Abstract
During the last two decades, the occurrence of fungal infections either superficial or systemic has been increasing. Moreover, fungal infections become more difficult to treat when they show coupling with immunogenic diseases like AIDS. Superficial fungal infections are associated with skin, nail and eye and are less prominent to systemic infection. However, it may be dangerous if not treated properly. It is usually observed that conventional formulations including cream, powder, gels etc. are used to treat skin fungal infections even for the deep seated fungal infections. However, these formulations show various side-effects on the application site like burning, redness and swelling. Further, due to the immediate release of drug from these formulations they can stimulate the immune system of body generating high impact allergic reactions. Deep seated fungal infections like invasive aspergillosis and invasive candidiasis may be more difficult to treat because the drug released from conventional topical formulation can not reach at the target site due to the low penetration capacity. Similarly, in case of fungal infection of nail and eye, conventional formulations show problem of less bioavailability. Thus, to overcome the drawbacks of conventional therapy a lot of research works have been carried out to develop novel formulations of antifungal drugs to deliver them superficially. Novel formulations explored for the skin delivery of antifungal drugs include liposomes, niosomes, ethosomes, microemulsions, nanoparticles, microspheres and micelles. These formulations show extended or sustained release of drug, minimizing the side effect on application site, enhancing bioavailability and reducing the dosing frequency. Further, these formulations also show penetration into the deep skin to treat invasive fungal infections. Novel formulations explored in treatment of fungal infections of eye are liposomes and nanoparticles and whether for nail fungal infections microemulsions are the choice. In present article, we have discussed about conventional treatment of superficial fungal infection and their comparison with the novel drug delivery systems.
Introduction
The incidences of fungal infections are increasing now-a-days especially in patients that are immunocompromized. This increase in the occurrence of fungal infection whether superficial or systemic is due to coupling of causative fungus with diseases like AIDS or due to excessive use of immunosuppressive drugs during the period when there is a technological advancement in the field of solid organ transplantation medicines, stem cell transplantation and neonatology (Li et al. Citation2007). Fungal infections are classified as superficial infections or systemic infections, affecting the body as a whole. Superficial infections are affecting the skin, hairs, nails or eyes of body whereas subcutaneous infections are mainly found in the subcutaneous tissue or dermis; however, they can reach deep into epidermis and may be invasive in nature (Kaur and Kakkar Citation2010).
Subcutaneous fungal infections
Subcutaneous infections are found in the skin. Various fungal skin infections are discussed below:
Tinea corporis
It is commonly known as “ring-worm”. It usually occurs in trunk and extremities. Three species are mainly responsible for this infection namely T. rubrum, T. tonsurans and T. mentagrophytes. Deep seated tinea corporis is also called as Majocchi granuloma. It is caused by T. mentagrophytes and mainly found in women. It is characterized by the erythematous, round, annular plaques with central and scaly border containing pustules and papules (Adams Citation2002).
Tinea pedis
Tinea pedis represents the most common form of dermatophytosis (Goldstein et al. Citation2000). It is most commonly found in people who use communal showers or locker rooms. T. rubrum is mainly responsible for the generation of tinea pedis (http://www.mycology.adelaide.edu.au.). Tinea pedis is usually classified into three types: (i) interdigital, (ii) moccasin, and (iii) vesiculobullos. Interdigital infection is found in the foot usually between the fourth and fifth toes. The infection can be usually dry and scaly showing its slight resemblance to psoriasis. It can be moist or macerated also. Moccasin type infection involves medial, heel or lateral region of foot. It can exist as silvery white scale on a red, thickened base resembling contact dermatitis. Vesciculobullos type of infection is usually found on the sole of the foot. Pustules may be present in the affected area and become usually macerated and get secondarily affected with bacteria (Svejgaard Citation1997).
Tinea faciei
It is found in the face mainly in the non-bearded region. Causative fungus for this infection is either T. rubrum or T. mentagrophytes. It is characterized by the itchy red coloured patches on the affected area. Erythematous eruptions are other characteristic of tinea faciei (Zuber and Baddam Citation2001).
Tinea manuum
It is not a highly susceptible fungal infection. Pathogenic fungus for this infection is T. tonsurans. The occurrence sites for this infection are palmer, dorsal or interdigital region of hands or fingernails. It is manly found in the one hand only and rarely both hands get affected. It can be easily characterized by eczema, psoriasis and callous formation (Pfaller et al. Citation2004).
Tinea cruris
The common name for the tinea cruris is “Jock-itch”. In this type of fungal infection usually hair follicles get affected. The causative fungus for this infection is T. rubrum. It mainly occurs in the summer season in the person wearing tight fitting clothes. This fungal infection is characterized by pruritic erythematous patch on the medial thighs along with the distinct sparing of penis and scrotum (Goldstein et al. Citation2000).
Tinea barbae
It is a very rare fungal infection that is associated with the hairy area of face. Causative fungus for this disease is T. violaceum or some time T. rubrum also. It is mainly found in persons working with pet animals. The causative fungus affects the hair shaft or the hair follicles forming crusted patches associated with partial alopecia (Adams Citation2002).
Tinea capitis
This fugal infection is mainly found in preadolescent children and less commonly in adults (Babel and Baughman Citation1989). It is a vector born infection which occurs due to direct contact with infected person or by using contaminated clothes, furniture or combs (Elewski Citation2000). It is caused by the fungus T. tonsurans. It is characterized by papules, pustules or the nodules on the scalp. Inflammation can occur after some time and if not treated properly it can generate secondary infection like scaling, erythema, edema or alopecia (Ghannoum et al. Citation2006).
Fungal infections of nails (Onychomycosis)
Onychomycosis is also known as tinea unguium. It is the fungal infection that affects the nail or causes dysfunctioning of nails (Hall et al. Citation1997). Various predisposing factor for onychomycosis are immunosuppression, diabetes, age greater than 60, obesity, smoking, genetic predisposition etc (Gupta et al. Citation2004, Kuvandik et al. Citation2007). Recently, it is found that psoriasis can be another predisposing factor for onychomycosis (Szepietowski and Salomon Citation2007). Clinically it can be divided into four types: (i) proximal subungual, (ii) distal subungual, (iii) white superficial, and (iv) muco-cutaneous candidiasis. Proximal subungual is mainly caused by either T. rubrum or T. megnini. Causative organisms for distal subungual infection are T. tonsurans and T. mentagrophyte. White superficial infection can be caused due to T. mentagrophytes or sometime due to molds like Aspergillus terreus. The fungal species responsible for the fourth type are usually Candida albicans or C. tropicalis. In the onychomycosis infection, foot nails get affected more as that of fingernails. In this fungal infection, nail plate becomes white in colour associated with subungual hyperkeratosis and proximal onycholysis (Faergemann and Baran Citation2003). This can be considered as early indication of HIV infection as it is rarely found in the healthy individual and can be found in the person with very weak immunity like in AIDS (Durden and Elewski Citation1997).
Fungal infections of eye
Fungal eye infections are less common as compared to infections caused by bacteria or viruses. Various regions of the eye that are attacked by the fungus are cornea (keratitis) and the interior segment of eye (endopthalmitis) (Whitcher et al. Citation2001). It has been found in a survey that fungal keratitis is the second most common cause of blindness in the developing countries (Chowdhary and Singh Citation2005). Contact lens wear in the developed countries may cause ocular keratitis associated with Candida fungus. These are filamentous fungi associated with contact lens wear (Rangel Citation1999). Fungus can not penetrate the corneal epithelium of the eye. They usually need an injury or trauma to cross this barrier. So such type of post-traumatic fungal infection is caused by the Candida species. Various fungus involved in the generation of fungal infection of eye are Candida, Aspergillus, Fusarium and Sporothix (Williamson et al. Citation1968).
Conventional treatment for superficial fungal infections
Treatment for subcutaneous fungal infection
Conventionally Tinea corporis and Tinea faciei can be treated by oral and topical antifungal preparations. Oral therapy is used when the disease shows the extensive harmful characteristics. For the treatment of these diseases cream formulation of various azole derivatives (clotrimazole, ketoconazole, miconazole, econazole) and terbinafine can be used. Orally used antifungals are griseofulvin, itraconazole, fluconazole and terbinafine (Stary and Sarnow Citation1998).
