Abstract
In this study, a novel carbon paste electrode (CPE) was prepared using the salt form of polyaniline (pani)–silicon dioxide composite that is sensitive to choline. Choline oxidase (ChO) enzyme was immobilized to modified carbon paste electrode (MCPE) by cross-linking with glutaraldehyde. Determination of choline was carried out by the oxidation of enzymatically produced H2O2 at 0.4 V vs. Ag/AgCl. The effects of pH and temperature were investigated, and the optimum parameters were found to be 6.0 and 60°C, respectively. The linear working range of the electrode was 5.0 × 10−7–1.0 × 10−5 M, R2 = 0.922. The storage stability and operation stability of the enzyme electrode were also studied.
Introduction
Carbon paste electrodes (CPEs) are widely used in electroanalysis owing to their low background current, wide potential window, chemical inertness, and simple and fast preparation from inexpensive materials. CPEs can also be easily modified with electrocatalysts or enzymes by means of simply mixing the modifier and the carbon paste matrix. In addition, the CPE offers a renewable electrode surface (Patia et al. Citation2007, Arduini et al. Citation2010).
Choline is a dietary essential nutrient (Zeisel Citation2000) involved in several body functions, the most relevant being the synthesis of cell membrane's phospholipids. In addition, choline acts as a major source of methyl groups and as a precursor of acetylcholine. Neonates, especially, have a high demand for choline for sustaining growth and brain development (Patia et al. Citation2007, AAP Citation1985, Zeisel Citation1987).
Choline deficiency can affect memory and easily cause liver dysfunction or cancer. Abnormal choline metabolism has been implicated in a number of neurodegenerative disorders such as Alzheimer's and Parkinson's diseases (Zhang et al. Citation2010, Michel et al. Citation2006, Zeisel Citation2004).
Choline determination can be performed using different methods including wet chemical determinations (Martinez Citation1983), microbiological assays (Lied and Braekkan Citation1975), gas chromatography (Saucerman et al. Citation1984), capillary zone electrophoresis (Carter and Trenerry Citation1996), and high-performance liquid chromatography (HPLC) (Ikarashi and Maruyama Citation1993).
Thus, choline detection and determination are the basic problems for food and clinical analyses. Generally, analytical methods used up to date are based on the determination of hydrogen peroxide liberated as a result of the enzymatic oxidation of choline by choline oxidase (ChO) (Özdemir et al. Citation2012).
In this study, a novel CPE was prepared using the salt form of polyaniline (pani)–silicon dioxide composite that is sensitive to choline. ChO enzyme was immobilized to CPE by cross-linking with glutaraldehyde. The optimum working conditions of biosensor were investigated with respect to the substrate concentration, the pH, and temperature. The storage stability and operation stability of the biosensor were investigated.
Material and methods
Equipment and reagents
The electrochemical studies were carried out using an Epsilon EC electrochemical analyzer with a three-electrode cell. The working electrode was a carbon paste (glass tubes with a diameter of 0.8 cm and length of 3 cm) electrode. The auxiliary and reference electrodes were a Pt wire and an Ag/AgCl electrode (3 M KCl), respectively. The pH values of the buffer solutions were measured with an Orion Model 720A pH/ion meter. Temperature control was achieved with a Grant W14 thermostat. ChO (EC 1.1.3.17, purified from the Alcaligenes Species and with an activity of 12.03 unit/ml) and choline were purchased from Sigma. Graphite powder and nujol were supplied by Merck and Sigma, respectively. All other chemicals were obtained from Sigma. All the solutions were prepared using double-distilled water.
Preparation of CPEs
CPE was prepared by thoroughly mixing 160 μL of nujol with 0.15 g of graphite powder in a mortar. For the preparation of CPEs, glass tubes (a diameter of 0.8 cm and length of 3 cm) were filled with the carbon paste. Height of the paste in the tubes was 0.5 cm. Electric contacts were made using platinum wire. The modified carbon paste electrode (MCPE) was prepared with 2 mg of polyaniline–silicon dioxide composite by thoroughly mixing 160 μL of nujol with 0.15 g of graphite powder in a mortar. Polyaniline–silicon dioxide composite was synthesized by Zengin and Erkan (Citation2010). The electrode surface was smoothed on a paper to produce a reproducible working surface.
