruments. Instruments used in this investigation were high-capacity, low-temperature centrifuge (Beckman); CARY50 UV-Vis spectrophotometer (Beckman); Master Flex UF (Millipore); high-performance liquid (Waters e2695/2489); Gel column Superdex200 (GE); Blood cell analyzer (Mindray); Delsa Nano (Beckman Coulter); and Hemox analyzer (TCS Scientific Corp).
Methods
The stroma-free human placenta hemoglobin was prepared as described previously (Li and Yang Citation2006).
The polymerization principle of hemoglobin and GDA.
The influencing factors of GDA-crosslinked-hemoglobin from the literatures (Adamson Citation2002, Rausch et al. Citation1999, Houtchens et al. Citation2000, Moore et al. Citation2005, Winslow Citation2003, MacDonald and Pepper Citation1994, Bonsen et al. Citation1975, Sehgal et al. Citation1986, Hsia Citation1988) were FGDA, [Hb], RGDA:Hb, GDA concentration(CGDA), the speed of feeding GDA, pH, reaction temperature, time, etc.
The polymerization conditions. The 100 ml stroma-free human placenta hemoglobin solution reacted with GDA at 1.00% in sterile and pyrogen-free water for 60 min at 4 ± 2 ℃, gently stirred in a 250 ml filter flask filled with N2, and followed by a reaction with NaBH4 at 1.56% under the same conditions for 30 min. by end of the reaction, the pH was adjusted to 7.30 ± 0.05. The product was stored at 4 ± 2 ℃.
Detection of the molecular weight distribution. The distribution of the molecular weight was detected using a standard method with standard marker (molecular weights: 669 kDa, 443 kDa, 200 kDa, 150 kDa, 66 kDa, and 29 kDa). After the samples diluted in ultrapure water to 0.01% and filtered in 0.22 μm membrane, the HPLC had run with the injected 20 μl sample at the flow rate of 0.5 mL/min for 60 min at 25 ℃C, and the detected absorbance was 280 nm.
![]() : Σ Molecular weight * the percentage of this molecular weight in the HPLC figure;
: Σ Molecular weight * the percentage of this molecular weight in the HPLC figure;
The content of super-weight molecular (CSW): The percentage of polymerized hemoglobin of molecular weight more than 600 kDa in the HPLC figure;
REff: The percentage of polymerized hemoglobin of molecular weight 90 kDa–600 kDa in the HPLC figure.
Statistics. Data are expressed as mean± SEM. Groups were compared using the analysis of unpaired Student's t-test and statistical significance was p < 0.05.
Results
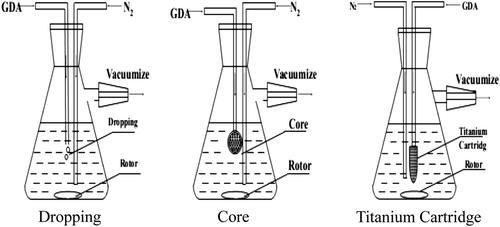
The approaches of feeding gluaraldehyde (FGDA)
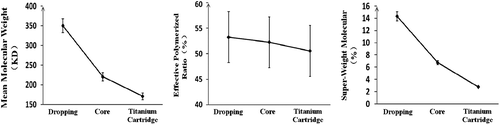
Polymerization conditions were CHb: 11.00 ± 0.30%, RGDA:Hb: 5.50 ± 0.30%, and FGDA was changed (dropping, sand core, titanium cartridge, see ).
shows that the different FGDA had no significant effect on REff (p > 0.10), but resulted in comparable decreases in ![]() (p < 0.01) and Csw (p < 0.01). Therefore, the optimal FGDA was titanium cartridge with
(p < 0.01) and Csw (p < 0.01). Therefore, the optimal FGDA was titanium cartridge with ![]() of 178.8 ± 18.02 kDa and Csw of 2.79 ± 0.29%.
of 178.8 ± 18.02 kDa and Csw of 2.79 ± 0.29%.
Hemoglobin concentration ([Hb])
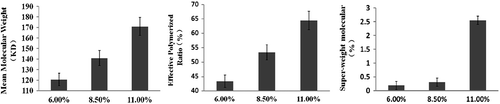
Polymerization conditions: RGDA:Hb: 5.50 ± 0.30%, FGDA was titanium cartridge, [Hb] was changed (11.00 ± 0.20%, 8.50 ± 0.20%, 6.00 ± 0.20%, respectively).
shows that the lower CHb resulted in lowering ![]() , Csw, and REff. Taking into account that decreasing
, Csw, and REff. Taking into account that decreasing ![]() was the primary goal in this stage, therefore, the optimal [Hb] was 6.00% with
was the primary goal in this stage, therefore, the optimal [Hb] was 6.00% with ![]() of 122.21 ± 60.09 kDa and CEff of 43.41 ± 3.39%.
of 122.21 ± 60.09 kDa and CEff of 43.41 ± 3.39%.
