Abstract
In this study, a Soluplus-coated colloidal silica nanomatrix (SCCSN) formulation for the entrapment of poorly water-soluble drugs was devised. The maximum supersaturation of the drug-loaded nanomatrix was higher than that of a physical mixture as indicated by the results of in vitro kinetic solubility studies. For atorvastatin calcium, dutasteride, and sorafenib tosylate, there were 2.8-, 326-, and 46.4-fold increases in solubility, respectively. For dutasteride, a promising 4.7-fold increase for in vivo oral drug absorption in the entrapped nanomatrix was observed as compared to the free physical mixture, supported by statistical significance testing of pharmacokinetic parameters.
Introduction
Nanomedicine seeks to offer nanotechnology-based solutions for medical problems, with the main aims of enhancing the bioavailability of drugs and reducing the side effects (Davis et al. Citation2008). Mesoporous silica nanoparticle–based drug delivery systems have been emerging as an active research area in recent years (Souris et al. Citation2010, Vivero-Escoto et al. Citation2010). In particular, it is interesting to note that there are some pharmaceutical additives that have nanostructures, such as colloidal silica (Aerosil and Sylysia) and magnesium aluminum silicate (Neusilin). Pharmaceutical additives can consist of either a non-ordered mesoporous network or nano-sized particles (Mamaeva et al. Citation2012). Drug adsorption or coating onto a nano-structured carrier with a large specific surface area is known to be a useful approach for improving the dissolution rate and absorption of poorly water-soluble drugs (Wang et al. Citation2006, Kinnari et al. Citation2011, Limnell et al. Citation2011, Miura et al. Citation2011, Zhang et al. Citation2012). In addition, the use of hydrophilic polymers (or surfactants) such as PEG 400 (Friedrich et al. Citation2006), pH-sensitive polymers (Jia et al. Citation2011), polyglycolized glycerides (Chauhan et al. Citation2005), and D-α-tocopherol polyethylene glycol 1000 succinate (van Eerdenbrugh et al. Citation2009) further enhances the biological performance of poorly water-soluble drugs.
In this study, a relatively new amphiphilic polymeric solubilizer, Soluplus, was applied as a solublizer. Soluplus is a graft copolymer comprising polyethylene glycol, polyvinyl acetate, and polyvinyl caprolactam (Nagy et al. Citation2012). Soluplus shows excellent solubilizing properties for biopharmaceutics classification system (BCS) class II substances (low solubility and high permeability) and offers the possibility of producing solid solutions by hot-melt extrusion (Linn et al. Citation2012). The objective of the present investigation was to fabricate a Soluplus-coated colloidal silica nanomatrix (SCCSN) in order to use Soluplus as a solubilizer for enhanced solubility and oral absorption of poorly water-soluble drugs. Atorvastatin calcium (a HMG-CoA reductase inhibitor), dutasteride (a 5-α reductase inhibitor), and sorafenib tosylate (an inhibitor of several tyrosine protein kinases) were used in this study as model hydrophobic drugs. The solubility of atorvastatin calcium (form I) in water is approximately 150 μg/mL (Kim et al. Citation2008). Dutasteride is insoluble in water, with a solubility below the quantitation limit of the assay (0.038 ng/mL) (Baek and Kim Citation2012). Sorafenib solubility in water is less than 25 ng/mL (Wang et al. Citation2011).
Materials and methods
Materials
Atorvastatin calcium (form I) was purchased from Zhejiang Jiangbei Pharmaceutical Co., Ltd, China (purity, 99.0%). Dutasteride was generously supplied by Dr. Reddy's laboratories Ltd., India (purity, 99.6%). Sorafenib tosylate (form I) was obtained from Nato Pharm Ltd., India (purity 99.7%). Soluplus was generously supplied by BASF, Germany. Aerosil 200 used as colloidal silica was procured from Degussa, Germany. HPLC grade organic solvents and analytical grade chemicals were used throughout this study.
Preparation of SCCSN
Various SCCSN encapsulating drugs were prepared using the rotary evaporation method based on a bottom-up approach (Jia et al. Citation2011, Zhang et al. Citation2012). The different compositions were prepared by dissolving each drug and Soluplus in methanol at 10 mg/mL concentration and then dispersing Aerosil 200 under stirring (drug/Soluplus/Aerosil 200 = 1:9:10 weight ratio). The methanol solvent was removed under reduced pressure in a rotary evaporator, and the resulting solid powder was pulverized. All samples were passed through a 100-mesh sieve and then stored in a desiccator at room temperature. For comparative purposes, physical mixtures of the non-entrapped drugs were also prepared by mixing the drug with excipients using a mortar and pestle until homogeneous mixtures were obtained.