Preparation of choline oxidase/modified carbon paste electrode
Fifty microliters of ChO enzyme (12.03 Unit/mL), 1 mg of bovine serum albumin, 50 μL of 0.1 M phosphate buffer at a pH of 6.0, and 30 μL of 2.5% glutaraldehyde were dropped upon MCPE. The electrode was dried at room temperature and washed with buffer solution (0.1 M phosphate buffers of pH 6.0) several times in order to remove the excess non-immobilized enzyme and glutaraldehyde. The electrode was kept in a refrigerator at 4°C in phosphate buffer when it was not in use.
Electrochemical measurements
The quantification of choline was achieved via electrochemical detection of the enzymatically released H2O2.
ChO/MCPE was immersed in the phosphate buffer (0.1 M) of pH 6.0. The solution contained 0.1 M sodium perchlorate as supporting electrolyte. The electrode was brought to equilibrium by keeping at 0.4 V (vs. Ag/AgCl electrode (3 M KCl)). Steady current (ia) was recorded. Choline solution was added to the cell using a micropipette, and the mixture was stirred. The currents (ib) values obtained at 0.4 V were recorded, and the values (∆i = ib–ia) were plotted against the choline concentration.
Results and discussion
In this study, a novel CPE was prepared using the salt form of polyaniline (pani)–silicon dioxide composite that is sensitive to choline. The parameters affecting the performance of the biosensor and optimum working conditions were investigated.
Amperometric responses of CPE and MCPE to hydrogen peroxide
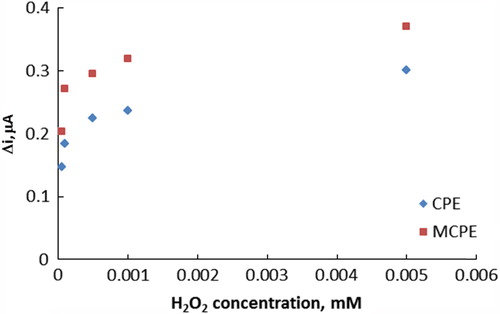
Amperometric responses of the CP and MCP electrodes to hydrogen peroxide were determined at different hydrogen peroxide concentrations. As shown in , when MCP was used, differences in the current were obtained higher than those obtained using CP.
The working potential
After preparing MCPE, the hydrogen peroxide oxidation was carried out at different potentials (0.3, 0.4, 0.5, 0.6, and 0.7 V).
Oxidation of hydrogen peroxide was investigated at the mentioned potentials using polyaniline–silicon dioxide composite by Arslan et al. and optimum potential was detected at 0.4 V (Arslan et al. Citation2012).
The effect of amount of the polyaniline–silicon dioxide composite to selectivity
Arslan et al. investigated the effect of the amount of the polyaniline–silicon dioxide composite on selectivity in another study, which is explained in the following paragraph (Arslan et al. Citation2012).
The selectivity is an important challenge in the development of a biosensor. In order to obtain the best compromise between sensitivity and selectivity, we evaluated the response of the electrode toward ascorbic and uric acids, and acetaminophen (Luque et al. Citation2005). Firstly, 5.0 × 10−4 M hydrogen peroxide was added to the cell. After the addition of hydrogen peroxide; 3.0 × 10−4 M uric acid, 1.0 × 10−4 M ascorbic acid and 1.0 × 10−4 M acetaminophen were also added to the cell. The interference was evaluated at 0.4 V using electrodes prepared with 1.0, 1.5, and 2.0 mg of polyaniline–silicon dioxide composite. It was observed that interference effect decreased when the amount of composite was increased. When the amount of composite was 3 mg, the electrode became very weak. Because of this composite, the amount was limited to 2 mg. The lowest percentage ratio of interference was found when 2 mg composite was used. Therefore, an amount of 2 mg of this composite was used in future studies.
