Abstract
In this study, 4’-(phenylurenyl/thiourenyl)chalcones (14–25) were prepared from 4’-(phenylurenyl/thiourenyl)acetophenones and benzaldehyde derivatives by Claisen-Schmidt condensation. In vitro inhibition effects of chalcone derivatives on purified carbonic anhydrase I and carbonic anhydrase II were investigated by using the CO2 hydration method of Maren. The result showed that all the synthesized compounds inhibited the CA isoenzymes activity. 18 and 19 were found to be most active (IC50 = 25.41 μM and 23.06 μM) for hCA I, respectively. For hCA II, 24 is the most active compound (IC50 = 14.40 μM).
Introduction
Glaucoma is an eye disease in which the optic nerve is damaged related to different risk factors and it can permanently damage vision in the affected eye(s). Elevated intraocular pressure (IOP) is known as the major risk factor in this disease. Therefore, targeting IOP with different pressure lowering agents is the main treatment strategy for this disease (Siesky et al. Citation2009).
Because IOP depends on the delicate balance between production of aqueous humor by the ciliary body and its drainage through the various pathways, selective activation of the carbonic anhydrase isoenzymes can increase IOP by facilitating aqueous formation and transport through cell membranes, as well as its release into the posterior chamber (Steele et al. Citation2009).
Together most of CA isoenzymes are related to treatment of glaucoma, they also involved in these processes which are important therapeutic targets with the potential to be inhibited to treat a range of disorders including edema, obesity, cancer, epilepsy and osteoporosis (Ashida and Brey Citation1995, Vullo et al. Citation2003, Nishimori Citation2004, Lehtonen et al. Citation2004, Vullo et al. Citation2005a, Citation2005b, Nishimori et al. Citation2005a, Citation2005b). Given the physiological importance of the CA, the metabolic impact of chemicals for crop production should receive greater study.
With the involvement of the carbonic anhydrase (CA) enzyme family catalyzing the physiological hydration of CO2 to yield bicarbonate and a proton in many physiological/pathological processes, development of specific inhibitors has been achieved for clinical application (Thiry et al. Citation2006, Supuran Citation2008).
The active site of most CAs contains a zinc ion (Zn2+), which is essential for catalysis. To inhibit this enzyme, it can be interacted with different electron donor agent such as coumarin derivatives (Karatas et al. Citation2012), a series anabolic compounds (Gencer et al. Citation2012a, Citation2012b), some macrocyclic thiocrown ethers (Cicek et al. Citation2012), some snalgesic drugs (Gokce et al. Citation2012), some oral contraceptives (Kıranoglu et al. Citation2007) and pesticides (Gencer et al. Citation2012a, Citation2012b).
As these compounds, chalcones (1,3-diaryl-2-propen-1-ones) have been studied widely for their interesting pharmacological properties (Di Carlo et al. Citation1999) and synthetic importances (Trivedi et al. Citation2008, Gezegen et al. Citation2010, Piotrowska et al. Citation2011) for a long time. In this context, a number of chalcone derivatives containing aromatic rings and unsaturated chain, which is responsible for many biological activities such as antitumoral (Cabrera et al. Citation2007) anticancer and antioxidant (Anto et al. Citation1995), chemoprotective (Forejtnikova et al. Citation2005), antifungal (Lahtchev et al. Citation2008), antinociceptic (Santos et al. Citation2008) and antimicrobial (Karaman et al. Citation2010) activities have been found to inhibit several important enzymes in cellular systems, including xanthine oxidase (Sogawa et al. Citation1994), aldose reductase (Iwata et al. Citation1999), heme oxygenase (Lee et al. Citation2006), protein tyrosine kinase (Yang et al. Citation2001, Nerya et al. Citation2004) and quinone reductase (Miranda et al. Citation2000).
On the other hand it has been reported that some urea derivatives have antiviral (Huang et al. Citation2004), antiinsecticide (Bassett Citation2004), antitumor (Sun et al. Citation2010) and tyrosine kinase inhibitor (Engen et al. Citation2010) activities. Especially, reactions of 4-aminochalcones with isocyanates give unsymmetrically substituted urea derivatives which are linked to a series of biological activities including antiglycating (Khan et al. Citation2009), MCH-R1 antagonists (Galiano et al. Citation2007), P2Y1 receptor antagonists (Thalji et al. Citation2010) and heparanase inhibitors (Pan et al. Citation2006) properties.
