Abstract
Death after severe hemorrhage remains an important cause of mortality in people under 50 years of age. Keratin resuscitation fluid (KRF) is a novel resuscitation solution made from keratin protein that may restore cardiovascular stability. This postulate was tested in rats that were exsanguinated to 40% of their blood volume. Test groups received either low or high volume resuscitation with either KRF or lactated Ringer's solution. KRF low volume was more effective than LR in recovering cardiac function, blood pressure and blood chemistry. Furthermore, in contrast to LR-treated rats, KRF-treated rats exhibited vital signs that resembled normal controls at 1-week.
Introduction
Though it was invented over one hundred years ago, lactated Ringer's (LR) solution remains the most widely used fluid for the treatment of hypovolemic shock (Young Citation2011). However, there is a significant and persistent debate on whether colloid solutions provide better outcomes than crystalloids. Despite the lack of clarity, emergency medicine and intensive care textbooks continue to advocate crystalloids, even if large volumes must be infused (Young Citation2011, Cabanas et al. Citation2011). When such fluid overloading is performed, perfusion is restored at the expense of resulting hemodilution, risking dilutional coagulopathy, diminished oxygen-carrying capacity of the blood, persistent hypo-oxygenation of tissues, and organ failure. Regardless of the fluid used, overly aggressive resuscitation has the potential to dilute clotting factors, dislodge clots, and thus interfere with the clotting cascade and exacerbate bleeding (Young Citation2011). Clinical studies have not found any difference in mortality between critically ill patients randomized to receive infusion of saline or 4% albumin (Finfer et al. Citation2004). However, human albumin solution may impair cardiac function due to a reduction in ionized calcium and in the ionized calcium/total calcium ratio (Kovalik et al. Citation1981) and, although it is now labeled as a no harm substitute, due to cost limitations, saline solutions continue to be more widely used as a resuscitation fluid in the emergency department, intraoperative and intensive care unit settings. Another colloidal option, hetastarch, is considered relatively safe at low dosages. Gan et al. defined a dosing limit of up to 5 L as determined in their clinical trial (Gan et al. Citation1999). However, its labeling establishes a safe limit of 20 ml/kg, which can be quite limiting in the emergency setting. Investigators have also reported prolonged partial thromboplastin time and decreased circulating factor VII after infusion of hetastarch, even to euvolemia (Stump et al. Citation1985, Kheirabadi et al. Citation2008). Patients with active hemorrhage have an increased probability of bleeding following starch-based colloid treatment, thus limiting effective dosing and resuscitation.
The United States military employs a colloid primed resuscitation protocol on the battlefield, with an initial infusion of 500 ml of 6% hetastarch for unstable patients, followed by a bolus of 500 ml of 6% hetastarch if hemodynamic stability is not achieved (Szul et al. Citation2004). However, if cardiovascular stability is still not attained, further infusion of hetastarch is avoided to prevent complications. LR infusion is then started in large volumes until blood transfusions can be given. Although no wide consensus has been reached regarding the proper use of colloids such as hetastarch, several studies have demonstrated significant advantages at the cellular level when compared to conventional crystalloids (Liang et al. Citation2010). Recent debate in the field has proposed damage control resuscitation (DCR) as the solution to mortality after severe hemorrhage. This approach is based on a timely diagnosis of incoming patients at high risk for developing coagulopathy and allows prompt treatment of the so-called “lethal triad” of hemorrhage: hypothermia, acidosis and coagulopathy (Holcomb Citation2007, Hess et al. Citation2006). However, DCR is a protocol for in-hospital use, and therefore large volume isotonic crystalloids continue to be the “gold standard” in pre-hospital resuscitation (Young Citation2011).
More broadly, cardiovascular research has produced insights that have significantly impacted clinical practice over the last decade, yet cardiovascular-related mortality remains a primary cause of trauma-related death. Shock and resuscitation are important topics in this field and the development of better treatment options is essential. Previous experiments in our laboratory demonstrated the viability of human keratin protein solutions for intravenous use (Nunez et al. Citation2011). Keratins represent a novel colloid that can be formulated at a desired viscosity with the potential to diminish the need for large volumes of resuscitation fluids by improving blood's osmotic profile, thereby reducing the risk for side effects described with the use of other colloid compounds (Dahn et al. Citation1979, Krausz Citation1995, Innerhofer et al. Citation2002, Weaver et al. Citation1978). Keratin proteins have been investigated more broadly as biomaterials for several medical applications and have shown excellent cell and tissue compatibility (de Guzman et al. Citation2011). Our recent study investigating keratin colloids for intravenous use showed encouraging results (Nunez et al. Citation2011). Nunez et al. showed that specific fractions of keratin were found to have a vasodilation effect while further separation of the molecules yielded a purified compound of high molecular weight dimers and tetramers that could serve as a hypotensive resuscitation fluid. These purified keratins offer potential advantages as a colloid including:
• High molecular weight prevents fast clearance from the circulation during periods of vascular pathology
• Hyperviscosity aids in the restoration of normal microcirculatory perfusion
• Purified keratin fractions are not vasoactive, which avoids further hypotension
• Keratin is compatible within the circulatory system and does not cause immunologic reactions or untoward acute reactions with blood
The aim of the present study was to examine the potential of KRF to recover hemodynamic parameters after hypovolemic shock. Our goal was to recreate conditions similar to pre-hospital course of treatment in which emergency medical technicians rely on crystalloids and for which there is no disagreement that new treatment options are needed. The hypothesis tested was that intravenous infusion of KRF would restore cardiovascular stability after maintained hypovolemic hypotension with less volume than LR solution.
