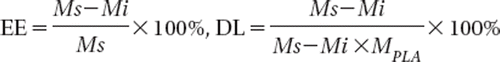Abstract
The oxaliplatin nanoparticles were prepared with polylactic acid matrix, orthogonal test was applied to optimize the prescriptions, and the qualities of oxaliplatin nanoparticles were characterized by the shape, particle size, encapsulation efficiency (EE), and drug loading (DL). Oxaliplatin nanoparticle was prepared by solution replacement method. The formation of 0.25% Tween80, DMF-water 1:8 (v/v), oxaliplatin-polylactic acid 1:5 (w/w), and 20 mg/ml polylactic acid showed the suitable EE (17.4 ± 0.47%), DL (3.52 ± 0.07%). We observed the shape of oxaliplatin nanoparticles through SEM. The average size of the particles was 120.5 ± 8.7 nm, which was detected by N5 submicron particle size analyzer.
Introduction
Oxaliplatin (OP) is a typical antitumor agent of the third generation platinum-based drug which has a broad anticancer spectrum. OP can inhibit the DNA replication and transcription through the formation of alkylation and the cross links between the inter-strand and intra-strand of DNA (Graham et al. Citation2004). OP is mainly applied to cure the colorectal cancer patients with metastasis and hepatocarcinoma. However, its side effects such as blood system toxicity, nausea, vomit, diarrhea, and peripheral neuritis, limit its clinical applications.
The purpose of the preparation of OP nanoparticles is to prolong the elimination time of OP and enhance the concentration of OP in the tumor with a reduction of toxicity in other healthy organs. Nanoparticle drug delivery system (NDDS) usually consists of drug and carrier material with a particle size ranging from 10 to 1000 nm (Mansour et al. Citation2009), which can prevent the premature degradation of the drug in the physiological environment. Nanoparticles have some special “size effect” (Mahapatro and Singh Citation2011), which could enhance the interaction of nanoparticles and physiological membrane proteins. The newly formed tumor vessels usually have abnormal architecture and form, which are poorly-aligned defective endothelial cells with wide fenestrations, lacking a smooth muscle layer, or innervation with a wider lumen, and lack of effective lymphatic return. Consequently, biodegradable material nanoparticles are easier to pass through the vessel and they attain a concentration that is higher in the tumor tissues than in other normal tissues; this effect is referred to as enhanced permeability and retention (EPR) (Matsumura and Maeda Citation1986, Vasey et al. Citation1999).
Polylactic acid (PLA) is a synthetic biodegradable and biocompatible polymer. PLA is metabolized to CO2 and H2 in physiological environment. For its safety and biocompatibility, PLA has been approved by the FDA for application in tissue engineering, medical material, and drug carrier (Lee et al. Citation2006). Solvent evaporation, solvent displacement, salting out, and solvent diffusion methods have been mostly applied to prepare PLA nanoparticles (Yang et al. Citation2003, Lu et al. Citation2006). OP is insoluble in most of the organic solvents except dimethyl formamide (DMF). Removing organic solvent in the nanoparticle solution by evaporation cannot be achieved due to the high boiling point of DMF; the salting out method is not suitable for the preparation of small molecular compound nanoparticles but suitable for the macromolecular bioactivator nanoparticles. Therefore, the solvent displacement method is used for the preparation of OP nanoparticles.
Materials
Reagents
Oxaliplatin (98.5%), Qilu pharmaceutical co., Ltd; PLA (Mw 20,000), Shandong institute of medical devices; Tween 80, Tianjin Fu Xing chemical industry research institute; PEG(pol-yethyleneglycol)-400, Tianjin Xing Hong Da chemical co., Ltd; Poloxamer, BASF.
Instruments
Magnetic stirrers (RCT), IKA; Electronic balance (BSl24S), Mettler Toledo; high speed emulsion shear machine, Shanghai Fluke company; dynamic laser particle spectrometers (LB550), HORIBA; scanning electron microscope (JSM-6490LA), JEOL; HPLC(LC-20), Shimadzu.
Methods and results
Preparation of OP nanoparticles
The organic phase was DMF solution of PLA and OP, the aqueous phase was 30 ml water solution of surfactant. Add the organic phase into the aqueous phase slowly under the high-speed shearing to get the OP nanoparticle suspension. Transfer the nanoparticle suspension into the bag filter (semi-permeable cellulose membrane with a cutoff of 8,000–10,000 Daltons), then dialyze against 500ml water to remove DMF.