Therefore, the investigation of clinically useful chalcones and ureas/thioureas is a growing field of interest. In this study, we describe a number of chalcones containing phenylurea/thiourea groups and study their properties as inhibitors of hCA I and hCA II purified from human erythrocytes.
Materials and methods
Materials
Sepharose 4B, L-tyrosine, sulphanilamide, synthetic starting material, reagents and solvents were of analytical grade and were purchased from Aldrich Chemical Co., Merck Chemical Co. 1-(4-acetylphenyl)-3-phenylureas (6–9) and chalcone derivatives (14–25) were synthesized according to the literature (Dominguez et al. Citation2005, Sönmez et al. Citation2011). All other chemicals used were of analytical grade and obtained from either Sigma or Merck.
General procedure for preparation of 6-9
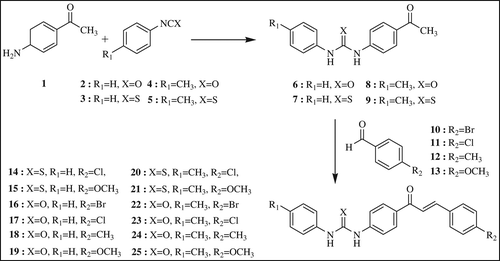
A mixture of the 4’-aminoacetophenone 1 (10 mmol) and phenylisocyanate or phenylisothiocyanate derivative 2–5 (10 mmol) was dissolved in dry toluene (20 ml). After the mixture was refluxed overnight, dry toluene was added until resulting solid was dissolved, and recrystallization afforded the desired 1-(4-acetylphenyl)-3-phenylurea and thiourea derivatives in pure form. Synthesis of phenylurea/phenylthiourea-substituted chalcone derivatives were prepared according to .
General procedure for preparation of 14–25
Phenylurea or phenylthiourea substituted chalcones 14–25 were synthesized by reacting equimolecular quantities of 1-(4-acetylphenyl)-3-phenylurea or thiourea derivatives 6-9 and the corresponding benzaldehyde derivatives 10–13 in the presence of an excess sodium hydroxide (2.5 mmol) in dry methanol (10 ml) and DMSO (10 ml). After the mixture was stirred overnight at room temperature, cold brine (30 ml) was added to this solution and resulting precipitate was filtered and dried in air. The precipitate was recrystallized from appropriate solvent to give phenylurea or phenylthiourea substituted chalcones 14–25 in pure form.
Preparation of hemolysate and purification from blood red cells
Blood samples (25 ml) were taken from healthy human volunteers. They were anticoagulated with ACD (acid- citrate-dextrose), centrifuged at 2000 × g for 20 min at 4˚C and the supernatant was removed. The packed erythrocytes were washed three times with 0.9% NaCI and then hemolysed in cold water. The ghosts and any intact cells were removed by centrifugation at 2000 × g for 25 min at 4˚C, and the pH of the hemolysate was adjusted to pH 8.5 with solid Tris-base. The 25 ml hemolysate was applied to an affinity column containing L-tyrosine-Sulphanilamide -Sepharose-4B (Arslan et al. Citation1996) equilibrated with 25 mM Tris–HCl/0.1M Na2SO4 (pH 8.5). The affinity gel was washed with 50 ml of 25 mM Tris–HCl/22 mM Na2SO4 (pH 8.5). The human CA (hCA) isozymes were then eluted with 0.1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6), which recovered hCA-I and hCA-II, respectively. Fractions of 3 mL were collected and their absorbance measured at 280 nm.
CA enzyme assay
Carbonic anhydrase activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 hydration (Maren Citation1960). The assay solution was 0.5 M Na2CO3/0.1 M NaHCO3 (pH 10.0) and Phenol Red was added as the pH indicator. CO2-hydratase activity (enzyme units (EU)) was calculated by using the equation t0-tc/tc where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
In vitro inhibition studies
For the inhibition studies of chalcone different concentrations of these compounds were added to the enzyme. Activity % values of carbonic anhydrase for different concentrations of each chalcone were determined by regression analysis using Microsoft Office 2000 Excel. Carbonic anhydrase enzyme activity without a chalcone solution was accepted as 100% activity.