Materials and methods
Fluid preparation
Alpha-keratin (the keratin used in KRF) was prepared as previously described (Nunez et al. Citation2011) and the solution prepared by aseptic reconstitution of the lyophilized keratin powder with phosphate buffered saline at a concentration to match human blood viscosity (3.88 cP at 10 dynes/cm2). Viscosity was determined with a DV-II+ Pro cone and plate viscometer (Brookfield Engineering Laboratories, Inc., Middleboro, MA). At the above-mentioned viscosity-matched concentration, the solution had an osmolality of 315–325 mosm/L. To ensure cardiovascular compatibility, KRF was clarified prior to infusion. This could not be accomplished via filtration because of the protein's high molecular weight, so solutions were repeatedly (i.e. 10X) centrifuged at 2000 rpm for 5 minutes and only the supernatant used for infusion.
Surgical preparation
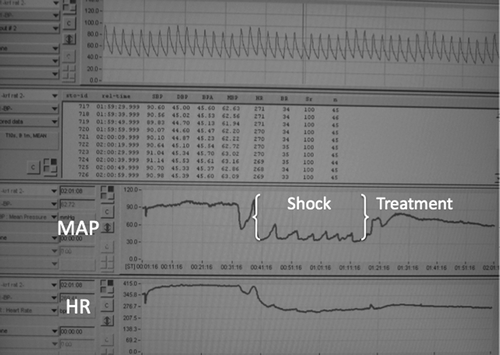
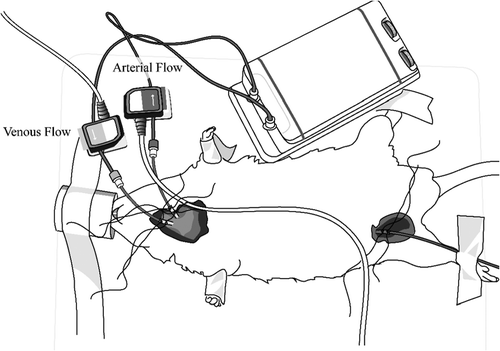
The animal research protocol was approved by the Wake Forest School of Medicine Institutional Animal Care and Use Committee. Thirty-seven male Sprague-Dawley rats (S-D; Charles River Laboratories International Inc, Wilmington, MA) weighing 327–528 g were studied. Anesthesia was induced with isoflurane at 3 volume % concentration and maintained at 1.5% isoflurane using a nose cone (95% O2/5%CO2). A femoral artery catheter (PE50) was inserted for blood pressure measurements, and a carotid artery to jugular vein extracorporeal loop was established and driven through a mechanical pump (Instech Laboratories, Plymouth Meeting, PA) at 10 ml/min. The extracorporeal loop tubing was primed with heparinized saline at 10 U/ml, the total content inside the loop is roughly 1.5 ml. Since minute flow through the system roughly represents 8–10% of the cardiac output, subjects exhibited a transient drop in blood pressure when the loop was started. Blood pressure recovered back to baseline within 5 minutes, however, all subjects were allowed to recover for 45 minutes before experimental test were initiated. Transit time flow probes continuously monitored the carotid outflow and the jugular inflow for changes in blood density (Transonic Systems, Inc, Ithaca, NY). Automatic cardiac output measurements using the ultrasound dilution method were triggered with a 0.3 ml bolus injection of saline into the jugular line () (de Boode et al. Citation2010).
Figure 1. Ultrasound Transit Flow Probes. A constant flow of 10 ml/min extracts blood from the carotid artery and returns it into the jugular vein. Saline injections into the venous line lowers blood density, which is detected by the venous flow probe and triggers automatic cardiac output measurement (Original Drawing).