Tinea pedis occurs to a large extentwhen compared to other topical fungal infections. Topically it can be treated with antifungal powders (tolnaftate, miconazole and clotrimazole) or antifungal gels (terbinafine, naftifine and ciclopirox) (Korting et al. Citation2007). It is found that topical treatment fails in most of the patients especially when the disease gets associated with onychomycosis (Hart et al. Citation1999). Oral therapy is used for severe or extensive disease conditions. Doses of various antifungal agents in the treatment of tinea pedis are: the use of 250 mg terbinafine daily for three to five weeks, 200 to 300 mg fluconazole once in a week for a period of five to six weeks, 500mg griseofulvin twice a day for six to eight weeks (Del Citation2000, McClellan et al. Citation1999). Tinea mannum can be effectively treated by using the topical antifungal drugs or keratolytics. The dosage regimen for oral antifungal in case of tinea manuum is similar as required for tinea pedis (Hall et al. Citation1997).
Tinea capitis is the disease for which only oral antifungal agents are effective for proper elimination. Tinea capitis can be treated in children/pediatric patients by using oral griseofulvin. It can be delivered in the form of crushable tablets or in oral suspension form (Roberts and Friedlander Citation2005). Terbinafine is also another antifungal agent that was approved by USFDA in 2007 for treatment of tinea capitis in children who were more than five years of age (Fleece et al. Citation2004). Topically it can be treated using an antifungal shampoo containing either ketoconazole or selenium sulphide. For the treatment of tinea barbae usually a dose of 0.5 to 1g/day of oral griseofulvin is given for three to four weeks. Regular shaving, use of effective antibacterial soap and proper hygiene can be very useful for total removal of this fungal infection (Gupta et al. Citation2004).
Treatment for onychomycosis
For the treatment of onychomycosis usually oral therapy is preferred over topical therapy because when the drug is employed topically it shows a poor penetration through nail bed. Early treatment of onychomycosis involves use of ciclopirox nail lacquer in the concentration range upto 8% (Gupta et al. Citation2000). Oral antifungal drugs used in the treatment of onychomycosis are fluconazole, itraconazole, terbinafine and griseofulvin (Elewski Citation1999). Dose of 250mg of terbinafine for the treatment of onychomycosis is administered daily for 6 to 8 weeks. 200mg of Itraconazole is given daily for 2 to 3 months (Evans and Sigurgeirsson Citation1999). Dose prescribed for this disease is 200 to 300mg of fluconazole once in a week for a period of five to six months. Doses can be varied according to the impact of infection (Scher et al. Citation2007).
Treatment for fungal infection of eye
The drugs for treatment of fungal infection of the eye are natamycin, fluconazole and amphotericin B. Natamycin is generally used in the form of suspension for treatment of filamentous fungal keratitis. Candida infection of the eye can be treated with the use of ophthalmic solution of fluconazole. Amphotericin B eye drops are used in the non-responding cases of infections. These eye drops may show toxic effect on the eye so these should be administered under the supervision of medical practitioners (Thomas Citation1994, Thomas et al. Citation1997). Various marketed formulations available for treatment of superficial fungal infection are shown in (http://www.rxlist.ca).
Table I. Marketed formulations available for treatment of superficial fungal infections.
Drawbacks of conventional therapy for the superficial fungal infection
Drawbacks of conventional topical formulations
Topical antifungal drugs, used in the form of cream, gels and ointments, may cause burning, stinging, erythema and redness of skin (Goldstein et al. Citation2000). The main difficulties with conventional topical antifungal treatment of ocular infections are poor ocular penetration, local bioavailability and drug toxicity (Le et al. Citation1998).
Drawbacks of conventional oral formulations
Oral antifungals show more adverse effect as compared to the topical antifungals. Some of them are very costly, a few of them can generate organ toxicity and their interaction with other therapeutic agent is more prominent (Amichai and Grunwald Citation1998, Brodell and Elewski Citation2000). Griseofulvin is a most commonly used oral antifungal. It may show side effects like hepatotoxicity when used in a long term treatment of onychomychosis. Other side effects include headache, photosensitivity, nausea and vomiting (Moossavi et al. Citation2001). It can show adverse drug interactions with various categories of drugs like barbiturates, alcohol, oral contraceptives and warfarin (Katz Citation1997). Hepatotoxicity is also associated with ketoconazole. Hepatotoxicity with ketoconazole occurs in one out of 10,000 to 12,000 people. Other side effects of this drug are haemolytic anaemia, impotence and the abdominal pain (Moossavi et al. Citation2001). Use of antihistaminic drug and the triazolam is usually contraindicated with this antifungal drug (Brodell and Elewski Citation2000). Side effects of itraconazole are similar to the ketoconazole but the hepatotoxicity is rarely reported (Moossavi et al. Citation2001). Side effects associated with fluconazole are more prominent in HIV-patients (Goldstein et al. Citation2000). These include dyspepsia, dizziness, abdominal pain, exfoliative skin disorders and anaphylaxis (Brodell and Elewski Citation2000). Terbinafine is also an orally active antifungal drug showing a number of adverse effects when used in the form of conventional formulation. These include neutropenia, visual disturbances, urticaria and dyspepsia. It can rarely generate the cholestatic liver disease or fulminant hepatic failure (Lazaros et al. Citation1996, Agarwal et al. Citation1999, Perveze et al. Citation2007).
General consideration related to superficial delivery of antifungal drugs
Considerations related to the delivery of drug to the skin
In case of topical fungal infection the causative fungus usually invades the stratum corneum layer of the skin. Topical agents can be used in the form of cream, lotion or gels. They can penetrate the stratum corneum to kill the causative fungus. They usually can be fungicidal (kill fungi) and fungistatic (inhibit the fungal division). Since the side effects of topical agents are less as those of systemic agents therefore topical therapy represents an attractive approach for treatment of skin infections. General adverse reactions after topical medication occur at the site of application, which are mild or transient (Gupta et al. Citation2003). Some important characteristics of antifungal drugs used topically are given in (http://www.drugbank.ca).
Table II. Important characteristics of antifungal drugs used topically.
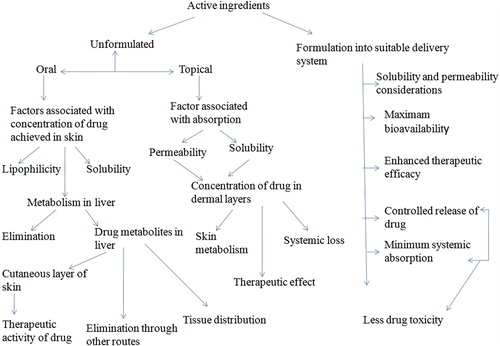
Another advantage of the topical formulations is its self administration by the patients. It can also avoid the drug-drug interaction that is more prominent in case of oral administration of drug. Drug must have some specific characteristics to be delivered in the form of topical preparation such as high lipophilic nature and poor aqueous solubility (e.g. clotrimazole, miconazole). When formulation of such drug is applied on the skin, a depot is formed in lipidic stratum corneum and releases the drug slowly to the underlying skin layers like dermis and epidermis which are aqueous in nature. Therefore, it is important to ensure the release rate of drug to achieve local therapeutic concentration and to provide prolonged pharmacological effect (Robert and Kalia Citation2006). The skin can be considered as a main target or major barrier for the topical and transdermal delivery of the drugs. The major problem associated with topical delivery of antifungal drug is the low permeability of stratum corneum. Several approaches have been employed to increase the permeation of stratum corneum. One of the new and sophisticated approaches is the use of vesicular systems like liposomes and niosomes whose effectiveness depends on their physicochemical characteristics (Blank et al. Citation1984). represents the fate of the drug in the skin before and after formulation in a suitable drug delivery system.