Results and discussion
All chalcone derivatives (14–25) containing phenylurea/ thiourea groups were synthesized from the appropriate benzaldehydes (l0–13) and acetophenone derivatives (6–9) which were obtained by reaction between 4'aminoacetophenone (1) and isocyanates/isothiocyanates (2–5) according to literature procedures (Dominguez et al. Citation2005, Sönmez et al. Citation2011).
In this study, we examined the effects of chalcone derivatives (14–25) on hCA I and hCA II. The result showed that all the synthesized compounds inhibited the CA isoenzymes activity. Furthermore, phenylureny/phenylthiourenychalcones containing electron-donating groups generally inhibited hCA I and hCA II isozymes at low IC50 values. The IC50 values of (14–25) analogues against hCA I and II were summarized in . Especially 24 inhibited both enzymes with 27.93 μM and 14.40 μM IC50 values. Besides 18 and 19 showed remarkable inhibition effect on hCA I at low IC50, 19 and 25 had inhibition effect at 19.44 and 17.59 μM IC50 values on hCA II.
Table I. The IC50 values of chalcone derivatives (14–25).
CA inhibitors lower intraocular pressure by reducing bicarbonate formation in the ciliary process, thus lowering Na+ transport and flow of aqueous humor: this is the basis for their use in glaucoma treatment. Unfortunately, systemic therapy with parenteral sulphonamides and their derivatives leads to significant side effects, many of them being probably due to inhibition of CA isoforms in other tissues. Acetazolamide is the most widely used inhibitor and has advantages over the others because it is 20 times less active against CAI than against CAII in erythrocytes. But the inhibition of various CA isoforms which present in tissues other than eye leads to an entire range of side effects, the most prominent being numbness and tingling of extremities, metallic taste, depression, fatigue, malaise, weight loss, decreased libido, gastrointestinal irritation, metabolic acidosis, renal calculi and transient myopia (Maren Citation1960, Arslan et al. Citation1997, Supuran and Scozzafava Citation2000). For similar reasons, designing of new drugs is essential for clinical application of treatment of glaucoma.
In summary, enzyme inhibition is more important issue for drug design and biochemical applications (Aydemir and Kavrayan Citation2009, Demir et al. Citation2012, Bytyqi-Damoni et al. Citation2012, Demirel and Tarhan Citation2004, Senturk et al. Citation2012). The results showed that new chalcone derivatives inhibited the hCA I and II enzyme activity. Therefore, our results suggested that phenylurenyl/thiourenyl chalcone derivatives are likely to be adopted as candidates to treat glaucoma and may be taken for further evaluation in in vivo studies.
Notice of Correction
This paper published online on 18 January 2013 contained an error in the list of references. The following references were written incorrectly as follows:
- Çiçek B, Ergün A, Gençer N. 2012. Synthesis and evaluation in vitro effects of some macro cyclic thiocrown ethers on erythrocyte carbonic anhydrase I and II. Asian J Chem. 24:3729–3731.
- Demir D, Gençer N, Er A. 2012. Purification and characterization of prophenoloxidase from Galleria mellonellaL. Artif Cells Blood Substit Immobil Biotechnol. 40:391–395.
- Gençer N, Ergün A, Demir D. 2012a. In vitro effects of some anabolic compounds on erythrocyte carbonic anhydrase I and II. J Enzyme Inhibition Med Chem. 27:208–210.
- Gençer N, Ergün A, Demir D. 2012b. In vitro effects of some pesticides on human erythrocyte carbonic anhydrase activity. Fresenius Environ Bull. 21:549–552.
- Gökçe B, Gençer N, Arslan O, Turkoglu SA, Alper M, Köçkar F. 2012. Evaluation of in vitro eff ects of some analgesic drugs on erythrocyte and recombinant carbonic anhydrase I and II. J Enzyme Inhib Med Chem. 27:37–42.
- Kıranoglu S, Sinan S, Gencer N, Köckar F, Arslan O. 2007. In vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biol Pharm Bull. 6:1048–1051.
These references should have been written as follows:
- Cicek B, Ergun A, Gencer N. 2012. Synthesis and evaluation in vitro effects of some macro cyclic thiocrown ethers on erythrocyte carbonic anhydrase I and II. Asian J Chem. 24:3729–3731.
- Demir D, Gencer N, Er A. 2012. Purification and characterization of prophenoloxidase from Galleria mellonella L. Artif Cells Blood Substit Immobil Biotechnol. 40:391–395.