HPLC assay
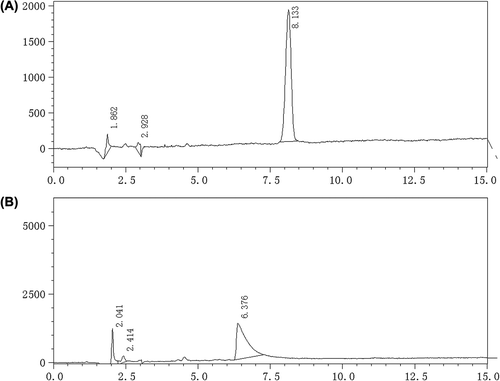
The content of OP was determined by HPLC. Chromatograph conditions: ODS-C18 column, 250 × 4.6 mm, 5 μm; mobile phase: methanol-water(5:95); detection wavelength: 250 nm; flow rate: 1 ml/min; Injection volume: 20 μl ().
The concentration of OP ranged from 3.75 to 60 μg/ml and had a good linearity, Y = 7240.6C-3395.7, R2 = 1.0000 (Y, peak area, C, OP concentration).
Precision tests
Injected 20 μl OP standard solution into chromatography 5 times in 1 d, then injected 20 μl OP standard solution into chromatography everyday in 5 days, the inner-day precision RSD was 0.78% and the intra-day precision RSD was 1.23%.
Recovery tests
Dissolved OP in blank dialysate to obtain 12.50 mg/ml OP solution, then transferred 250, 500, 1000 μl into 50 ml flask, diluted with nanoparticle dialysate to obtain recovery sample solutions. Measured the OP content by HPLC, is the recovery results of the method.
Table I. Results of the recovery tests.
Measurements of entrapment efficiency and drug loading
The EE of OP nanoparticle was determined using the dialysis technique for separating the non-entrapped OP from nanoparticles. Added 30 ml nanoparticles suspension into the bag filter to dialyze against 500 ml water for 210 min, then the concentration of OP in the dialysis solution was measured by HPLC. The entrapment efficiency (EE) and drug loading (DL) were calculated according to the following equations.
Where Ms is the total amount of OP that was added, and Mi is the amount of OP in the dialysis solution, MPLA is the total amount of PLA that was added.
The preparation optimization of the OP nanoparticles
The EE, DL, and particle size were the indicators of the optimization study on the preparation of OP nanoparticles. The factors, including surfactant concentrations, OP concentrations, the shear velocity, DMF-water volume ratio, OP-PLA weight ratio, temperature, pH and PLA concentrations, were considered to optimize the main influencing factors which affect the preparation of OP nanoparticles. Integrated the results of the single factor investigations, the factor A (surfactant concentration), factor B (PLA concentration), factor C (DMF-water volume ratio), and factor D (OP-PLA weight ratio) were selected as the principal factors of the orthogonal tests for the preparation of OP nanoparticles ( and ); each factor has three levels to prepare OP nanoparticles at pH6.8 and 25°C.
Table II. Factors and levels of orthogonal tests.
Table III. Arrangement and results of orthogonal tests according to L9(34).
When the EE and DL of nanoparticle were treated as the indictors, the intensity orders of the factors which affect the nanoparticle were both D> A> B> C, therefore the DL statistics values alone were considered. However, the intensity order was D> C = B> A when the particle size was treated as the indictor. Factor A1 could lead to the higher DL and smaller particle size. Factor B3 could lead to the highest DL and the smaller particle size. Factor C3 could lead to the smallest particle size and the smallest quantity of DMF is easy to be removed; Factor C3 was the optimal option and Factor D1 could enhance the DL with the larger particle sizewhile factor D3 could reduce the particle size with the lower DL at the same time; considering the balance between DL and particle size, factor D2 was the optimal option. The result of optimal combination was A1B3C3D2, which were 0.25% Tween80, 20mg/ml PLA, 1:8 (DMF-water volume ratio), and 1:5 (OP-PLA weight ratio).
Evaluations of OP nanoparticles
We prepared 3 batches of OP nanoparticles (No.12081201, 12081202, and 12081203) according to the optimal conditions and determined their shapes, particle size, EE, and DL.
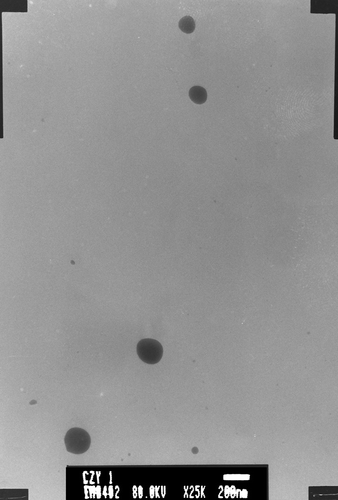
OP nanoparticles suspension was diluted with 5 times volume of distilled water and dropped in a microscopic copper grid, negatively stained with 0.03% phosphotungstic acid (PTA) and dried, and then the stained sample was observed through TEM. The micrographs showed that the nanoparticles were round or nearly round with an estimated diameter of 110 nm ().