Scanning electron microscopy (SEM)
The morphology of particles was examined using SEM (JSM-7100f; Jeol Ltd., Japan). Samples were first distributed evenly on conductive carbon adhesive tape and then coated with a thin layer of gold.
Surface area analysis
The specific surface area of samples was determined by nitrogen adsorption using a surface area analyzer (ASAP 2010; Micromeritics Instrument Corporation, USA). Surface area calculations were made based on the Brunauer– Emmett–Teller equation using the software provided.
Powder X-ray diffraction (PXRD)
X-ray diffraction patterns were recorded using a D8 Advance X-ray diffraction system (Bruker AXS GmbH, Germany) with Ni-filtered Cu-Kα radiation. The 2θ scan range was 5–50° with a step size of 0.02° at a rate of 3°/min.
Differential scanning calorimetry (DSC)
DSC measurements were performed using a Sinco model S-650 DSC system (Sinco, Korea). Each sample (3–5 mg) was placed in a crimp-sealed aluminum pan, and measurements were made over a temperature range of 30–300°C at a heat rate of 5°C/min under nitrogen flow (20 mL/min).
Drug-loading percentage
Drug content in the nanomatrix was determined using HPLC. A sample of 19.5–21.5 mg was dissolved in 100 mL of methanol. HPLC analyses of in vitro samples were performed using an HPLC system consisting of an LC10ADvp pump, a SIL-10A autoinjector, and a SPD-10ADvp UV detector (Shimadzu, Japan). A C18 analytical column (Luna C18(2), 5 μm, 250 × 4.6 mm; Phenomenex, USA) was used. The injection volume was 20 μL, and the eluent flow rate was 1.0 mL/min. For atorvastatin calcium, the mobile phase was composed of a 60:40 mixture of acetonitrile/50 mM sodium acetate in water, where the pH was adjusted to 4.0 with glacial acetic acid. The signal was monitored at 245 nm. For dutasteride, the mobile phase was 60% acetonitrile and 40% water. For sorafenib tosylate, the mobile phase consisted of a 70:30 mixture of acetonitrile/2% v/v triethylamine in water, where the pH was adjusted to pH 5.4 with phosphoric acid. The signal was monitored at 265 nm. The drug-loading percentage of drug in SCCSN was calculated using the following equation:
Kinetic solubility test
Kinetic solubility tests were performed using a USP rotating paddle apparatus with a VK 7000 dissolution testing station and VK 750d heater/circulator (Vankel, USA) at 37°C and a rotating speed of 100 rpm. Excess solid (299.5–300.5 mg of drug) was placed in 300 mL of water. Then, 3-mL aliquot samples were collected at different time intervals and filtered using a 0.11-μm nylon syringe filter. The filtrate was diluted with methanol, and the drug content was quantified using HPLC using the method described above. The experiments were carried out in triplicate per formulation.
In vivo oral absorption study
Male Sprague–Dawley rats weighing 200–220 g were obtained from Samtaco Bio Korea Inc. (Korea). All experiments were performed according to the guidelines of the committee on care and use of laboratory animals of Inje University. The study protocol was approved by the ethics committee of Inje University. The rats were anesthetized with Zoletil (25 mg/kg i.m.), and then, their jugular veins were cannulated using a 23-gauge polyethylene cannula. Rats in the first group (n = 4) were orally administered SCCSN containing dutasteride dispersed in 1 mL of water, whereas rats in the second group (n = 4) received 1 mL of water containing a physical mixture of non-entrapped dutasteride. The oral dose of dutasteride was 2 mg/kg. Serial blood samples (approximately 350 μL each) were collected from the jugular vein at different time intervals. The blood samples were placed in heparinized tubes, and the plasma was separated by centrifugation. Dutasteride concentrations in the plasma samples were determined using liquid chromatography with tandem mass spectrometry (LC–MS/MS), as reported previously (Baek and Kim Citation2012). The area under the concentration–time curve (AUC0→24h) was calculated through non-compartmental analysis/model independent approach using the WinNonlin 2.1 software (Pharsight Corp., USA). The values of maximal plasma concentration (Cmax) and the time to reach Cmax (Tmax) were obtained directly from the plasma data. A Student's t-test for unpaired means was conducted at the 95% confidence interval (α = 0.05) using SPSS 12.0 software (SPSS, USA).