- Gencer N, Ergun A, Demir D. 2012a. In vitro effects of some anabolic compounds on erythrocyte carbonic anhydrase I and II. J Enzyme Inhibition Med Chem. 27:208–210.
- Gencer N, Ergun A, Demir D. 2012b. In vitro effects of some pesticides on human erythrocyte carbonic anhydrase activity. Fresenius Environ Bull. 21:549–552.
- Gokce B, Gencer N, Arslan O, Turkoglu SA, Alper M, Kockar F. 2012. Evaluation of in vitro effects of some analgesic drugs on erythrocyte and recombinant carbonic anhydrase I and II. J Enzyme Inhib Med Chem. 27:37–42.
- Kıranoglu S, Sinan S, Gencer N, Kockar F, Arslan O. 2007. In vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biol Pharm Bull. 6:1048–1051.
The error has been corrected for this version.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anto RJ, Sukumaran K, Kuttan G, Rao MNA, Subbaraju V, Kuttan R. 1995. Anticancer and antioxidant activity of synthetic chalcones and related compounds. Cancer Lett. 97:33–37.
- Arslan O, Küfrevioglu OI, Nalbantoglu B. 1997. Synthesis and investigation of inhibition effects of new carbonic anhydrase inhibitors. Bioorg Med Chem. 3:515–518.
- Arslan O, Nalbantoglu B, Demir N, Ozdemir H, Küfrevioglu OI. 1996. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Turk J Med Sci. 26:163–166.
- Ashida M, Brey P. 1995. Role of the integument in insect defense - pro-phenol oxidase cascade in thecuticular matrix. Proc Natl Acad Sci USA. 92:10698–10702.
- Aydemir T, Kavrayan D. 2009. Purification and characterization of glutathione-S-transferase from chicken erythrocyte. Artif Cells Blood Substit Immobil Biotechnol. 37:92–100.
- Bassett JE. 2004. Role of urea in the postprandial urine concentration cycle of the insectivorous bat Antrozous pallidus. Comp Biochem Phys A. 137:271–284.
- Bytyqi-Damoni A, Genç H, Zengin M, Beyaztas S, Gençer N, Arslan O. 2012. In vitro effect of novel β-lactam compounds on xanthine oxidase enzyme activity. Artif Cells Blood Substit Immobil Biotechnol. 40:369–377.
- Cabrera M, Simoens M, Falchi G, Lavaggi L, Piro LE, Castellano EE, et al. 2007. Synthetic chalcones, lavanones, and flavones as antitumoral agents: Biological evaluation and structure–activity relationships. Bioorg Med Chem. 15:3356–3367.
- Cicek B, Ergun A, Gencer N. 2012. Synthesis and evaluation in vitro effects of some macro cyclic thiocrown ethers on erythrocyte carbonic anhydrase I and II. Asian J Chem. 24:3729–3731.
- Demir D, Gencer N, Er A. 2012. Purification and characterization of prophenoloxidase from Galleria mellonella L. Artif Cells Blood Substit Immobil Biotechnol. 40:391–395.
- Demirel LAB, Tarhan L. 2004. Dismutation properties of purified and GDA modified CuZnSOD from chicken heart. Artif Cells Blood Substit Immobil Biotechnol. 32:609–624.
- Di Carlo G, Mascolo N, Izzo AA, Capasso F. 1999. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 65: 337–353.
- Dominguez JN, Leon C, Rodrigues J, Dominguez NG, Gut J, Rosenthal PJ. 2005. Synthesis and Evaluation of new antimalarial phenylurenyl chalcone derivatives. J Med Chem. 48:3654–3658.
- Engen W, O’Brien TE, Kelly B, Do J, Rillera L, Stapleton LK, et al. 2010. Synthesis of aryl-heteroaryl ureas (AHUs) based on 4-aminoquinoline and their evaluation against the insulin-like growth factor receptor (IGF-1R). Bioorg Med Chem. 18:5995–6005.
- Forejtnikova H, Lunerova K, Kubinova R, Jankovska D, Marek R, Kares R, et al. 2005. Potentials of synthetic and natural Chemoprotective and toxic chalcones and dihydrochalcones in vitro. Toxicology. 208:81–93.