The molar ratio of glutaraldehyde and hemoglobin (RGDA:Hb)
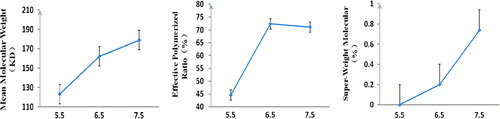
Polymerization conditions were [Hb]: 6.00 ± 0.30%, FGDA: titanium cartridge, RGDA:Hb was changed (5.50 ± 0.03, 6.50 ± 0.03, and 7.50 ± 0.03, respectively).
shows that there was significant difference in ![]() and Csw in RGDA:Hb with 5.5, 6.5, and 7.5 (p < 0.05); and significant differences on REff in RGDA:Hb with 5.5 and 6.5 (p < 0.01), but was not statistically significant in 6.5 and 7.5 (p > 0.10). So, the optimal RGDA:Hb was selected as 6.50 with
and Csw in RGDA:Hb with 5.5, 6.5, and 7.5 (p < 0.05); and significant differences on REff in RGDA:Hb with 5.5 and 6.5 (p < 0.01), but was not statistically significant in 6.5 and 7.5 (p > 0.10). So, the optimal RGDA:Hb was selected as 6.50 with ![]() of 158.60 ± 8.70 kDa, REff of 69.50 ± 3.70%, and Csw of 0.26 ± 0.25%.
of 158.60 ± 8.70 kDa, REff of 69.50 ± 3.70%, and Csw of 0.26 ± 0.25%.
Expansion experiments
The optimal conditions were chosen on the basis of the above experiments. FGDA: titanium cartridge; CHb: 6.00 ± 0.20%; pH: 7.30 ± 0.05; RGDA:Hb: 6.50; the reaction time: 60 min; temperature: 4.0 ± 2.0 ℃; the flux of feeding glutaraldehyde: 1.00 ml/min; and CGDA: 1.00 ± 0.03%. The scale-up experiments were implemented after expanding 30 times (3000 ml) in the above conditions. The GDA-crosslinked product was purified as the ultrafiltration after 100 kDa (Millipore). The pH was adjusted to 7.35 ± 0.05, and the final product was bulk-filtered with a 0.8 μm filter followed by two sequential 0.2 μm sterile filtration steps. The final product was concentrated to 6.0 g/dl into 10 ml sterile glass bottles. The product was stored at −40 ℃. contains the molecular weight distribution of the poly-hemoglobin and final products based on optimal process.
Table I. The molecular weight distribution of poly-hemoglobin and final products based on optimal process (n = 3).
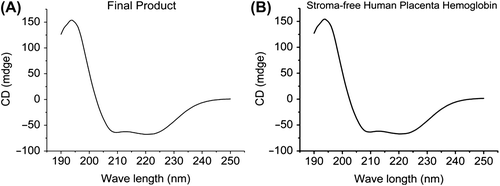
The rest of physical and chemical items of final products based on optimal process accorded with the internal standards of Institute of Tianjin Union Biotechnology Development. is the secondary structure of final products and stroma-free human placenta hemoglobin with circular dichroism.
Figure 5. Secondary structure of final products with circular dichroism (A). Compared with the secondary structure of the storma-free human placenta hemoglobin (B), the secondary structure of final products is consistent. This can be very obviously explained by the process of crosslinking without disrupting the secondary structure.
Discussion
The results reported here indicate that human placenta Hb crosslinked by GDA with optimized condition can be produced with high yield and lower ![]() .
.
GDA is a promising candidate for the crosslink agent of the blood substitutes because it is easy to industrialize and is less expensive (Moore et al. Citation2005, Winslow Citation2003, MacDonald and Pepper Citation1994). For example, GDA has been used as the crosslink agent in both Hemopure 201(Biopure) and PolyHeme (Northfield) and no related side effects were reported due to the presence of trace of the GDA with less than 2 ppm.