Considerations related to delivery of drugs to the nails
Nail is an important skin appendage that is highly susceptible to fungal attack. Fungal infections of nail are very difficult to treat by using the conventional formulation because of very low permeability of nail plate. Conventional formulation like cream or gels get removed from the nail surface through mechanical contact like rubbing and washing causing more reduction in their efficacy. Water can act as a plasticizer for the nail plate. So the formulations having capacity to increase the nail hydration can be good candidate for the treatment of fungal infections of nail. Recent studies show that diffusion of water and the other material increases from the skin and the nail as the skin or the nails become more hydrated (Blank et al. Citation1984, Kasting and Barai Citation2003).
Consideration related to delivery of drugs to the eye
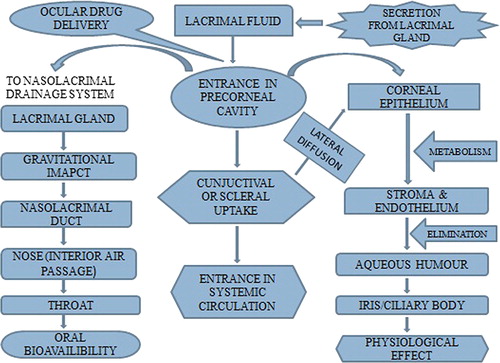
Topical treatment of ocular fungal infection is the most difficult because of poor penetration, drug toxicity and low corneal permeability. Barriers associated with pre-corneal area include blinking reflex, tear turn over and corneal permeability (Le et al. Citation1998). The drug usually penetrates the cornea through diffusion mechanism. Structural components affecting penetration of drug through the cornea are epithelium, endothelium and the stroma. Epithelium and endothelium are generally lipophilic in nature while the stroma is hydrophilic in nature. Epithelium is considered as rate-limiting barrier for transcorneal diffusion of the drug. For the drug molecules that are smaller in size, having lipophilic nature and capable of crossing the epithelium, stroma or endothelium play an important role especially endothelium. For the drug molecules having higher size, stroma acts as a greater barrier as compared to the endothelium (Prausnitz and Noonan Citation1998, Kaur et al. Citation2008). Activity of the ocular antifungal drugs depends upon the various factors like its molecular mass, duration of contact with the target tissue, concentration of drug and the ability of the drug to get penetrated in the eye. It is found that compound with molecular mass greater than 500 Daltons (Da) penetrate the corneal epithelium with difficulty like amphotericin B (924.10 Da), natamycin (665.75 Da) and ketoconazole (531.44 Da). Drug molecules having the comparable size like miconazole (416.12 Da) or fluconazole (306.30 Da) can easily cross the corneal epithelium (Nagarsenker et al. Citation1999, Manzouri et al. Citation2001). Highly lipophilic drugs like itraconazole can easily cross the lipidic endothelial and epithelial layers along with the blood aqueous barrier, while hydrophilic drugs can more easily cross the corneal stroma and the amphiphilic compounds can cross all the ocular layers. Therefore, amphiphilic drugs are good candidates for the treatment of ocular fungal infections (Friedberg et al. Citation1991). The fate of the drugs used to treat infections of the eye is shown in . This figure also shows factors responsible for poor bioavailability of drugs in ocular system.
Novel drug delivery system in the treatment of subcutaneous fungal infections
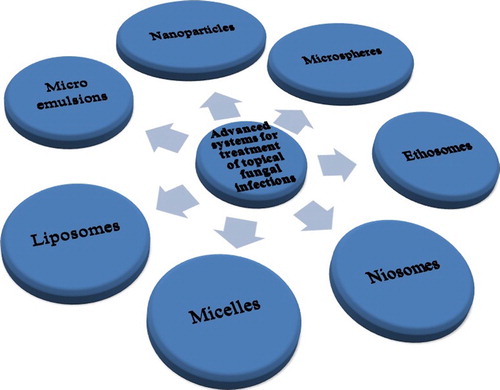
Various novel drug delivery systems have been explored for the treatment of subcutaneous fungal infections (). A brief overview of novel drug delivery system of various antifungal drugs used subcutaneously is shown in .
Table III. A brief overview of novel drug delivery systems of various antifungal drugs used subcutaneously.
Liposomes
Liposomes are the microscopic sphere having an aqueous core surrounded by one or more than one outer shell composed of lipids arranged in a bilayer configuration. Therapeutic applications of liposomes were recognized about 25 years ago (Sessa and Weissmann Citation1968, Norbert et al. Citation2001).
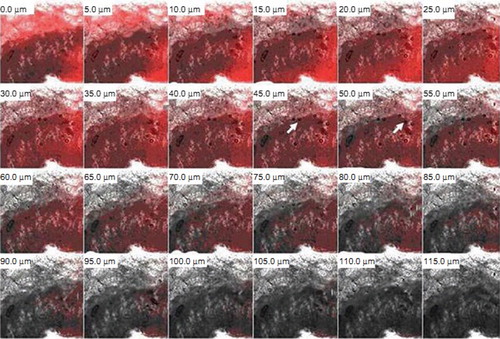
First liposomal preparation that came into market was of antifungal drug Amphotericin B (AmB). It was marketed under the name AmBisomeR, produced by Nexatar Company USA in 1990. It was marketed in certain countries of Europe (Gulati et al. Citation1998). Other products of Amphotericin B introduced were ABLC, AbelcetR (AmB lipid complex produced by the Liposome Company USA) and ABCD, AmphotecR or AmphocilR (AmB Colloidal dispersion produced by SEQUUS Pharmaceuticals USA). These formulations have shown that when they are employed for the treatment of fungal infections they show reduction in side effect of Amphotericin B while antifungal activity of drug is retained (Arikan and Rex Citation2001). AmBisomeR was found effective in both for the treatment of the intracellular (histoplasmosis and leishmaniasis) or intercellular (aspergillosis or candidiasis) systemic infections (Johnson et al. Citation2002). Compared to AmBisomeR other liposomal preparation of AmB (L-AMP-LRC-1) was found to be more effective in the treatment of neonatal candidiasis. All these Amphotericin B liposomal preparations are administered parenterally for treatment of fungal infections (Kotwani et al. Citation2003). Recently, a lot of research work has been carried out on liposomes for the treatment of topical fungal infections also. Liposomes are now-a-days a centre of attraction for the scientists to deliver the drug topically for treatment of various skin infections. A comparative study between miconazole nitrate (MCZ) loaded topical liposomes and conventional cream formulation was carried out for antifungal efficacy. Two lipids namely phosphatidyl choline saturated 97.3% content (PCS) and phosphatidyl choline unsaturated 98.0% content (PCU) were used for the formulation of the liposomes. Liposomes composed of the PCS showed higher retention as compared to the liposomes composed of PCU. Prepared systems had good size, stability characteristics and enhanced permeation properties (Agarwal and Katare Citation2002) Comparison between niosomal and liposomal gel of clotrimazole was done for vaginal antifungal efficacy. Optimized formulations were having higher drug: lipid ratio (> 85 mg/mg with liposome; > 75 mg/mg with noisome) and were stable at storage temperature 4 ± 1°C for 24 h. Liposomal and niosomal gel did not show any adverse effect on the vaginal morphology at 24 h post application. (Ning et al. Citation2005). Further, carbopol gel containing liposomes of ketoconazole were also prepared. Liposomal gel showed more drug retention compared with plain gel and plain drug cream (Patel et al. Citation2009). Later on, liposomes and niosomes of fluconazole were also prepared for eradication of cutaneous candidiasis. Further, these systems were incorporated into 1% w/w carbopol gel. Liposomal gel produced 14.2 folds higher drug accumulation when compared with that of plain gel while accumulation was 3.3 folds more in case of niosomal gel. These novel systems were considered effective for elimination of cutaneous candidiasis (Gupta et al. Citation2010). Liposomal gel of fluconazole was also developed and process parameters for production of this system were optimized by using factorial design. Liposomes were found stable at freezing temperature and gel showed increase in skin permeation and deposition compared to control (Mitkari et al. Citation2010). Liposomes of ciclopirox olamine were incorporated in various gel systems using different gelling polymer like 0.5% w/V Agar base, 0.5% w/V Carrageenan and 0.5% w/V Carrageenan/Agar in 1:1 ratio. Optimum phosphatidylcholine (PC) and cholesterol (CHOL) ratio was used for development of liposomes as high CHOL level could disturb entrapment of drug loaded inside the vesicle. Antifungal gels were very effective in reducing the number of fungal colonies when their in-vitro antifungal activity was checked (Verma and Palani Citation2010). Further, our group also prepared oleic acid vesicles of fluconazole and evaluated vesicles for topical antifungal efficacy. Penetration capacity of the vesicle into skin was determined by using the confocal microscopic study. Results of the study showed that vesicles could penetrate the stratum corneum and could retain the drug accumulated in epidermal part of skin. shows confocal microscopic images of oleic acid vesicle in rat skin. Images indicate presence of oleic acid vesicles in stratum corneum of skin. From the figure it is clear that fluorescence intensity was detectable up to a thickness of 55–60 μm (Zakir et al. Citation2010). Deformable membrane vesicles (DMVs) of griseofulvin were prepared and tested for effective dermal treatment of dermatophytosis. They were compared with liposomes for drug permeation, skin retention and histopathological studies and were found to be more promising than those of liposomes (Aggarwal and Goindi Citation2012).