Parameters
• Blood pressure and heart rate were continuously monitored and stored with IOX2 Software (EMKA Technologies, Falls Church, VA).
• Cardiac output was calculated using Transonic ICU software (Transonic Systems, Inc, Ithaca, NY) software at baseline, 1 minute before treatment was given, and at 5, 15, 30, 60, 90 and 120 minutes after treatment was initiated. Stroke volume was calculated by dividing cardiac output by the heart rate at the time of each measurement.
• Cardiac index was obtained by dividing cardiac output by the total body surface area, which was obtained using the Meeh constant formula (Gilpin Citation1996).
• Blood-filled microhematocrit tubes (Fisher Scientific, Pittsburgh, PA) were spun at 3000 rpm for 5 minutes and hematocrits measured using an International Microcapillary Reader (International Equipment Company, Boston, MA) at baseline, 1 minute before treatment, 30 minutes after treatment, and 120 minutes after treatment. An estimate of oxygen delivery was obtained by multiplication of cardiac output and hematocrit. The volume needed for each hemocrit measure was 50 μL (200 μL total).
• Blood gases were analyzed 120 minutes after initiation of resuscitation treatment using an IRMA Trupoint blood analysis system (ITC, Edison, NJ).
• Actively circulating volume (ACV) and central blood volume (CBV) were calculated posthoc by Transonic Systems Inc (Ithaca, NY) (Wilkins et al. Citation2001). These parameters correlate with severity of cardiac instability during hypovolemia (Secher and Van Lieshout Citation2005).
• Volume restoration measurements were obtained by dividing the restored ACV by the volume of resuscitation fluid infused intravenously (IV). Restored ACV was obtained by averaging ACV after 15 minutes of treatment and subtracting the ACV level before treatment.
Low-volume resuscitation, short-term assessment (experiment #1)
Eleven Male Sprague-Dawley rats were studied. Blood was withdrawn from the carotid arterial catheter until a mean arterial blood pressure of 40 mmHg was reached. This pressure was maintained for 30 minutes (). After 30 minutes of hypovolemic shock, rats were given the equivalent of 20% of their total blood volume of a randomly assigned fluid, LR or KRF. Total blood volume was calculated as 6.5% of body weight. Two hours after treatment arterial blood gases were measured and the rats were euthanized.
High-volume resuscitation, short-term assessment (experiment #2)
Ten male Sprague-Dawley rats were treated as described previously and were randomized to receive either total volume resuscitation of shed blood (total blood removed during hemorrhage was replaced by KRF) or 3X the volume of shed blood replaced by LR. Outcomes measured were the same as in the low volume group described above and animals were euthanized after 2 hours.
Long-term assessment of recovery (experiment #3)
Sixteen male Sprague Dawley rats were anesthetized and treated as described in experiment #1. Treatment conditions consisted of 3× the shed blood volume replaced by LR (i.e. same as high volume treatment) or 20% of total blood volume replaced by KRF (i.e. same as low volume treatment). After two hours, instrumentation was removed and the rats were returned to the vivarium. Seven days later the rats were re-instrumented and hemodynamic parameters, arterial blood gases, hemoglobin, hematocrit and electrolytes were assessed. Arterial oxygen content was obtained using values obtained from arterial blood gas analysis (Levitzky Citation2007). Comparisons with healthy controls were made for reference purposes but no statistical comparison was made to the treatment groups.
Histology
At the end of experiment #3, the rats were infused with saline solution followed by 10% neutral buffered formalin. Brain, heart, liver and kidney were harvested for histologic evaluation. The tissues were embedded in paraffin blocks, cut and stained with hematoxylin and eosin (H&E) for optical microscopy examination.
Statistical analyses
A two-way repeated measures analysis of variance (ANOVA; treatment group vs time) was performed to compare the treatment groups for all parameters measured repeatedly during the experiments. Main effect measured differences in mean squares between the two treatment groups. All separate time points were analyzed using matched pairs Bonferroni post-hoc tests if a main effect was found to be significant. Interaction analysis determined that the difference is slopes of recovery between the two groups.
Because the purpose of the study was to determine recovery after hemorrhage, all main effect analyses between groups as well as interactions between time and recovery were done starting at the before treatment timepoint and thereafter. Therefore, trends in recovery are drawn starting 30 minutes after hemorrhage and including that timepoint.
A one-tailed Student's test was performed to determine mean differences between parameters measured at the end of the experiment.
Results
Low-volume resuscitation, short-term assessment (experiment #1)
Hypovolemic shock
Rats were hemorrhaged 10.9 ml equivalent to 40% of estimated total blood volume (± 6.22%) to a mean arterial pressure of 38 mmHg (± 5.6 mmHg) for 35 minutes (± 2 minutes). There was no significant difference in volumes of hemorrhage or time spent in a hypotensive state between experimental groups. The mean volumes given IV were 5.94 ml and 5.63 ml in the LR and KRF groups, respectively.