Although it is more promising than the other crosslink agents, the clinical potential of GDA has not yet been fully demonstrated, in part because of the unwanted alternative of high yield or lower ![]() . Meanwhile, the higher
. Meanwhile, the higher ![]() may might have caused the products to be unstable and have poor microvascular flow, even embolism. Therefore, in this study, reducing
may might have caused the products to be unstable and have poor microvascular flow, even embolism. Therefore, in this study, reducing ![]() was the primary purpose, at the same time significantly increasing REff had been achieved. It was a dramatically successful point in this optimization process, and may provide favorable conditions of expandable production.
was the primary purpose, at the same time significantly increasing REff had been achieved. It was a dramatically successful point in this optimization process, and may provide favorable conditions of expandable production.
FGDA, a major influencing factor, impacts on the homogeneity of the poly-Hb attributed to the GDA dispersion in the hemoglobin solution. Compared with the dropping and the core, the titanium cartridge made the GDA disperse faster and more uniform which increased the ordering of the crosslink reaction, so the titanium cartridge has the same CEff but declining Csw and ![]() . The other two approaches, in contrast, maybe because the local concentration of GDA was too high thus the heterogeneity of the poly-Hb was exacerbating, resulting in increasing of CSW and
. The other two approaches, in contrast, maybe because the local concentration of GDA was too high thus the heterogeneity of the poly-Hb was exacerbating, resulting in increasing of CSW and ![]() .
.
In addition, according to the chemical reactions equilibrium theory and the law of mass action, with the increasing of substrate concentration, the reaction rate will increase under the certain conditions, and the chemical equilibrium moves in the direction of the positive reaction. In this report, the higher the CHb was, the faster the reaction rate was, and the reaction equilibrium favored movement to the direction of a positive reaction. So CEff increased as the hemoglobin concentration increased. Increasing RGDA:Hb was equivalent to increasing the GDA concentration, thus making the reaction equilibrium move in direction of the positive reaction, resulting in increasing product ![]() and CEff.
and CEff.
Conclusion
In summary, compared with the original process, the optimal process improved CEff and reduced ![]() , and this optimal process has apparent advantages in product scale up.
, and this optimal process has apparent advantages in product scale up.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Supported by National High-tech R&D Program of China (863 Program) (Grant No. 2012AA021903).
References
- Adamson GJ, (Georgetown, CA). 2002. US Patent 6500930 B2.
- Bonsen P (Los Alton,CA), Laver MB (Weston, MA), Morris KC (Mountain View,CA). 1975. US Patent 4001200.
- Chang TMS. 2000. Red blood cell substitutes. Bailieres Best Pract Res Clin Haematol. 13:651–667.
- Estep T, Bucci T, Farmer M, Greenburg G, Harrington J, Kim HW, et al. 2008. Basic Science focus on blood substitutes: a summary of the NHLBI Division of Blood Diseases and Resources Working Group Workshop, March 1. 2006. Transfusion. 48: 777–780.
- Houtchens RA (Milford, MA) Rausch CW (Medford, MA). 2000. US Patent 6150507A.
- Hsia JC, 10 Cherry Hills Road, Concord, Ontario, Canada, M4K 2M4. 1988. US Patent 4857636.
- Kocian R, Spahn DR. 2008. Haemoglobin, oxygen carriers and perioperative organ perfusion. Best Pract Res Clin Anaesthesiol. 22:63–80.
- Li T, Yang CM. 2006. Purificationand viral inactivation of hemoglobin from human placenta blood. J Biomed Eng. 23:641.
- MacDonald SL, Pepper DS. 1994. Hemoglobin polymerization. Methods Enzymol. 231:287–308.
- Moore EE, Johnson JL, Cheng AM, Masuno T, Banerjee A. 2005. Insights from studies of blood substitutes in trauma. Shock. 24: 197–205.
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. 2008. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 299:2304–2312.
- Phibin N, Rice J, Gurney J, McGwin G, Arnaud F, Dong F, et al. 2005. A hemoglobin-based oxygen carrier, bovine polymerized hemoglobin (HBOC-201) versus hetastarch (HEX) in a moderate severity hemorrhagic shock swine model with delayed evacuation. Resusciatation. 66:367–378.
- Rausch CW (Medford, MA), Gawryl Maria S (Charlestown, MA), Houtchens RA (Milford, MA), Laccetti AJ (North Andover, MA), Light WR (Natick, MA). 1999. US Patent 5955581A.
- Sehgal LR, De Woskin RE (both of Cook Country), Moss GS, Gould SA (both of Lake Country), Rosen AL, Sehgal H (both of Cook Country, all of ρ). 1986. US Patent 4826811.
- Winslow RM. 2003. Current status of blood substitute research: towards a new paradigm. J Intern Med. 253:508–517.