Ethosomes
Ethosomes are similar to liposomes but have high ethanol content in them. Ethosomes are very soft vesicles that contain phospholipids, ethanol and water. These systems get easily penetrated through the skin. This is due to reason that ethanol cause fluidization of both ethosomal lipids and intercellular lipid of stratum corneum (Barry Citation2001). Touitou et al. (Citation2000) discovered and investigated lipid vesicular systems embodying ethanol in relatively high concentration and named them ethosomes. This system was developed for the topical delivery of minoxidil. The basic difference between liposomes and ethosomes lies in their composition. The synergistic effect of combination of relatively high concentration of ethanol (20%–50%) in vesicular form was suggested to be the main reason for their better skin permeation ability (Touitou et al. Citation2000). Recently, it has been noted that ethosomes can act as good carriers for small, medium and large sized molecules (Maestrelli et al. Citation2009). These are new carriers for delivering the drugs through dermal route of varying lipophilicity. Preparation of ethosomes is easier and there is no use of any sophisticated instrument for preparation of these carriers. So these can be scaled up easily up-to industrial level (Verma and Fahr Citation2004). Ethosomes of fluconazole were prepared and a comparative study of ethosomal gel with liposomal gel, marketed product and hydroethanolic solution of the drug was carried out. The drug diffused from ethosomes was twice than that from liposomes and three times higher than that from hydroethanolic solution across rat skin. Ethosomal formulation was found clinically effective compared to liposomes and hydroethanolic solution (Bhalaria et al. Citation2009). Amphotericin B ethosomes, liposomes and transferosomes were developed and evaluated for their antifungal efficacy. Transferosomal formulation showed better in-vitro antifungal activity and skin permeation so they were considered effective for the elimination of deep seated fungal infection (Devi et al. Citation2011). Further, ethosomes and ultradeformable liposomes of clotrimazole were prepared for transdermal delivery to treat the topical fungal infections. After in-vitro testing against Candida fungus, ethosomal formulation was found more effective when compared to ultradeformable liposomes (Maheshwari et al. Citation2012). Recently, econazole nitrate ethosomes were dispersed in carbopol 934 NF gel for treatment of invasive fungal infections. Prepared gel was compared with liposomal or hydroethanolic gels. Ethosomal gel showed controlled release of econazole nitrate for 12 h across the rat skin and percent drug diffused from ethosomes was nearly two folds higher than liposomal and hydroethanolic gels. Stability studies of gel were carried out for 180 days and it showed very low aggregation and insignificant growth in vesicular size as compared to liposomal and hydroethanolic gels (Verma and Pathak Citation2012).
Microemulsions
Microemulsions are homogeneous, transparent, thermodynamically stable dispersions of water and oil, stabilized by a surfactant, usually in combination with a cosurfactant (Kumar and Mittal Citation1998). The size of the dispersion is usually 10–140 nm. In this type of system, the two liquids tend to separate out in two layers and to avoid this, a third substance called as an emulsifier is added which is, in general, surface-active agent or surfactant (Paul and Moulik Citation1997). In 1959, Schulman & his co-worker gave the term “microemulsion” to describe a multiphase system consisting of water, oil and surfactant which is usually transparent in nature (Schulman et al. Citation1959). Medicinal importance of microemulsion came into existence during 1970s (CitationShinoda et al. 1975). Microemulsions can deliver a greater amount of water and various agents applied to skin when compared to water alone or the other old delivery systems like creams and lotions. This is due to the fact that microemulsion acts as a reservoir for poorly water soluble drugs through their capacity of enhanced solubilization (Derle and Sagar Citation1975). It has been proved now that microemulsion increases the cutaneous absorption of both lipophilic and hydrophilic drug when compared with conventional formulations like emulsions and pure oils. So the microemulsion can be a good choice for delivering the antifungal drug topically to treat local fungal infections (Kreilgaard Citation2002). Charged submicron emulsion of drug combination econazole and miconazole were investigated for topical antifungal activity in earlier period. Extensive increase in topical bioavailability of drug was observed in case of charged microemulsion. It was due to binding affinity of charged emulsion droplets to the skin (Piemi et al. Citation1999). Further, microemulsion-based gel of fluconazole was developed for the treatment of invasive fungal infections. Brij 96 and Jojoba oil were employed as surfactant and oil phase, respectively, for preparation of microemulsion gel. Percutaneous absorption and stability of gel was found to be very high. It was due to the combined effect of both the lipophilic and hydrophilic domains as well as the particle size of the microemulsion (Laithy and Shaboury Citation2002). Microemulsion of the fluconazole was prepared again by using isopropyl myristate as oil phase, labrasol as surfactant and plurol oleique as co-surfactant. Percutaneous permeability of the microemulsion was five folds higher as compared to the marketed formulation of the same drug (Shah et al. Citation2009). Further, ketoconazole o/w microemulsion was prepared and effects of vehicle on the in-vitro skin permeation of drug were studied. It was found that particle size of microemulsion could affect its antifungal efficacy and small particle size could make microemulsion an excellent carrier for promoting in-vitro skin permeation of ketoconazole (KTZ) (Patel et al. Citation2010). Gel containing ciclopirox olamine microemulsion was developed for treatment of vaginal candidiasis. In-vivo antifungal activity of gel was determined on female rabbits by inducing candidal fungal infection in them. Microemulsion-based gel showed good antifungal activity in rabbits when compared with marketed formulation (1% clotrimazole gel) (Nair et al. Citation2010). Itraconazole microemulsion was developed by using transcutol as a surfactant, benzyl alcohol as oil and ethanoland phasphatidyl choline (3:2) as co-surfactant. This microemulsion was incorporated into hydrogel prepared from xanthun gum and carbopol mixture. It was found that permeation of drug and skin drug content increased with higher drug loading in microemulsion. Optimized microemulsion with 1.5% w/w drug loading was considered beneficial for the topical delivery of the itraconazole (Lee et al. Citation2010). Later on, comparative studies between emulsion, emulgel, lipogel and microemulsion-based hydrogels of fluconazole were carried out for topical antifungal efficacy. Propylene glycol and diethyleneglycol monoethyl ether were used as penetration enhancers in the formulations. Microemulsion-based hydrogels were found most effective dosage form to deliver the fluconazole topically as compared to other dosage form. Microemulsion gel containing diethyleneglycol monoethyl ether as penetration enhancer showed best performance in the antifungal activity test compared with the one containing propylene glycol (Salerno et al. Citation2011). Microemulsion-based gel of clotrimazole was prepared and evaluated for its thermodynamic stability, pH, refractive index, droplet size, viscosity and in-vitro antifungal activity. Prepared microemulsion-based hydrogel of the clotrimazole showed high skin retention and high in-vitro antifungal activity against Candida albicans as compared to conventional cream. After clinical evaluation of gel, it was found that clinical efficacy was good in 93.31% of patients who completed the study (Hashem et al. Citation2011). Microemulsion (o/w) of itraconazole was also developed and it was incorporated in polymeric gel using different polymers like Lutrol F127, xanthun gum, carbopol 934. Microemulsion-based gel with Lutrol F127 was found most effective for delivery of drug by transdermal route as it showed high skin retention and high antifungal activity (Chudasama et al. Citation2011). Voriconazole microemulsion was also developed for topical delivery to treat deep invasive fungal infections. Penetration enhancer namely sodium deoxycholate or oleic acid were used in formulations. Use of the penetration enhancer improved the transdermal delivery of the drug. Sodium deoxycholate was found more effective than oleic acid for enhancing the voriconazole permeation (El-Hadidy et al. Citation2011).