Cardiac index
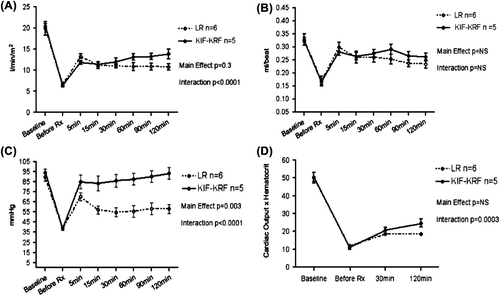
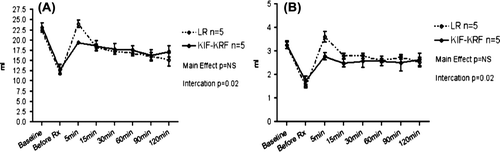
No significant main effect due to treatment was found (F = 1.43, p = 0.26) (). A statistically significant interaction between time and treatment group showed that the KRF group had an increasing slope in the recovery of cardiac index from 11.9 L/min/m2 at 15 minutes to 13.9 L/min/m2 at 120 minutes, while the LR group had a decreasing slope from 13.3 L/min/m2 to 10.8 L/min/m2 (F = 7.75, p < 0.0001) during the same period. This finding indicates that there was an improving slope in cardiac index in the KRF group compared to the LR group, which was in decline ().
Stroke volume
There was no difference between treatment groups in recovery of stroke volume following resuscitation ().
Heart rate
There was no difference between treatment groups in recovery of heart rate following resuscitation.
Mean arterial pressure
Blood pressure was significantly elevated after KRF treatment compared to treatment with LR (; F = 16.33, p = 0.003). A statistically significant interaction was noted between time and treatment group (F = 8.37, p < 0.0001),
Hematocrit and oxygen delivery
There was no difference in hematocrit between treatment groups. However, a steeper slope in calculated oxygen delivery was demonstrated by a significant interaction between time and treatment group (; F = 12.93, p = 0.0003).
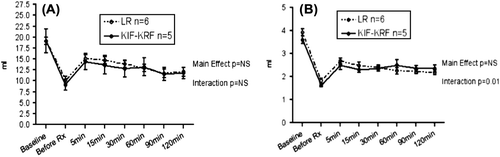
Changes in ACV and CBV were similar between the two groups (p = 0.73 and p = 0.99, respectively). However, a statistically significant interaction was found between time and treatment group in the recovery of CBV (F = 2.97, p = 0.01) ().
Blood gases
A one-tailed Student's test of blood gases after 120 minutes of treatment showed that the KRF group had arterial blood gas values more closely resembling normal than did the LR group: pH 7.16 vs. 7.09 (Normal = 7.35 – 7.45)(t = 2.43, p = 0.02) and pCO2 89.0 vs. 108.2 (NL = 35–45 mmHg) (t = 2.46, p = 0.02). No significant differences were found among other blood gas parameters ().
Table I. Hemodynamics and arterial blood gases: low volume resuscitation, short term assessment.
Volume restoration
KRF restored higher ACV per ml of resuscitation fluid infused but the difference was not significant (p = 0.16) ().
High-volume resuscitation, short-term assessment (experiment #2)
Hypovolemic shock
Rats were hemorrhaged 11.1 ml equivalent to 39% of estimated total blood volume (± 7.6%) to a mean arterial pressure of 39 mmHg (± 2.5 mmHg) for 39 minutes (± 2 minutes). There was no significant difference in volumes of hemorrhage or time spent in a hypotensive state between the groups. The mean volumes given IV were 34.5 ml and 10.6 ml in the LR and KRF groups, respectively. The high volume of resuscitation treatment took longer to infuse, hence the difference in resuscitation time between the first and second set of experiments.
Cardiac index
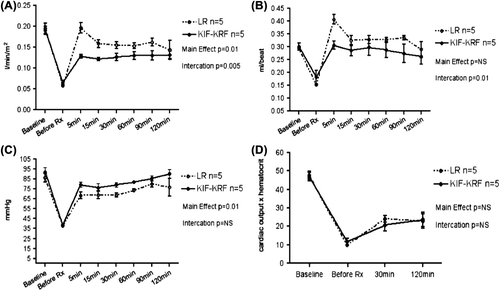
Main effect analysis showed that administration of LR resulted in improved overall recovery of cardiac index compared to KRF (F = 9.93, p = .01). However, a significant interaction between time and treatment group showed that the KRF group maintained a steadily increasing cardiac index from 12.2 L/min/m2 5 minutes after treatment to 13.2 L/min/m2 2 hours after treatment, while the LR group showed a decreasing slope from 15.9 L/min/m2 to 14.4 L/min/m2 (F = 3.56, p = 0.005) during the same period ().