Results and discussion
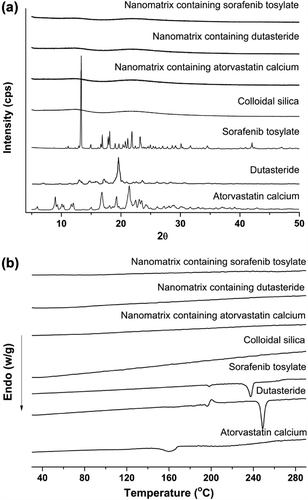
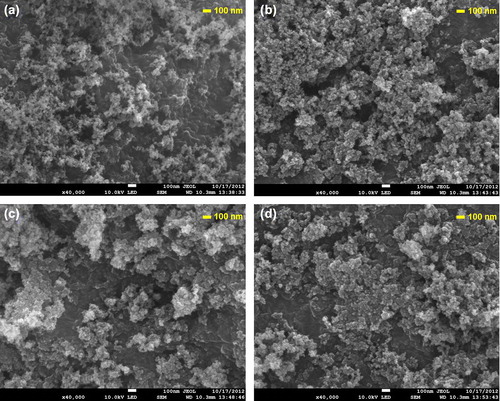
In this study, SCCSN containing different drugs was prepared using the rotary evaporation method based on a bottom-up approach. Aerosil 200 was used as the hydrophilic colloidal silica, which consists of primary particles of about 12 nm in size, resulting in a very high specific surface area of 200 m2/g (Product Information, Degussa). After coating Soluplus and each drug onto colloidal silica nanoparticles, the specific surface areas of the atorvastatin calcium, dutasteride, and sorafenib tosylate nanomatrices formed were 86.34, 90.29, and 88.53 m2/g, respectively. As shown in , SCCSNs existed as aggregates consisting of primary nanoparticles. Therefore, it is possible that the reduced particle size and increased surface area may have been responsible for the enhanced drug solubility of the nanomatrix. In addition, as shown in , DSC and PXRD results showed that each of the 3 drugs was present in an amorphous form within the nanomatrix.
Figure 1. SEM of Aerosil 200 (a) and Soluplus-coated colloidal silica nanomatrix (SCCSN) containing atorvastatin calcium (b), dutasteride (c), and sorafenib tosylate (d).
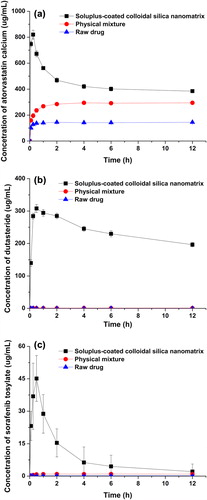
HPLC analysis was used to determine the exact content of drug in the nanomatrix, and revealed that the drug- loading percentage of drug in SCCSN was in good agreement with theoretical values (5.0%); in addition, no chemical degradation of the drugs was observed. Kinetic solubility studies were performed in water for raw drug, SCCSN, and a physical mixture of each of the 3 drugs (). All SCCSNs had rapid dissolution rates, with all 3 drugs tested reaching maximum supersaturation (solubility) within 30 min. For atorvastatin calcium, the maximum supersaturated concentration (solubility) of SCCSN was significantly higher than that of the physical mixture and the raw material, with approximately 2.8- and 5.7-fold increases in solubility, respectively. For sorafenib tosylate, the maximum supersaturated concentration (solubility) of SCCSN was also increased by 46.4-fold compared to its physical mixture. In particular, SCCSN containing dutasteride showed a dramatic enhancement of maximum supersaturated concentration; it was increased by 326-fold compared to its physical mixture. For raw dutasteride, no drug was detected in the dissolution medium tested during the period of determination, due to its very low solubility. After maximizing supersaturation, the supersaturated concentration (solubility) of amorphous drug was decreased due to solvent-mediated conversion from the amorphous form to the crystalline form for all the 3 drugs tested, as shown in . Nevertheless, a high solubility of drug, of above 200 μg/mL, was maintained for at least 12 h for dutasteride. Generally, a supersaturated drug solution is thermodynamically unstable and has a tendency to return to the equilibrium state in accordance with drug precipitation (Kojima et al. Citation2012). It was suggested that Soluplus may adsorb onto the surface of small embryo particles and inhibit crystal growth by blocking the active surface, providing steric stabilization and/or hydrogen bonding between the drug and Soluplus during drug precipitation (Wen et al. Citation2005, Overhoff et al. Citation2008, Ilevbare et al. Citation2012).