Niosomes
Niosomes are non-ionic surfactant vesicles having microscopic lamellar structure. They are formed due to admixture of non ionic surfactant (alkyl or dialkylpolyglycerol ether class) and cholesterol with subsequent hydration in aqueous media (Jaydeep et al. Citation2011). In these vesicular systems non-ionic surfactant is usually stabilized by addition of cholesterol or by using small amount of anionic surfactant like dicetyl phosphate (Malhotra and Jain Citation1994). These systems are found to be osmotically stable due to their neutral nature thus they enhance the stability of the entrapped drug (Arora and Jain Citation2007). There are a number of advantages of niosomes over liposomes such as low toxicity due to non-ionic nature, no requirement of special precaution and condition for their formulation and preparation so they can be considered good carrier for topical delivery as that of liposomes (Hu and Rhodes Citation1999). Recently, griseofulvin niosomes were incorporated in carbopol gel and clinical efficacy of this gel was evaluated against tinea corporis. It was found that niosomal gel of griseofulvin showed highest mycological cure rates (about 80%) as compared to liposomal gel (about 50%) for the treatment time of two weeks (Kassem et al. Citation2005). Further, griseofulvin was again formulated in proniosomal gel by using span (20, 40, 60, 80) as non ionic surfactant. Proniosomes prepared from span 40 showed highest entrapment efficiency and least leakage of drug from it. It was found that the proniosomal gel made from span 40 showed six folds higher skin permeation as that of plain formulation (Gupta et al. Citation2009). Later on, niosomal gel of terbinafine hydrochloride was developed by using tween (20, 40, 60 and 80) as non ionic surfactant and cholesterol as stabilizer for the preparation of the niosomes. Gels were prepared by using carbopol 934 (1% w/w) and Sodium carboxy methyl cellulose (3% w/w) as gel bases. Gel containing tween 40 niosomes (carbopol) showed maximum zone of inhibition against Aspergillus niger fungus (Sathali and Rajalakhmi Citation2010). Itraconazole niosomes were also prepared by using non ionic surfactant Span 60 and Brij 58. Niosomes prepared from the Span 60 showed highest entrapment of drug and topical antifungal efficacy (Ataei et al. Citation2011). Miconazole, an effective topical antifungal was incorporated into niosomes using Span 60 as surfactant and cholesterol as vesicle stabilizer. The niosomes were incorporated into gel by using sodium carboxy methyl cellulose as gelling polymer. Optimized formulation showed 92.10% drug release in 24 h so was considered effective in elimination of topical fungal infections (Firthouse et al. Citation2011).
Nanoparticles
Nanoparticles are the particulate dispersion or the solid particles with a size range of 10–1000nm. Drug is either dissolved, entrapped, encapsulated or attached to a nanoparticle matrix. They may be further classified into the fine particles (sized between 2,500 and 100 nanometers) and ultrafine particles (sized between 100 and 1 nanometers) (Mohanraj and Chen Citation2006). Michael Faraday provided the first description, in scientific terms, of the optical properties of nanometer-scale metals in his classic 1857 paper (Faraday Citation1857).
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are mostly employed forms of the nanoparticles for delivery of drug to the skin (Muller et al. Citation2002a). Solid lipid nanoparticles (SLN) are usually spherical in shape having diameter 10 to 1000 nm. These have solid lipid core matrix that usually can dissolve the lipophilic molecules. The example of the solid lipids employed in the formulation of SLNs are triglycerides (e.g. tristearin), diglycerides (e.g. glycerol bahenate), monoglycerides (e.g. glycerol monostearate), fatty acids (e.g. stearic acid), steroids (e.g. cholesterol), and waxes (e.g. cetyl palmitate) (Utreja and Jain Citation2001). Advantages of SLNs are the use of physiological lipids, the avoidance of organic solvents, a potential wide application spectrum (dermal and intravenous usually) and the high pressure homogenization as an established production method. (Muller et al. Citation2000). Nanostructured lipid carriers (NLCs) are another type of nanocarriers that may be effective in delivering the drug topically. NLCs consist of a lipid matrix with a special nanostructure. It is found that the NLCs increase the drug loading and firmly incorporate the drug during storage (Muller et al. Citation2002b). These nanocarriers can be produced by using high-pressure homogenization technique. This process can be modified to generate lipid particle dispersion with solid content from 60–80%. So NLC can be a good choice of carrier for drugs which are employed for treatment of topical skin infections (Müller et al. Citation2005). SLN and NLC of clotrimazole were prepared for treatment of topical fungal infections using hot high pressure homogenization technique. NLCs showed high entrapment efficiency and faster release profile as compared to SLNs with the same concentration of lipid while SLNs showed a better occlusive capacity than that of NLCs at the same concentration of lipid. Stability studies of formulations were carried out for three months and it was found that the mean diameter of these systems did not show any significant changes and thus was considered physically stable (Souto et al. Citation2004). Similarly, SLNs and NLCs of the ketoconazole were also prepared for topical delivery by employing Compritol 888 ATO as a solid lipid and natural antioxidant α-tocopherol as liquid lipid compound. It was found that prepared SLNs were stable on storage for three months but they got degraded on exposure to the light while NLCs stabilized the drug but aqueous NLC dispersion showed size increase during storage (Souto and Muller Citation2005). Later on, Solid lipid nanoparticles of miconazole were prepared for the treatment of superficial fungal infections. Compritol 888 ATO (glyceryl behenate) was used as solid lipid and propylene glycol (PG) was used to increase drug solubility in lipid. Optimized formulation of SLNs was incorporated into carbopol (934) gel. It was found that the prepared gel showed enhanced skin targeting effect in comparison to marketed gel (Bhalekar et al. Citation2009). Miconazole nitrate (MN) loaded SLNs were developed and evaluated for effective elimination of invasive fungal infections by using tristearin as solid lipid and modified solvent injection as method of preparation. SLNs were incorporated into carbopol 934 hydrogel. In-vivo activity of formulation was done by using rats infected with Candida fungal strains. Formulation showed promising effects in removal of induced fungal infections in rats (Jain et al. Citation2010). Recently, a novel SLN-Dextran hydrogel of Ketoconazole was developed and studied for its therapeutic effect. Lipid phases (compritol, precirol and a mixture precirol/almond oil) were used to prepare SLNs and formulations were characterized for their mean particle diameter, polydispersion index and zeta potential. It was found that the prepared system was biocompatible in nature whose rheological properties could be conveniently and easily adjusted by changing the derivatization degree or the concentration of the polymer. Prepared novel gel was found effective against candidal infections in rabbits (Paolicellia et al. Citation2011). Further, fluconazole SLNs were prepared for treatment of cutaneous candidiasis by using solvent diffusion as a method of preparation and Compritol 888 ATO as a lipid. Prepared SLNs showed sustained release fashion over 24 h. Fluconazole SLNs prepared from the Compritol 888 ATO showed improved dermal localization and good antifungal efficacy. So, they were considered as promising carriers for the treatment of skin fungal infection (Gupta et al. Citation2011). A comparative study between SLNs & NLCs of fluconazole for effective treatment of cutaneous candidiasis was carried out recently. Results of the study revealed that NLCs of fluconazole showed higher drug retention in skin along with a good skin targeting effect in comparison to the SLNs. Therefore, NLCs were considered as better alternatives over SLNs to deliver antifungal drug topically (Gupta and Vyas Citation2012). Finally it can be concluded that nanoparticulate carriers may be a good choice for delivering antifungal drugs topically.