Stroke volume
There was no significant main effect of treatment on stroke volume. However, a significant interaction between time and treatment group was found (F = 3.17, p = 0.01) (). This interaction statistically describes diverging slopes in stroke volume recovery, this interaction is mainly found due to LR rapid recovery and fall in this parameter with a more flat slope in the KR group.
Heart rate
There was no difference between treatment groups in recovery of heart rate following resuscitation.
Mean arterial pressure
Blood pressure was significantly increased after KRF treatment compared with LR (F = 11.38, p < 0.01) ().
Hematocrit and oxygen delivery
There was no difference between treatment groups in hematocrit or oxygen delivery (p = 0.4 and p = 0.9 respectively) ().
Actively circulating volume and central blood volume
No main effect was found between treatment groups in ACV or CBV. LR produced a rapid increase in ACV and CBV determined at 5 minutes, which was followed by a steep drop. ACV and CBV equilibrated at levels similar to those in the KRF group (p > 0.05). A significant interaction between time and treatment group was found in both ACV and CBV recovery analyses (F = 4.02, p = 0.002 and F = 4.19, p = 0.002 respectively) (). This interaction indicates that KRF was able to maintain steady circulating volumes while circulating volumes in the LR group decrease significantly over two hours. This effect is expected given that LR tends to escape into the interstitium (Young Citation2011).
Volume restoration
KRF showed a significantly higher ratio of ACV compared to LR (p = 0.01) ().
Table II. Hemodynamics and arterial blood gases: full volume resuscitation, short term assessment.
Blood gases
There were no differences between treatment groups in any blood gas parameters ().
Long-term assessment of recovery (experiment #3)
Hypovolemic shock
Rats were hemorrhaged 10.9 ml equivalent to 41.8% of their total blood volume (± 5.9%) to a mean arterial pressure of 39 mmHg (± 4 mmHg) for 35 minutes (± 2 minutes). There was no significant difference in volumes of hemorrhage or time spent in hypotension between groups. The mean volumes given IV were 32.4 ml and 5.6 ml in the LR and KRF groups, respectively.
Cardiac index
No difference was found between the two groups and both exhibited CI similar to healthy controls ().
Table III. Hemodynamics, arterial blood gases and electrolytes: long term assessment.
Stroke volume
KRF-treated rats recovered to a higher stroke volume compared to the LR rats (t = 2.86, p = 0.006). The KRF group was similar and the LR group slightly lower than healthy controls ().
Heart rate
The KRF group showed slightly lower mean heart rate than the LR group, the difference was not significant (t = 1.59, p = 0.06). The KRF group had similar mean heart rates whereas the LR group was significantly higher compared to healthy controls ().
Mean arterial pressure
No difference in mean arterial pressure was found between the two groups. Both groups exhibited mean arterial pressures similar to healthy controls ().
Actively circulating volume and central blood volume
The KRF group showed slightly higher ACV and CBV than the LR group, but the difference was not statistically significant (p = 0.15 for ACV and p = 0.09 for CBV). The LR group had similar ACV and CBV and the KRF group higher compared to healthy controls ().
Blood gases
There were no significant differences in arterial blood gases between groups ().
Electrolytes
No abnormalities were noted in any treatment group ().
Arterial oxygen content and myocardial oxygen delivery
No difference was noted between groups in arterial oxygen content. Myocardial oxygen delivery was higher in the KRF group (t = 2.91, p = 0.006).
Histology
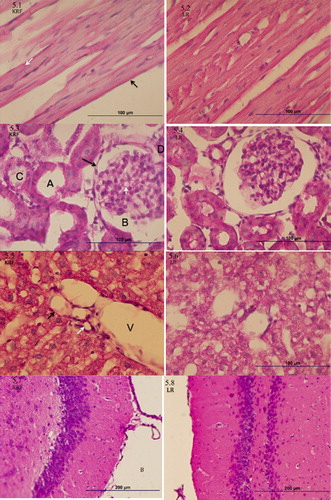
Histologic features of the brain, heart, liver and kidney were similar between the two treatment groups and resembled normal healthy tissue ().