Figure 3. Kinetic solubility of raw material, physical mixture, and SCCSN for atorvastatin calcium (a), dutasteride (b), and sorafenib tosylate (c) (data represented as mean ± SD, n = 3).
The oral absorption of SCCSN was evaluated in rats. Dutasteride was assessed because it showed the highest maximum supersaturation among the drugs tested. The mean plasma concentration profile of dutasteride after oral administration of SCCSN containing dutasteride and its physical mixture was plotted as a function of time and is shown in . For SCCSN, the AUC0→24 h, Cmax, and Tmax were 2858.2 ± 587.5 ng·h− 1∙mL− 1, 180.4 ± 22.4 ng/mL, and 4.0 ± 2.2 h, respectively. The oral drug absorption of SCCSN was significantly higher than that of the physical mixture, with approximately 4.7- and 3.3-fold increases in AUC0→24 h and Cmax, respectively (). Results of statistical significance testing are presented in . There is a significant difference between the AUC0→24 h (p < 0.001) and Cmax (p < 0.001) of the drug entrapped in SCCSN and the physical mixture at the 95% confidence interval. No significant difference was detected for Tmax (p = 0.606). The bioavailability of SCCSN was markedly higher than that of the physical mixture. It could be hypothesized that this enhancement in bioavailability is a consequence of the higher supersaturation and sustained supersaturated concentration as indicated by the results of the kinetic solubility studies (Warren et al. Citation2010, Kim et al. Citation2011).
Figure 4. Plasma concentration–time profile of dutasteride in rats (data represented as mean ± SD, n = 4).
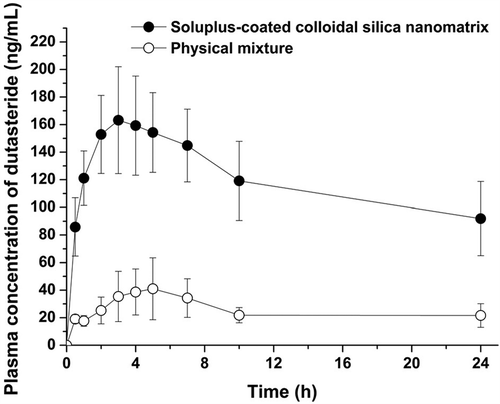
Table I. Pharmacokinetic parameters of dutasteride in rats (data represented as mean ± SD, n = 4).
Conclusion
In this study, a SCCSN was developed for the enhancement of solubility and oral absorption of poorly water-soluble drugs. For atorvastatin calcium, dutasteride, and sorafenib tosylate, the maximum supersaturated concentration (solubility) of SCCSN was significantly higher than that of a physical mixture of each drug, with approximately 2.8-, 326-, and 46.4-fold increases in solubility, respectively. In addition, the oral drug absorption of SCCSN containing dutasteride was significantly higher than that of the drug's physical mixture, with approximately 4.7- and 3.3-fold increases in AUC0→24 h and Cmax, respectively. The results from this preliminary study suggest that the SCCSN system could be a promising approach to improve the supersaturation and oral absorption of poorly water-soluble drugs.
Declaration of interest
The author reports no declarations of interest. The author alone is responsible for the content and writing of the paper.
References
- Baek IH, Kim MS. 2012. Improved supersaturation and oral absorption of dutasteride by amorphous solid dispersions. Chem Pharm Bull. 60:1468–1473.
- Chauhan B, Shimpi S, Paradkar A. 2005. Preparation and evaluation of glibenclamide-polyglycolized glycerides solid dispersions with silicon dioxide by spray drying technique. Eur J Pharm Sci. 26:219–230.
- Davis ME, Chen ZG, Shin DM. 2008. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 7:771–782.
- Friedrich H, Fussnegger B, Kolter K, Bodmeier R. 2006. Dissolution rate improvement of poorly water-soluble drugs obtained by adsorbing solutions of drugs in hydrophilic solvents onto high surface area carriers. Eur J Pharm Biopharm. 62:171–177.