Microspheres
Microspheres are characteristically free flowing powders consisting of proteins or synthetic polymers which are biodegradable in nature and ideally having a particle size less than 200 μm (Alagusundaram et al. Citation2009). Microsphere-based drug delivery has received considerable attention in the recent years. The most important characteristic of microsphere is the microphase separation morphology which endows it with a controllable variability in degradation rate and also drug release (Singh et al. Citation2011). Selection of polymer is important for production of microspheres. Biodegradable polymers are usually employed to avoid toxic effect of preparation in body. Natural polymers such as proteins and polysaccharides undergo enzymatic degradation in the body. Most synthetic biodegradable polymers contain hydrolysable linkages like amides, esters, ureas and urethanes. Polypeptides undergo enzymatic degradation while synthetic polyesters such as poly(lactic acid) and poly(glycolic acid) degrade by simple hydrolysis. Due to the biodegradable nature of microsphere they can be used for the treatment of topical fungal infections (Okada and Toguchi Citation1995). Fluconazole, an effective topical antifungal was encapsulated in microspheres by spray-drying process and effects of various process parameters on the physical characteristics of microsphere were studied. It was found that drug loading in microsphere is greatly affected by the physical state of the drug in which it exists in the matrix of the carrier. After differential thermal analysis or X-ray powder diffraction studies, it was concluded that when the drug was in crystalline form, the loading of the drug was high and the loading of the drug was low when it was used in amorphous form/molecular dispersion form (Rivera et al. Citation2004). Later on, mucoadhesive microspheres of econazole nitrate were also developed for the vaginal delivery of drug. Comparative studies were carried out by using different mucoadhesive polymers like chitosan, sodium carboxymethylcellulose and poloxamers (Lutrol® F68 and F127). It was found that microspheres prepared from the polaxamers showed improvement in solubility and in-vitro bioavailability of econazole. Polaxamer microspheres also showed high mucoadhesive strength and good in-vitro antifungal activity against Candida albicans as compared to microspheres prepared from other polymers (Albertini et al. Citation2009). Further, fluconazole microspheres were prepared by using alginate facilitated (water in oil)-in-water emulsion technology and were investigated for the effects of various processing variables on the properties of microspheres. It was found that alginate increased the entrapment efficacy of microspheres. This was because alginate could enhance the viscosity of internal aqueous phase and was able to prevent the expulsion of drug from internal aqueous phase to external aqueous phase during processing due to high viscosity and thus could enhance the entrapment of drug inside microsphere. Microspheres were found effective against Candida albicans so were considered as good carriers to deliver fluconazole topically or systemically (Maiti et al. Citation2009). Recently, chitosan-based microcapsules of the miconazole nitrate were also developed. Different ratios of chitosan and drug were taken for the formulation of microspheres and it was found that formulation containing 25 mg of drug showed the highest encapsulation efficiency (52.47%) and the acceptable mean particle size (5.65 μm). In-vitro antifungal activity of microcapsules was determined against Aspergillus niger species. Microcapsules showed higher antifungal activity compared to the commercial antifungal ointment of miconazole (Yeun et al. Citation2011).
Micelles
A micelle is defined as an aggregate of surfactant molecules dispersed in a liquid colloid. In an aqueous solution, the aggregate is formed due to mounting of hydrophilic head of surfactant molecule towards the outer solvent and sequestering of the hydrophobic tails towards the centre of micelle (Sutton et al. Citation2007). Micelles are approximately spherical in shape. Other phases, including shapes such as ellipsoids, cylinders, and bilayers are also possible. The shape and size of a micelle is a function of the molecular geometry of its surfactant molecules and solution conditions such as surfactant concentration, temperature, pH, and ionic strengths (Jones and Leroux Citation1999). The process of forming micelles is known as micellization and forms part of the phase behaviour of many lipids according to their polymorphism. This usually takes place in soaps when the hydrophilic occupies the water and the hydrophobic catches hold of the dirt in clothes that are washed. The micelle can be another good choice of carrier to deliver antifungal drug topically (Kazunori et al. Citation2001). Recently, aqueous micelle solution of clotrimazole, econazole and fluconazole were prepared using novel amphiphilic methoxy-poly-(ethylene glycol)-hexyl-substituted polylactide (MPEG-hexPLA) block copolymers for enhancement of their cutaneous bioavailability. Econazole nitrate showed highest entrapment in the micelles. Micellar formulation of econazole nitrate was compared with Pevaryl® cream (1% w/w, econazole nitrate), a marketed liposomal formulation for topical application. Micellar formulation enhanced cutaneous bioavailability of azole antifungal to a greater extent as compared to Pevaryl® cream. (Bachhav et al. Citation2011).
Novel treatment of onychomycosis
For the treatment of onychomycosis, microemulsions have been investigated as a vehicle for delivery of antifungal drugs like terbinafine and econazole. Terbinafine was tested for its effectiveness against onychomycosis in the form of microemulsion based gel. It was found that terbinafine from gel permeated three times more than that of the commercial cream in human cadaver skin. The gel also showed better antifungal efficacy against Candida albicans and Trichophyton rubrum when compared to conventional cream (Barot et al. Citation2011). Recenly, microemulsion-based gel econazole nitrate was prepared for treatment of onychomycosis. Microemulsion-based gel was evaluated for its rheology, globule size, zeta potential, spreadability and in-vitro antifungal activity. Results of all parameters were in acceptable range & microemulsion-based gel showed good antifungal activity against Aspergillus niger fungus compared to the marketed cream. Hence, the microemulsion-based gel so prepared was considered better when compared to the marketed product for treatment of nail fungal infection (Jaya et al. Citation2012). Results of these studies clearly suggest that microemulsions are good choices to deliver antifungal drug on the nail surface.
Novel treatment for the fungal infection of the eye
Vesicular drug delivery systems used in ophthalmic carrier, such as liposomes and niosomes, help in providing prolonged and controlled action at the corneal surface and preventing the metabolism of the drug by enzymes present at the tear or corneal surface (Kaur et al. Citation2004). Liposomes can enhance corneal drug absorption, which is achieved through their ability to come into intimate contact with the corneal and conjunctival surfaces (Dharma et al. Citation1986). So, liposomes are a good choice of novel career to deliver antifungal drug in ocular system.
Fluconazole-loaded liposomes were investigated for treatment of Candida keratitis in rabbits. Reverse phase evaporation technique was used for preparation of liposomes. The liposomes were delivered in the form of eye drops. Results of fluconazole-loaded liposomes were compared with plain fluconazole solution. Fuconazole-loaded liposomal formulations were found better than fluconazole solution because viscosity of liposomal formulation was higher than that of the solution. The higher viscosity may lead to an increase in residence time compared with the solution form (Fawzia et al. Citation2010). Further, effects of formulation design and freeze-drying on properties of fluconazole liposomes were studied. The effects of cholesterol molar ratio, charge-inducing agents, and a-tocopherol acetate on encapsulation efficiency values and in vitro drug release of multilamellar liposomes were studied. Freeze-dried liposomal products were prepared with or without cryoprotectants. Results showed that incorporation of stearylamine resulted in an increased entrapment of fluconazole, whereas incorporation of dicetyl phosphate decreased the drug entrapment efficiency. Physical stability studies showed superior potentials of the lyophilized product after reconstitution in comparison with those of a solution product (Ola et al. Citation2010). Recently, natamycin-encapsulated lecithin/chitosan mucoadhesive nanoparticles were prepared for prolonged ocular application. The ocular pharmacokinetics of nanoparticles and marketed formulation were evaluated in rabbits. The ocular bioavailability was increased up to 1.47-fold and clearance was decreased up to 7.4-fold as compared to marketed suspension (Bhatta et al. Citation2012).