Determination of optimum pH
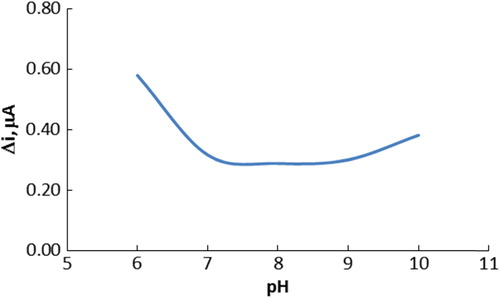
Since enzyme activity is dependent on the ionization state of the amino acids in the active site, pH plays an important role in maintaining the proper conformation of an enzyme. The effect of pH on the response of the choline biosensor was determined in 0.1 M phosphate buffer of pH range 6.0–10.0. The measurements were taken at a constant choline concentration of 5.0 × 10− 3 M. shows that the maximum response was obtained at pH 6.0. Because the isoelectric point of ChO was between pH 4.0 and 5.5, pH values lower than 6.0 were not studied. For choline biosensor, pH values different from 6.0 were employed in the literature (pH 9.0, 7.4, and 8.0) (Özdemir et al. Citation2012, Razola et al. Citation2003, Langer et al. Citation2004). This was attributed to the fact that the used polymer and the type of immobilization were different.
Determination of optimum temperature
Enzymes are known to be sensitive to changes in temperature. The relationship between reaction rate of an enzyme and temperature is exponential. Temperature's influence on the response of choline enzyme electrode was tested between 20°C and 65°C at pH 6.0 using constant choline concentration of 5.0 × 10− 3 M. As seen in , the current difference increases with temperature up to 60°C and decreases afterward. The highest electrode response was obtained at 60°C. For choline biosensor, temperature values were employed in the literature (37 and 30°C) (Razola et al. Citation2003, Langer et al. Citation2004).
Figure 3. The effect of temperature on the response of the biosensor (at pH 6.0, 5.0 × 10− 3 M choline at 0.4-V operating potential).
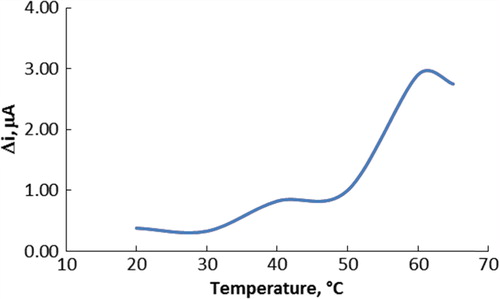
The study was carried out at 25°C due to the difficulties involved in working at 60°C. The temperature of 25°C was chosen as a working temperature for all further experiments. ChO enzyme kept its activity even at high temperatures, when it was immobilized to polyaniline–silicon dioxide composite.
When literatures were examined, it was seen that the polyaniline film which was obtained by the electropolymerization of aniline became a good microenvironment around the enzyme. It was observed that the enzyme was stronger even in high temperatures because of the microenvironment (Arslan et al. Citation2011, Shi et al. Citation2009, Chen et al. Citation2006).
Effect of substrate concentration on response of biosensor and calibration curve
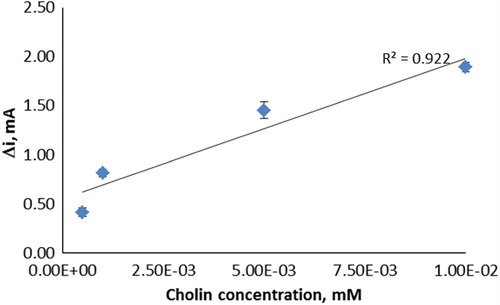
The effect of the substrate concentration on the reaction rate, catalyzed by immobilized ChO, was studied using varying concentrations (5.0 × 10− 7–1.0 × 10− 3 vM) of choline ().
Figure 4. The effect of choline concentration upon the amperometric response of the biosensor (in pH 6.0 phosphate buffer and at a 0.4-V operating potential, 25°C).