- Galiano S, Ceras J, Cirauqui N, Perez S, Juanenea L, Rivera G, et al. 2007. Novel series of substituted biphenylmethyl urea derivatives as MCH-R1 antagonists for the treatment of obesity. Bioorg Med Chem. 15:3896–3911.
- Gencer N, Ergun A, Demir D. 2012a. In vitro effects of some anabolic compounds on erythrocyte carbonic anhydrase I and II. J Enzyme Inhibition Med Chem. 27:208–210.
- Gencer N, Ergun A, Demir D. 2012b. In vitro effects of some pesticides on human erythrocyte carbonic anhydrase activity. Fresenius Environ Bull. 21:549–552.
- Gezegen H, Dingil A, Ceylan M. 2010. Three-step synthesis of 2, 4-diaryl-5,6,7,8-tetrahydroquinoline derivatives. J Heterocyclic Chem. 47:1017–1024.
- Gokce B, Gencer N, Arslan O, Turkoglu SA, Alper M, Kockar F. 2012. Evaluation of in vitro effects of some analgesic drugs on erythrocyte and recombinant carbonic anhydrase I and II. J Enzyme Inhib Med Chem. 27:37–42.
- Huang PP, Randolph JT, Klein LL, Vasavanonda S, Dekhtyar T, Stoll VS, Kempf DJ. 2004. Synthesis and antiviral activity of P10 arylsulfonamide azacyclic urea HIV protease inhibitors. Bioorg Med Chem Lett. 14:4075–4078.
- Iwata S, Nagata N, Omae A, Yamaguchi S, Okada Y, Shibata S, Okuyama T. 1999. Inhibitory effect of chalcone derivatives on recombinant human aldose reductase. Biol Pharm Bull, 22, 323–5.
- Karaman I, Gezegen H, Gürdere BM, Dingil A, Ceylan M. 2010. Screening of biological activities of a series of chalcone derivatives against human pathogenic microorganisms. Chem Biodivers. 7:400–408.
- Karatas MO, Alici B, Cakir U, Cetinkaya E, Demir D, Ergün A, et al. 2012. Synthesis and carbonic anhydrase inhibitory properties of novel coumarin derivatives.J Enzyme Inhibition Med Chem. Early Online: 1–6 (In press). doı:10.3109/14756366.2012.677838.
- Khan KM, Saeed S, Ali M, Gohar M, Zahid J, Khan A, et al. 2009. Unsymmetrically disubstituted urea derivatives: a potent class of antiglycating agents. Bioorg Med Chem. 17:2447–2451.
- Kiranoglu S, Sinan S, Gencer N, Kockar F, Arslan O. 2007. In vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biol Pharm Bull. 6:1048–1051.
- Lahtchev KL, Batovska DI, Parushev StP, Ubiyvovk VM, Sibirny AA. 2008. Antifungal activity of chalcones: a mechanistic study using various yeast strains. Eur J Med Chem. 43:2220–2228.
- Lee SH, Seo GS, Kim HS, Woo SW, Ko G, Sohn DH. 2006. 2’,4’,6’-Tris(methoxymethoxy)chalcone attenuates hepatic stellate cell proliferation by a heme oxygenase-dependent pathway. Biochem Pharmacol. 72:1322–1333.
- Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT, et al. 2004. Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem. 279:2719–2727.
- Maren TH. 1960. A simplified micromethod for the determination of carbonic anhydrase and its inhibitors. J Pharm Exp Ther. 130: 2629–2634.
- Miranda CL, Aponso GLM, Stevens JF, Deinzer ML, Buhler DR. 2000. Prenylated chalcones and flavanones as inducers of quinone reductase in mouse Hepa 1c1c7 cells. Cancer Lett. 149:21–29.
- Nerya O, Musa R, Khatib S, Tamir S, Vaya J. 2004. Chalcones as potent tyrosinase inhibitors: the effect of hydroxyl positions and numbers. Phytochemistry. 65:1389–1395.
- Nishimori I. 2004. Acatalytic CAs: carbonic anhydrase-related proteins. In: Supuran CT, Scozzafava, A, Conway J, Eds. Carbonic Anhydrase-Its Inhibitors and Activators. Boca Raton: CRC Press, pp. 25–43.
- Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Claudiu CT. 2005a. Carbonic anhydrase inhibitors. Carbonic anhydrase inhibitors: Inhibition of the transmembrane isozyme XIV with sulphonamides. Bioorg Med Chem Lett. 15:3828–3833.
- Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT. 2005b. Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. J Med Chem. 48:7860–7866.
- Pan W, Miao HO, Xu YJ, Navarro EC, Tonra JR, Corcoran E, et al. 2006. 1-[4-(1H-Benzoimidazol-2-yl)-phenyl]-3-[4-(1H-benzoimidazol- 2-yl)-phenyl]-urea derivatives as small molecule heparanase inhibitors. Bioorg Med Chem Lett. 16:409–412.
- Piotrowska DG, Cieslak M, Krolewska K, Wroblewski AE. 2011. Design, synthesis and cytotoxicity of a new series of isoxazolidines derived from substituted chalcones. Eur J Med Chem. 46:1382–1389.
- Santos L, Lima LA, Cechinel-Filho V, Correa R, Buzzi FC, Nunes RJ. 2008. Synthesis of new 1-phenyl-3-{4-[(2E)-3-phenylprop-2-enoyl]phenyl}-thiourea and urea derivatives with anti-nociceptive activity. Bioorg Med Chem. 16:8526–8534.
- Senturk M, Alici HA, Beydemir S, Kufrevioglu OI. 2012. In vitro and in vivo effects of some benzodiazepine drugs on human and rabbit erythrocyte carbonic anhydrase enzymes. J Enzyme Inhibition and Med Chem. 27:680–684.
- Siesky B, Harris A, Brizendine E, Marques C, Loh J, Mackey J, et al. 2009. Literature review and meta-analysis of topical carbonic anhydrase inhibitors and ocular blood flow. Surv Ophthalmol. 54:33–46.
- Sogawa S, Nihro Y, Ueda H, Miki T, Matsumoto H, Satoh T. 1994. Protective effects of hydroxychalcones on free radical-induced cell damage. Biol Pharm Bull. 17:251–256.
- Sönmez F, Sevmezler S, Atahan A, Ceylan M, Demir D, Gencer N, et al. 2011. Evaluation of new chalcone derivatives as polyphenol oxidase inhibitors. Bioorg Med Chem Lett. 21:7479–7482.
- Steele RM, Benedini F, Biondi S, Borghi V, Carzaniga L, Impagnatiello F, et al. 2009. Nitric oxide-donating carbonic anhydrase inhibitors for the treatment of open-angle glaucoma. Bioorg Med Chem Lett. 19:6565–6570.
- Sun M, Wu X, Chen J, Cai J, Cao M, Ji M. 2010. Design, synthesis, and in vitro antitumor evaluation of novel diarylureas derivatives. Eur J Med Chem. 45:2299–2306.
- Supuran CT, Scozzafava A. 2000. Carbonic anhydrase inhibitors and their therapeutic potential. Exp Opta Ther Patents. 10:575–579.
- Supuran CT. 2008. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov. 7:168–181.
- Thalji RK, Aiyar N, Davenport EA, Erhardt JA, Kallal LA, Morrow DM, et al. 2010. Benzofuran-substituted urea derivatives as novel P2Y1 receptor antagonists. Bioorg Med Chem. 20:4104–4107.
- Thiry A, Dogne JM, Masereel B, Supuran CT. 2006. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci. 27:566–573.
- Trivedi AR, Dodiya DK, Ravat NR, Shah VH. 2008. Synthesis and biological evaluation of some new pyrimidines via a novel chalcone series. ARKIVOC. 11:131–141.
- Vullo D, Franchi M, Gallori E, Pastorek J, Scozzafava A, Pastorekova S, Supuran CT. 2003. Carbonic anhydrase inhibitors: Inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulphonamides. Bioorg Med Chem Lett. 13:1005–1009.
- Vullo D, Innocenti A, Nishimori I, Pastorek J, Scozzafava A, Pastorekova S, Supuran CT. 2005a. Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides - a new target for the design of antitumor and antiglaucoma drugs?Bioorg Med Chem Lett. 15:963–969.
- Vullo D, Voipiob J, Innocenti A, Riverab C, Rankib H, Scozzafavaa A, et al. 2005b. Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isozyme VII with aromatic and heterocyclic sulphonamides. Bioorg Med Chem Lett. 15:971–976.
- Yang EB, Guo YJ, Zhang K, Chen YZ, Mack P. 2001. Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochim Biophys Acta. 1550:144–152.