Clinical potential of new delivery system in the treatment of superficial fungal infections
The concentration of antifungal agent reached the target tissues like skin, nail and eye after application, which is the main factor that affects its clinical efficacy. Therapeutic level of the drug in the target tissue can be achieved by frequent administration of the drug. Frequent superficial administration of antifungal drug may cause irritation or other side effects. Thus, the novel delivery approaches have given a new direction for the development of these antifungal agents as ophthalmic/cutaneous formulations. New delivery approaches give a better chance to repackage and suitably deliver these agents so as to enhance their bioavailability. Novel drug delivery systems improve permeability, and decrease dosage frequency/improve patient compliance and side effects. This will improve the safety, cost and tolerance of the antifungal therapy. Clinical effectiveness of ethosomal fluconazole gel for treatment of topical fungal infection was checked at SSG Hospital, Baroda Medical College, Vadodara, over a period of one month on 8 patients suffering from candidiasis. Clinical evaluation was carried out based on the reduction in the dimension of lesions. Reduction in skin lesion was 50%–75% with fluconazole ethosomal gel, 35%–60% with fluconazole liposomal gel, 25%–30% with marketed fluconazole cream, 15%–20% with hydroethnolic solution of drug (Bhalaria et al. Citation2009). It is clear from the results that novel formulation like ethosomes and liposomes have high clinical efficacy compared to conventional market formulation. Therefore, the new delivery approaches can be considered as clinically safe & effective. A few patents regarding novel drug delivery systems for treatment of superficial fungal infections are enlisted in (http://www.freepatentsonline.com).
Table IV. List of patents associated with novel treatment of superficial fungal infections.
Conclusions
Novel drug delivery systems show high therapeutic potential in the treatment of superficial fungal infections. They are capable of releasing the drug in controlled or sustained fashion thus minimising the adverse effect of drugs like burning, itching, swelling and other allergic reactions on skin. So for subcutaneous fungal infections any of the novel drug delivery systems can be considered as better alternative compared to conventional marketed products. It is clear from the literature that microemulsions show good penetration and spreadability on the nail surface so effective in treatment of onychomycosis as compared to conventional products like cream or gels. Similarly novel drug delivery systems like liposomes and solid lipid nanoparticles also show good corneal residence time and better ocular bioavailability as compared to conventional delivery systems like eye drops. In conditions like AIDS, these delivery systems might be highly beneficial to treat the superficial fungal infections because they do not activate immune system due to controlled release of drug. The chances of drug interactions from these delivery systems are negligible which are more prominent in case of oral antifungal agents. Thus novel drug delivery systems can be considered as highly efficient and better alternatives to treat superficial fungal infections.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adams BB. 2002. Tinea corporis gladiatorum. J Am Acad Dermatol. 47:286–290.
- Agarwal K, Manas DM, Hudson M. 1999. Terbinafine and fulminant hepatic failure. N Engl J Med. 340:1292–1293.
- Agarwal R, Katare OP. 2002. Preparation and in-vitro evaluation of miconazole nitrate loaded topical liposomes. Pharm Tech. 1–12.
- Aggarwal N, Goindi S. 2012. Preparation and evaluation of antifungal efficacy of griseofulvin loaded deformable membrane vesicles in optimized guinea pig model of Microsporum canis—Dermatophytosis. Int J Pharm. 437:277–287.
- Alagusundaram M, Madhu SCC, Umashankari K, Attuluri VB, Lavanya C, Ramkanth S. 2009. Microspheres as a novel drug delivery system- A review. Int J Chem Tech Res. 1:526–534.
- Albertini B, Passerini N, Sabatino MD, Vitali B, Brigidi P, Rodriguez R. 2009. Polymer–lipid based mucoadhesive microspheres prepared by spray-congealing for the vaginal delivery of econazole nitrate. Eur J Pharm Sci. 36:591–601.
- Amichai B, Grunwald M. 1998. Adverse drug reactions of the new oral antifungal agentsterbinafine, fluconazole, and itraconazole. Int J Dermatol. 37:410–415.
- Arikan S, Rex JH. 2001. Lipid-based antifungal agents: current status. Curr Pharm Res. 7:393–415.
- Arora R, Jain CP. 2007. Advances in niosomes as drug a carrier: A review. Asi J Pharm. 1: 29–39.
- Ataei S, Moazeni E, Gilani K, Ghaffari A, Asgharian R, Najafabi A. 2011. In-vitro evalauation of itraconazole loaded vesicles prepared from non ionic surfactant. J Pharm Sci. 1:50–52.
- Available at: http://www.drugbank.ca. Accessed on 20 February, 2012.
- Available at: http://www.mycology.adelaide.edu.au. Accessed on 12 January, 2012.
- Available at: http://www.rxlist.ca. Accessed on 20 February, 2011.
- Available at: http://www.freepatentsonline.com. Accessed on 15 December, 2012.
- Babel DE, Baughman SA. 1989. Evaluation of the adult carrier state in juvenile tinea capitis caused by Trichophyton tonsurans. J Am Acad Dermatol. 21:1209–1212.
- Bachhav YG, Mondon K, Kalia YN, Gurny MM. 2011. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J Control Rel. 153:126–132.
- Barot BS, Parejiya BP, Patel HK, Gohel MK, Shelat KP. 2011. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: optimization of formulation using D-optimal design. AAPS Pharm Sci Tech.
- Barry BW. 2001. Is transdermal drug delivery research still important today?. Drug Discov Today. 6:967–971.
- Bhalaria MK, Naik S, Misra AN. 2009. Ethosomes: a novel delivery system for antifungal drugs in treatment of topical fungal disease. Ind J Exp Bio. 47:368–375.
- Bhalekar MR, Pokharkar V, Ashwini M, Patil N. 2009. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS Pharm Sci Tech. 10:289–296.
- Bhatta RS, Chandasana H, Chhonker YS, Rathi C, Kumar D, Mitra K, Shukla PD. 2012. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in-vitro and pharmacokinetics studies. Int J Pharm. 432:105–112.
- Blank IH, Moloney J, Emslie AG. 1984. The diffusion of water across the stratum corneum as a function of its water content. J Invest Dermatol. 82:183–190.
- Brodell R, Elewski B. 2000. Antifungal drug interactions: avoidance requires more than memorization. Postgrad Med. 107:41–43.
- Chowdhary A, Singh K. 2005. Spectrum of fungal keratitis in North India. Cornea. 24:8–15.
- Chudasama A, Patel VK, Nivsarker M, Vasu K, Shishoo C. 2011. Investigation of microemulsion system for transdermal delivery of itraconazole. J Adv Pharm Tech Res. 2:30–38.
- Del RJ. 2000. Current management of onychomycosis and dermatomycoses. Curr Infect Dis Rep. 2:438–445.
- Derle DV, Sagar BSH. 1975. Microemulsion as a vehicle for transdermal permeation of nimesulide. Ind J Pharm Sci. 68:622–625.
- Devi M, Kumar SM, Mahadevan N. 2011. Amphotericin-B loaded vesicular systems for the treatment of topical fungal infection. Int J Rec Adv Pharm Res. 4:37–46.
- Dharma SK, Fishman PM, Peyman GA. 1986. A preliminary study of corneal penetration of I125- labeled iodoxuridine liposomes. Acta Ophthalmol (Copenh). 64:298–301.
- Durden F, Elewski B. 1997. Fungal infections in HIV-infected patients. Semin Cutan Mecl Surg. 16:200–212.
- Elewski B. 1999. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 133:1172–1173.
- Elewski B. 2000. Tinea capitis: a current perspective. J Am Acad Dermatol. 42:1–20.
- El-Hadidy GN, Ibrahi HK, Mohamed MI, El-Millig MF. 2011. Microemulsions as vehicles for topical administration of voriconazole: formulation and in vitro evaluation. Drug Dev Ind Pharm. 1–9.
- Evans E, Sigurgeirsson B. 1999. Double-blind, randomised study of continuous terbinafine comp intermittent itraconazole in treatment of toenail onychomycosis. BMJ. 318:1031–1035.
- Faergemann J, Baran R. 2003. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 149:1–4.
- Faraday M. 1857. Experimental relations of gold (and other metals) to light. Phil Trans Roy Soc Lond. 147:145–181.
- Fawzia SH, Ehub AF, Mohamed SAR, Dina F. 2010. Liposomes as an ocular delivery system of fluconazole: in-vitro studies. Acta Ophthalmol. 88:901–904.