Figure 7. Tissue Histology. 5.1-5.2 Cardiac Muscle Histology. Endocardial simple squamous epithelium marked by black arrow, intercalated disk marked by white arrow, normal muscle striations are evidence throughout the tissue. 5.3-5.4 Renal Histology. Simple squamous epithelium (black arrow), Bowman space (B), glomerular basement membrane (white arrow tip), loop of Henle tubule (A) and proximal convoluted tubule (C), arterial pole (D). 5.5-5.6 Liver Histology. Portal triad composed of Portal vein (V), hepatic artery (black arrow) and bile duct (white arrow). 5.7-5.8 Brain Histology. Coronal cut through the medial thalamus (A) and surrounding third ventricle (B).
Discussion
Data demonstrating the efficacy of in-hospital DCR support lower volume usage pre-hospital, and recent trends suggest that mean resuscitation fluid volume infused before packed red blood cells are given started to decline after 2007 (Heidary et al. Citation2012). However, there is consensus opinion that the lack of an ideal colloid has clouded the debate around pre-hospital resuscitation protocols and that a search for new colloids is certainly warranted.
With that objective in mind, the present study shows that low volume resuscitation with KRF proved to be more effective in recovering cardiac function, blood pressure, and blood chemistry than equivalent volumes of LR solution. This result was not surprising given LR's isotonicity and the tendency for the fluid to leak out of the vasculature. KRF, on the other hand, contains dimers and tetramers with molecular weights well above that of serum albumin (i.e. > 120 K Da). At 3X volume resuscitation, LR showed improved capacity to restore some cardiovascular parameters in the short term, but a significant interaction between time and treatment suggests that the LR volume likely moves into the interstitium, thus diminishing the ability of LR to maintain proper preload over time. This finding may explain why LR needs to be infused at three times the shed volume to maintain cardiovascular stability. KRF infusion at a level equivalent to shed blood volume maintained cardiac index similar to that obtained with high-volume LR after 2 hours.
KRF quickly and steadily increased MAP from immediate post-hemorrhage values and although heart function was also improved, the increases in cardiac index, stroke volume or heart rate were not equivalent in amplitude to the increase in pressure. An increase in circulating volume usually restores cardiac function to a greater extent than it does blood pressure. This finding might suggest that KRF has some other mechanism to raise pressure while not significantly improving cardiac contractility, aside from restoration of intravascular volume. This observation is counter-intuitive because in previous research, fractions of keratin different from those used in KRF induced significant vasodilation and did not increase vascular resistance (Nunez et al. Citation2011). It is difficult to determine from these data why KRF specifically increases blood pressure better than cardiac index or why it recovers cardiac index to a better extent than it does stroke volume or heart rate. Perhaps the additive effect of moderate increases in stroke volume and heart rate sums up to the effect seen in cardiac index. Specific pharmacodynamic studies would be needed to determine specific receptor interactions to further explain this scenario. Cardiac index was slightly improved when KRF was given in lower volumes versus higher volumes (13.9 L/min/m2 vs 13.2 L/min/m2 at 2 hours), but the difference was not statistically significant. During low volume resuscitation with KRF, blood pressure increased more quickly and was maintained at a higher level than with 1X volume resuscitation using KRF. No significant difference was found (p = 0.06). Although no explanation for these phenomena can be found in these data, it is possible that higher doses (volumes) of intravenous keratin proteins could blunt cardiac dynamics and diminish its advantages seen with low volume replacement. Further research is necessary to delineate the mediators/mechanisms of hemodynamic effects of KRF over a range of volumes.
During 1X volume resuscitation, KRF did not improve cardiac index as well as LR. However, LR caused a profound dilution of hematocrit such that estimated oxygen delivery was equivalent in both treatments. This phenomenon was also reflected by the fact that blood gases, direct markers of tissue perfusion and multi-organ function, did not differ with either treatment.
The rationale behind low volume resuscitation is to restore hemodynamics just enough to provide tissue perfusion to vital organs while diminishing complications from resulting dilution, including dilution of clotting factors and exacerbation of bleeding. Low volume resuscitation with KRF might not be as beneficial when compared to 3X volume LR in the short term, but this comparison is irrelevant if the most important long-term outcomes are achieved. Long-term assessment of resuscitation provides information on the course of hospitalization and some of this information can be extrapolated to assess how recovery, discharge and return to normal activities might happen. This experiment provided important information on survival as well as effectiveness of the treatments.
The results of long-term assessment demonstrated that low volume KRF provides sufficient cardiovascular support to allow the system to compensate for hemorrhage and recover from a loss of 40% of estimated blood volume. The fact that the KRF group in this set of experiments had higher stroke volume and lower heart rate than the LR group suggests that the animals in the KRF group were recovering more completely. When compared to healthy controls, the LR rats show equal cardiac index but at the expense of a higher heart rate (). During periods of hypovolemia, energy conservation becomes essential to survival. Therefore, the hemodynamic changes observed in the LR group could exacerbate myocardial oxygen deficits and trigger heart failure.