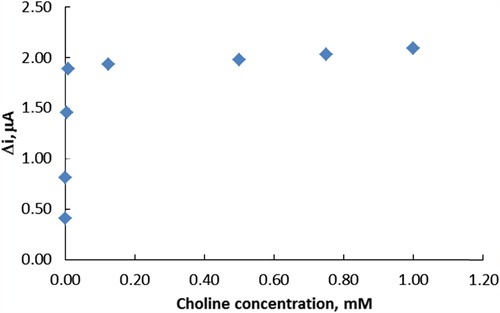
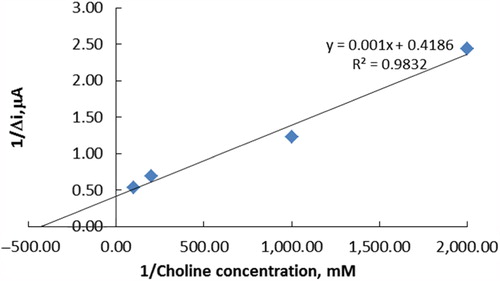
The linear working range of the electrode was 5.0 × 10− 7– 1.0 × 10− 5 M, R2 = 0.922 (). It was shown that the linearity of graphs was highly satisfactory and it could be used for the quantitative determination of choline. The detection limit of the biosensor was 1.0 × 10− 7 M and the response time of the biosensor was 200 s. Kinetic parameters Imax(app) and Km(app) for the enzyme biosensor were calculated as 2.39 μA/min and 2.39 × 10− 3 mM, respectively, from Lineweaver–Burk plots (). Km values for immobilized ChO presented in the literature are 5.58 × 10− 4 mM, 0.4 mM, 2.32 mM, and 0.083 mM (Özdemir et al. Citation2012, Langer et al. Citation2004, Gülce et al. Citation2003, Shi et al. Citation2006). This was attributed to the fact that the polymer used and the type of immobilization were different.
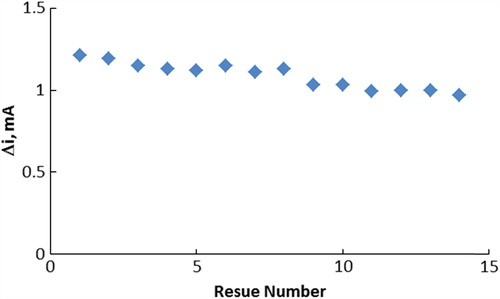
The operational stability of the enzyme electrode
The operational stability of the biosensor was studied by performing the activity assay (under optimum conditions) 14 times in the same day (). The relative standard deviation obtained after 14 measurements at a constant choline concentration of 5.0 ×10 − 3 M was found to be 7.38%.
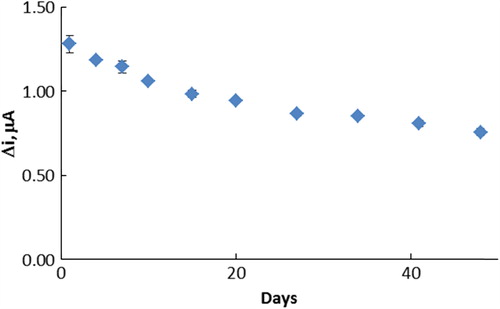
The storage stabilization of the enzyme electrode
Storage stability of the biosensor was determined by performing activity assays within 48 days. The activity assay was applied within 48 days to determine the storage stability of the immobilized enzyme. As shown in , immobilized enzyme retained 59.09% of its initial activity after 48 days.
Conclusion
Choline biosensor prepared in this study is useable in a large concentration range 5.0 × 10− 7–1.0 × 10− 5 M (R2 = 0.922). It has a very low detection limit (1.0 × 10− 7 M) and an acceptable response time for a biosensor (200 s). Choline biosensor gives perfect reproducible results after 14 measurements with 7.38% relative standard deviation. Also, it has good storage stabilization (gives 59.09% of the initial amperometric response at the end of the 48th day). The optimum pH and temperature parameters for immobilized enzyme were found to be 6.0 and 60°C, respectively. The Km(app) and Imax(app) values of ChO enzyme immobilized in polyaniline–silicon dioxide composite were 2.39 × 10− 3 mM and 2.39 μA/min, respectively. Choline biosensor prepared in this study is easy to prepare and is highly cost-effective.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- AAP (American Academy of Pediatrics). 1985. Pediatric Nutrition Handbook, 2nd ed. In: Forbes GB, Woodruff CW, Eds, Committee on Nutrition. Academy of Pediatrics: Elk Grove Village, IL.
- Arduini F, Di Giorgio F, Amine A, Cataldo F, Moscone D, Palleschi G. 2010. Electroanalytical characterization of carbon black nanomaterial paste electrode: development of highly sensitive tyrosinase biosensor for catechol detection. Anal Lett. 43:1688–1702.