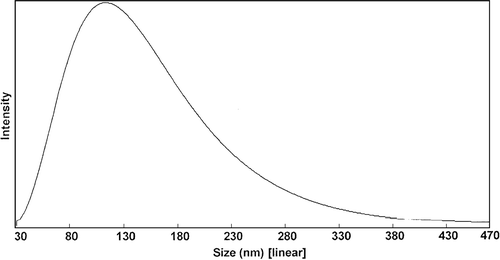
1 ml OP nanoparticles suspension was diluted with 2 ml distilled water and determined by N5 submicron particle size analyzer. The average particle size of 3 batches was 120 ± 42 nm (, ).
Table IV. The result of EE, DL and particle size (![]() x ± s, n = 3).
x ± s, n = 3).
Discussions
When using PEG-400 as surfactant for the preparation of nanoparticles, the transparency of colloidal solution was better, but the DL and EE were lower than when Tween80 or Poloxamer188 was used as surfactant. The DL and EE had no significant difference when Tween80 or Poloxamer188 was used as surfactant to prepare drug-loaded nanoparticles. A single factor study showed that the surfactant concentration was 0.25%∼4%, the DL and EE of the nanoparticles had a downward trend when the concentration of Tween80 was decreased, but if the concentration was too low, the stability of nanoparticles will be influenced (Liu et al. Citation2003). As the surfactant with high concentration has the potential risk of hemolysis, the concentration of orthogonal experiment was set from 0.25% to 1%.
When the ratio of DMF to aqueous phase was decreased, the DL and EE increased accordingly. If the volume of DMF was too small, the drug content of the nanoparticles may be decreased, which may lead to an excessive drug dosage; but if the volume of DMF was too large, the organic solvent content in nanoparticles may increase, which was not easy to be removed; therefore, the ratio of DMF to water was set from 1:8 to 1:3. Changing pH had no significant effect on the formation of nanoparticle, which was inconsistent with that reported in literature (Feng and Huang Citation2001). This may be because the dissociation of OP in this range has no significant difference.
Three methods are usually used to determine entrapment efficiency of nanoparticles; centrifugation, dialysis, and gel column chromatography. Because of the high boiling point of DMF, it is unfavorable to remove it by evaporation, this experiment removed DMF in nanoparticles suspension by dialysis method to achieve good separation of DMF from the nanoparticles. The method is simple, with good reproducibility, and can accurately determine the EE and DL of nanoparticles.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the 2010 Foundation of Shandong Province Plan of Science and Technology, 2010GWZ20202.
References
- Feng SS, Huang GF. 2001. Effects of emulsifiers on the controlled release of paclitaxel (Taxo1) from nanospheres of biodegradable polymers. J Control Release. 71:53–69.
- Graham J, Mushin M, Kirkpatrick P. 2004. Oxaliplatin. Nat Rev Drug Discov. 3:11–12.
- Lee WC, Li YC, Chu IM. 2006. Amphiphilic poly(D,L-lactic acid)/poly(ethylene glycol)/poly(D,L-lactic acid) nanogels for controlled release of hydrophobic drugs. Macromol Biosci. 6: 846–854.
- Liu MX, Ma L, Liu YQ, Zh Q. 2003. Preparation and characterization of biodegradable poly (lactic acid) nanoparticles. Chem World. 44:78–80.
- Lu HX, Xu CJ, Li B, Kang Y, Huang Q, Li LM, Chen QH. 2006. The inhibitory effect of paclitaxel nanoparticles on ovarian cancer xenografts and lymphatic targeting. Beijing Da Xue Xue Bao. 38:483–486.
- Mahapatro A, Singh DK. 2011. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 9:55.
- Mansour HM, Rhee YS, Wu X. 2009. Nanomedicine in pulmonary delivery. Int J Nanomedicine. 4:299–319.
- Matsumura Y, Maeda H. 1986. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46:6387–6392.
- Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, et al. 1999. Phase I clinical and pharmacokinetic study of PK1[N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin Cancer Res. 5:83–94.
- Yang K, Wen Y, Wang C. 2003. The study of cucurbitacin BE polylactic acid nanoparticles delivering cucurbitacin BE to metastasized cervical lymph nodes in mice with oral cancer. Hua Xi Kou Qiang Yi Xue Za Zhi. 21:477–480.