For the short-term assessments, low-volume KRF did not restore ACV to the same degree that 1X volume KRF did (p = 0.04). However, for the long-term assessments 1X KRF actually restored ACV and CBV to slightly higher levels than high-volume LR. This effect on ACV suggests two things: 1) overly aggressive volume expansion might not be necessary for proper recovery after hypovolemia, and 2) KRF has a longer lasting effect than previously anticipated. This finding is supported by the observation that seven days after treatment, the KRF group showed higher ACV than healthy controls. ACV provides proper preload and cardiac output, thus preventing the required tachycardia to maintain adequate heart function that is seen during hypovolemia. This improved cardiac function provides efficient oxygen delivery and allows the restoration of normal myocardial oxygen balance based on our calculations.
A 3X volume of LR would be expected to contribute to an exacerbation of active bleeding due to dilution of clotting factors (Young Citation2011). However, under the conditions of a controlled hemorrhage the deleterious effect of dilutional coagulopathy might not be observed. Several studies state that colloids restore cardiac function better than some crystalloids but survival is not improved (Exo et al. Citation2009). These observations, along with the higher costs of colloids are the main reasons why crystalloids continue to be employed as the primary resuscitation fluid in civilian emergency departments.
Conclusions
Our studies evaluated a novel keratin colloid that allows easy manipulation for improved viscosity and osmotic parameters. Keratins are relatively easy to isolate and purify from human hair fibers and KRF does not cause electrolyte disturbances (). During hematocrit measurements, visual inspection of supernatant plasma did not exhibit pink/red hue or unexpected changes in hematocrit, both of which indicate hemolysis. In addition, KRF is not toxic and does not stimulate chronic inflammatory reactions such as those frequently elicited by some synthetic polymers (Alexander et al. Citation1996).
Our results are somewhat expected given that most colloids restore hemodynamic parameters with lower volumes than crystalloids. A real advantage over other colloids would be appropriate to analyze in subsequent studies, especially regarding cost and side effects, which are colloids main downfall. However, given the current scarce use of colloids in civilian hospitals, it might not be a relevant comparison until albumin or other colloids are adopted as first line fluids for resuscitation.
Human-hair keratin is an inexpensive source of material to produce a colloid fluid. We believe cost advantage over most expensive colloids used today paired with a low side effect profile will expand keratin-derived resuscitation fluids indications greatly to cover emergency room, intraoperative and intensive care unit resuscitation. In this study KRF showed several statistically improved cardiac outcomes, while tissue perfusion was presumably maintained as histological finding showed no signs of cell or tissue damage due to its use.
Although our results suggest that there is a measurable benefit to low-volume KRF resuscitation, life-threatening hemorrhage might benefit even further from additional fluid infusion. Further studies should be designed to develop a more severe form of hemorrhage to determine: (1) the most efficient volumes of KRF for resuscitation following life-threatening blood loss, (2) the difference in mortality between low-volume and full-volume KRF resuscitation, and (3) whether a combined KRF/crystalloid protocol for resuscitation is beneficial. Moreover, an active bleeding protocol should be designed to contrast the behavior of both KRF and LR in a realistic trauma scenario.
Declaration of interest
Author Mark van Dyke holds stock and is an officer in the company, KeraNetics LLC. Author Luke Burnett is currently an employee of KeraNetics LLC. Wake Forest School of Medicine has a potential financial interest in KeraNetics through licensing agreements. Drs. Van Dyke and Burnett have a potential conflict of interest.
Funding Sources
Partial funding for this project was provided by KeraNetics LLC and NIH/NHLBI (grant no. 1R43HL099010-01A1).
References
- Alexander H, Brunski JB, Cooper SL; Hench LL, Hergenrother RW, Hoffman AS, et al. 1996. Classes of materials used in medicine. In: Ratner BD, AS Hoffman; Schoen FJ, Lemons JE, Eds. Biomaterials Science: An Introduction to Materials in Medicine, 1st ed. Orlando, FL: Elsevier Science, pp. 37–132.
- Cabanas JG, Manning JE, Cairns CB, 2011. Fluid and blood resuscitation. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, Eds. Tintinalli’s Emergency Medicine: A Comprehensive Guide, 7th ed. New York, NY: The McGraw-Hill Companies, Inc., http://www.accessmedicine.com.go.libproxy.wfubmc.edu/content.aspx?aID=6358094. Accessed November 29, 2012.
- Dahn MS, Lucas CE, Ledgerwood AM, Higgins RF. 1979. Negative inotropic effect of albumin resuscitation for shock. Surgery. 86:235–241.