- Arslan F, Ustabas S, Arslan H. 2011. An amperometric biosensor for glucose determination prepared from glucose oxidase immobilized in polyaniline-polyvinylsulfonate film. Sensors. 11:8152–8153.
- Arslan H, Özdemir M, Zengin H, Zengin G. 2012. Glucose biosensing at carbon paste electrodes containing polyaniline-silicondioxide composite. Int J Electrochem Sci. 7:10205–10214.
- Carter N, Trenerry VC. 1996. The determination of choline in vitamin preparations, infantformula and selected foods by capillaryzone electrophoresis within direct ultraviolet detection. Electrophoresis. 17:1622–1626.
- Chen C, Jiang Y, Kan J. 2006. Anon interference polypyrrole glucose biosensor. Biosens Bioelectron. 22:639–643.
- Gülce H, Aktas YS, Gülce A, Yıldız A. 2003. Polyvinyl ferrocenium immobilized enzyme electrode for choline analysis. Enzyme Microb Technol. 32:895–899.
- Ikarashi Y, Maruyama Y. 1993. Liquid chromatography with electrochemical detection for quantitation of bound choline liberated by phospholipase D hydrolysis from phospholipids containing choline in rat plasma. J Chromatogr. 616:323–326.
- Langer JJ, Filipiak M, Kecin´ska J, Jasnowska J, Włodarczak J, Buładowski B. 2004. Polyaniline biosensor for choline determination. Surface Sci. 573:140–145.
- Lied E, Braekkan OR. 1975. Determination of total choline in biologicalmaterials. Int J Vitamin Nutr Res. 45:438–447.
- Luque L, Rodrıguez MC, Rivas GA. 2005. Glucose biosensors based on the immobilization of copperoxide and glucose oxidase with in a carbon paste matrix. Talanta. 66:467–471.
- Martinez SEV. 1983. Simultaneous determination of choline and betaine in some fish materials. Analyst. 108:1114–1119.
- Michel V, Yuan Z, Ramsubir S, Bakovic M. 2006. Choline transport for phospholipid synthesis. Exp Biol Med. 231:490–504.
- Özdemir M, Arslan F, Arslan H. 2012. An amperometric biosensor for choline determination prepared from choline oxidase immobilized in polypyrrole-polyvinyl-sulfonate film. Artif Cells Blood Substit Biotechnol. 40:280–284.
- Patia S, Quinto M, Palmisano F. 2007. Flow injection determination of choline in milk hydrolysates by an immobilized enzyme reactor coupled to a selective hydrogen peroxide amperometric sensor. Analytica Chimica Acta. 594:234–239.
- Razola SS, Pochet S, Grosfils K, Kauffmann JM. 2003. Amperometric determination of choline released from rat submandibularglandacinar cells using a choline oxidase biosensor. Biosens Bioelectron. 18:185–191.
- Saucerman JR, Winstead CE, Jones TM. 1984. Quantitative gas chromatographic headspace determination of choline in adultand infant formula products. J Assoc Anal Chem. 67:982–985.
- Shi H, Yang Y, Huang J, Zhao Z, Xu X, Anzai J-I, et al. 2006. Amperometric choline biosensors prepared by layer-by-layer deposition of choline oxidase on the Prussian blue-modified platinum electrode. Talanta. 70:852–858.
- Shi Q, Wang P, Jiang Y, Kan J. 2009. Glucose biosensor based on polyaniline synthesized in ionic liquid Biocatal. Biotransfor. 27:54–59.
- Zeisel SH. 1987. Diet and brainfunction: available information and misinformation. In: Grand RJ, Sutphen JL, Dietz WH, Eds. Pediatric Nutrition: Theory and Practice. Boston, MA: Butterworths, pp. 801–808.
- Zeisel SH. 2000. Choline: an essential nutrient for humans. Nutrition. 16:669–671.
- Zeisel SH. 2004. Nutritional importance of choline for brain development. J Am Coll Nutr23:621S–626S.
- Zengin H, Erkan B. 2010. Synthesis and characterization of polyaniline/ silicon dioxide composites and preparation of conductive films. Polym Adv Technol. 21:216–223.
- Zhang Z, Wang J, Wang X, Wang Y, Yang X. 2010. A sensitive choline biosensor with supramolecular architecture. Talanta. 82:483–487.