- Ilevbare GA, Liu H, Edgar KJ, Taylor LS. 2012. Understanding polymer properties important for crystal growth inhibition—Impact of chemically diverse polymers on solution crystal growth of ritonavir. Crystal Growth Des. 12:3133–3143.
- Jia Z, Lin P, Xiang Y, Wang X, Wang J, Zhang X, Zhang Q. 2011. A novel nanomatrix system consisted of colloidal silica and pH-sensitive polymethylacrylate improves the oral bioavailability of fenofibrate. Eur J Pharm Biopharm. 79:126–134.
- Kim MS, Jin SJ, Kim JS, Park HJ, Song HS, Neubert RH, Hwang SJ. 2008. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur J Pharm Biopharm. 69:454–465.
- Kim MS, Kim JS, Park HJ, Cho WK, Cha KH, Hwang SJ. 2011. Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process. Int J Nanomedicine. 6:2997–3009.
- Kinnari P, Mäkilä E, Heikkilä T, Salonen J, Hirvonen J, Santos HA. 2011. Comparison of mesoporous silicon and non-ordered mesoporous silica materials as drug carriers for itraconazole. Int J Pharm. 414:148–156.
- Kojima T, Higashi K, Suzuki T, Tomono K, Moribe K, Yamamoto K. 2012. Stabilization of a supersaturated solution of mefenamic acid from a solid dispersion with EUDRAGIT(®) EPO. Pharm Res. 29:2777–2791.
- Limnell T, Santos HA, Mäkilä E, Heikkilä T, Salonen J, Murzin DY, et al. 2011. Drug delivery formulations of ordered and nonordered mesoporous silica: comparison of three drug loading methods. J Pharm Sci. 100:3294–3306.
- Linn M, Collnot EM, Djuric D, Hempel K, Fabian E, Kolter K, Lehr CM. 2012. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur J Pharm Sci. 45:336–343.
- Mamaeva V, Sahlgren C, Lindén M. 2012. Mesoporous silica nanoparticles in medicine-Recent advances. Adv Drug Deliv Rev. doi:10.1016/j.addr.2012.07.018.
- Miura H, Kanebako M, Shirai H, Nakao H, Inagi T, Terada K. 2011. Influence of particle design on oral absorption of poorly water- soluble drug in a silica particle-supercritical fluid system. Chem Pharm Bull. 59:686–691.
- Nagy ZK, Balogh A, Vajna B, Farkas A, Patyi G, Kramarics A, Marosi G. 2012. Comparison of electrospun and extruded Soluplus®-based solid dosage forms of improved dissolution. J Pharm Sci. 101:322–332.
- Overhoff KA, McConville JT, Yang W, Johnston KP, Peters JI, Williams RO III. 2008. Effect of stabilizer on the maximum degree and extent of supersaturation and oral absorption of tacrolimus made by ultra-rapid freezing. Pharm Res. 25:167–175.
- Souris JS, Lee CH, Cheng SH, Chen CT, Yang CS, Ho JA, et al. 2010. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials. 31:5564–5574.
- Product Information, Degussa. Available at: http://www.aerosil.com. Accessed on 18 Dec 2012.
- Van Eerdenbrugh B, Van Speybroeck M, Mols R, Houthoofd K, Martens JA, Froyen L, et al. 2009. Itraconazole/TPGS/Aerosil200 solid dispersions: characterization, physical stability and in vivo performance. Eur J Pharm Sci. 38:270–278.
- Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VS. 2010. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 6:1952–1967.
- Wang L, Cui FD, Sunada H. 2006. Preparation and evaluation of solid dispersions of nitrendipine prepared with fine silica particles using the melt-mixing method. Chem Pharm Bull. 54:37–43.
- Wang XQ, Fan JM, Liu YO, Zhao B, Jia ZR, Zhang Q. 2011. Bioavailability and pharmacokinetics of sorafenib suspension, nanoparticles and nanomatrix for oral administration to rat. Int J Pharm. 419:339–346.
- Warren DB, Benameur H, Porter CJ, Pouton CW. 2010. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: A mechanistic basis for utility. J Drug Target. 18:704–731.
- Wen H, Morris KR, Park K. 2005. Hydrogen bonding interactions between adsorbed polymer molecules and crystal surface of acetaminophen. J Colloid Interface Sci. 290:325–335.
- Zhang Y, Wang J, Bai X, Jiang T, Zhang Q, Wang S. 2012. Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs. Mol Pharm. 9:505–513.