- de Boode WP, van Heijst AF, Hopman JC, Tanke RB, van der Hoeven HG, Liem KD. 2010. Cardiac output measurement using an ultrasound dilution method: a validation study in ventilated piglets. Pediatr Crit Care Med. 11:103–108.
- de Guzman RC, Merrill MR, Richter JR, Hamzi RI, Greengauz-Roberts OK, Van Dyke ME. 2011. Mechanical and biological properties of keratose biomaterials. Biomaterials. 32:8205–8217.
- Exo JL, Shellington DK, Bayir H, Vagni VA, Janesco-Feldman K, Ma L, et al. 2009. Resuscitation of traumatic brain injury and hemorrhagic shock with polynitroxylated albumin, hextend, hypertonic saline, and lactated Ringer's: Effects on acute hemodynamics, survival, and neuronal death in mice. J Neurotrauma. 26:2403–2408.
- Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. 2004. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 350:2247–2256.
- Gan TJ, Bennett-Guerrero E, Phillips-Bute B, Wakeling H, Moskowitz DM, Olufolabi Y, et al. 1999. Hextend, a physiologically balanced plasma expander for large volume use in major surgery: a randomized phase III clinical trial. Hextend Study Group. Anesth Analg. 88: 992–998.
- Gilpin DA. 1996. Calculation of a new Meeh constant and experimental determination of burn size. Burns. 22:607–611.
- Heidary B, Bell N, Ngai JT, Simons RK, Chipperfield K, Hameed SM. 2012. Temporal trends in the treatment of severe traumatic hemorrhage. Am J Surg. 203:568–573.
- Hess JR, Holcomb JB, Hoyt DB. 2006. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion.[Editorial]. 46:685–686.
- Holcomb JB. 2007. Damage control resuscitation. J Trauma.[Review]. 62:S36–S37.
- Innerhofer P, Fries D, Margreiter J, Klingler A, Kuhbacher G, Wachter B, et al. 2002. The effects of perioperatively administered colloids and crystalloids on primary platelet-mediated hemostasis and clot formation. Anesth Analg. 95:858–865.
- Kheirabadi BS, Crissey JM, Deguzman R, Perez MR, Cox AB, Dubick MA, Holcomb JB. 2008. Effects of synthetic versus natural colloid resuscitation on inducing dilutional coagulopathy and increasing hemorrhage in rabbits. J Trauma. 64:1218–1228; discussion 28–29.
- Kovalik SG, Ledgerwood AM, Lucas CE, Higgins RF. 1981. The cardiac effect of altered calcium homeostasis after albumin resuscitation. J Trauma. 21:275–279.
- Krausz MM. 1995. Controversies in shock research: hypertonic resuscitation–pros and cons. Shock. 3:69–72.
- Levitzky MG. 2007. The Transport of Oxygen & Carbon Dioxide in the Blood.Pulmonary Physiology, 7th ed. New York: McGraw-Hill.
- Liang L, Xu G, Zhang Y, Chen W, Li J, Liang T. 2010. Resuscitation with hydroxyethyl starch solution prevents bone marrow mononuclear apoptosis in a rat trauma-hemorrhagic shock model. J Trauma. 68:655–661.
- Nunez F, Trach S, Burnett L, Handa R, van Dyke M, Callahan M, Smith T. 2011. Vasoactive properties of keratin-derived compounds. Microcirculation. 18:663–669.
- Secher NH, Van Lieshout JJ. 2005. Normovolaemia defined by central blood volume and venous oxygen saturation. Clin Exp Pharmacol Physiol. 32:901–910.
- Stump DC, Strauss RG, Henriksen RA, Petersen RE, Saunders R. 1985. Effects of hydroxyethyl starch on blood coagulation, particularly factor VIII. Transfusion. 25:349–354.
- Szul AC, Davis LB, Maston BG, Wise D, Sparacino LR, Shull J. 2004. Shock and Resuscitation. In: Szul AC, Davis LB, Eds. Emergency War Surgery, 3rd ed. Washington DC: Borden Institute, Walter Reed Army Medical Center, Department of Defense, pp. 7.1-7.12.
- Weaver DW, Ledgerwood AM, Lucas CE, Higgins R, Bouwman DL, Johnson SD. 1978. Pulmonary effects of albumin resuscitation for severe hypovolemic shock. Arch Surg. 113:387–392.
- Wilkins PA, Gleed RD, Krivitski NM, Dobson A. 2001. Extravascular lung water in the exercising horse. J Appl Physiol. 91:2442–2450.
- Young WF. 2011. Shock. In: Stone C, Humphries R, Eds. Current Diagnosis & Treatment: Emergency Treatment, 6th ed. New York: McGraw-Hill.