- Firthouse PUM, Halith SM, Wahab SU, Sirajudin M, Mohideen SK. 2011. Formulation and evaluation of miconazole niosomes. Int J Pharm Tech Res. 3:1019–1022.
- Fleece D, Gaughan JP, Aronoff SC. 2004. Griseofulvin versus terbinafine in the treatment of tinea capitis: a meta-analysis of randomized, clinical trials. Pediatrics. 114:1312–1315.
- Friedberg ML, Pleyer U, Mondino BJ. 1991. Device drug delivery to the eye. Collagen shields, iontophoresis and pumps. Ophthalmology. 98:725–732.
- Ghannoum M, Isham N, Sheehan D. 2006. Voriconazole susceptibility of dermatophyte isolates obtained from a large worldwide tinea capitis clinical trial. J Clin Microbiol. 44:2579–2580.
- Goldstein A, Smith K, Ives T. 2000. Mycotic infections: effective management of conditions involving the skin, hair, and nails. Geriatrics. 55:40–52.
- Gulati M, Bajad S, Singh S, Ferdous AJ, Singh M. 1998. Development of liposomal Amphotericin B formulation. J Microencapsul. 15:137–151.
- Gupta AK, Chow M, Daniel CR, Aly R. 2003. Treatments of tinea pedis. Dermatol Clin. 21:431–462.
- Gupta AK, Cooper EA, Ryder JE. 2004. Optimal management of fungal infections of the skin, hair, and nails. Am J Clin Dermatol. 5:225–237.
- Gupta AK, Fleckman P, Baron R. 2000. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 43:S70–;S80.
- Gupta M, Goyal AK, Paliwal SR, Paliwal R, Mishra N, Vaidya B, et al. 2010. Development & characterization of effective topical liposomal system for localized treatment of cutaneous candidiasis. J Lip Res. 20:341–350.
- Gupta M, Tiwari S, Vyas SP. 2011. Influence of various lipid core on characteristics of SLNs designed for topical delivery of fluconazole against cutaneous candidiasis. Pharm Dev Tech. doi:10.3109/10837450.2011.598161.
- Gupta M, Vyas SP. 2012. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem Phys Lipids. 165:454–461.
- Gupta S, Ahirwar D, Sharma NK, Jadhe D. 2009. Proniosomal gel as a carrier for improved transdermal delivery of griseofulvin: preparation an in-vitro characterization. Available at: http://www.pharmainfo.net/deenanath-jhade/publications/proniosomal-gel-carrier-improved transdermal-delivery-griseofulvin-prep. Accessed on 12 March, 2012.
- Hall M, Monka C, Krupp P. 1997. Safety of oral terbinafine: results of a postmarketing surveillance study in 25,884 patients. Arch Dermatol. 133:1213–1219.
- Hart R, Bell-Syer SE, Crawford F. 1999. Systematic review of topical treatments for fungal infections of the skin and nails of the feet. BMJ. 319:79–82.
- Hashem FM, Shaker DS, Ghorab MK, Nasir M, Ismail AA. 2011. Formulation, characterization, and clinical evaluation of microemulsion containing clotrimazole for topical delivery. AAPS Pharm Sci Tech. 12:879–886.
- Hu C, Rhodes DG. 1999. Proniosomes:a novel drug carrier preparation. Int J Pharm. 185:23–35.
- Jain S, Jain S II, Khare P, Gulbake A, Bansal D, Jain SK. 2010. Design and development of solid lipid nanoparticles for topical delivery of an anti-fungal agent. Drug Del. 17:443–451.
- Jaya RK, Deboroh E, Chuah CW, Selvadurai M, Sokkalingam AD. 2012. Development and evaluation of microemulsion based gel (MBGs) containing econazole nitrate for nail fungal infection. J Pharm Res. 5:2385–2390.
- Jaydeep DY, Priyanka RK, Kumar AV, Gurubas TS. 2011. Niosomes: a review. J Pharm Res. 4:632–636.
- Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, et al. 2002. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 137:105–109.
- Jones MC, Leroux JC. 1999. Polymeric micelles- a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 48:101–111.
- Kassem MAA, Esmat S, Bendas ER, El-Komy MHM. 2005. Efficacy of topical griseofulvin in treatment of tinea corporis. Mycoses. 49:232–235.
- Kasting GB, Barai ND. 2003. Equilibrium water sorption in human stratum corneum. J Pharm Sci. 92:1624–1631.
- Katz H. 1997. Possible drug interactions in oral treatment of onychomycosis. J Am Podiatr Med Assoc. 87:571–574.
- Kaur IP, Garg A, Singla AK, Aggarwal D. 2004. Vesicular systems in ocular delivery: an overview. Int J Pharm. 269:1–14.
- Kaur IP, Kakkar S. 2010. Topical delivery of antifungal agents. Expert Opin Drug Deliv. 7:1303–1327.
- Kaur IP, Rana C, Singh H. 2008. Development of effective ocular preparations of antifungal agents. J Ocul Pharmacol Ther. 24: 481–494.
- Kazunori K, Harada A, Nagasaki Y. 2001. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Del Rev. 47:113–131.
- Korting HC, Kiencke P, Nelles S. 2007. Comparable efficacy and safety of various topical formulations of terbinafine in tinea pedis irrespective of the treatment regimen: results of a meta-analysis. Am J Clin Dermatol. 8:357–364.
- Kotwani RN, Bodhe PV, Kirodian BG, Mehta KP, Ali US, Kshirsagar NA. 2003. Treatment of neonatal candidiasis with liposomal amphotericin B (L-AMP-LRC-1): phase II study. Ind Pediatr. 40:545–550.
- Kreilgaard M. 2002. Influence of microemulsions on cutaneous drug delivery. Bull Technique Gattefosse. 95:79–100.
- Kumar P, Mittal KL. 1998. Hand Book of Microemulsions: Science and Technology. New York, NY: Marcel Dekker, pp. 20–22.
- Kuvandik G, Cetin M, Genctoy G. 2007. The prevalence, epidemiology and risk factors for onychomycosis in hemodialysis patients. BMC Infect Dis. 7:102.
- Laithy HM, Shaboury KMF. 2002. Development of cutina lipogels and gel microemulsion for topical administration of fluconazole. AAPS Pharm Sci Tech. 3:article 35.
- Lazaros GA, Papatheodoridis G, Delladetsima JK. 1996. Terbinafine-induced cholestatic liver disease. J Hepatol. 24:753–756.
- Le BC, Acar L, Zia H. 1998. Ophthalmic drug delivery systems–recent advances. Prog Retin Eye Res. 17:33–58.
- Lee EA, Balakrishnan P, Song CK, Choi JH, Noh GY, Park GC, et al. 2010. Microemulsion-based hydrogel formulation of itraconazole for topical delivery. J Pharm Invest. 40:305–311.
- Li L, Redding S, Dongari BA. 2007. Candida glabrata, an emerging oral opportunistic pathogen. J Dent Res. 86:204–215.
- Maestrelli F, Capasso G, Maria L, Rodríguez G, Rabasco AM, Ghelardini C, Mura P. 2009. Effect of preparation technique on the properties and in vivo efficacy of benzocaine-loaded ethosomes. J Lip Res. 1–8.
- Maheshwari RGS, Tekade KR, Sharma PA, Darwhekar G, Tyagi A, Patel PR, Jain DK. 2012. Ethosome and ultradeformable liposomes for transdermal delivery of clotrimazole: a comparative assessment. Saudi Pharm J20:161–170.
- Maiti S, Dey P, Kaity S, Ray S, Maji S, Sa B. 2009. Investigation on processing variables for the preparation of fluconazole-loaded ethyl cellulose microspheres by modified multiple emulsion technique. AAPS Pharm Sci Tech. 10:703–715.
- Malhotra M, Jain NK. 1994. Niosomes as drug carriers. Ind Drugs. 31:81–86.
- Manzouri B, Vafidis GC, Wyse RK. 2001. Pharmacotherapy of fungal eye infections. Expert Opin Pharmacother. 2:1849–1857.
- McClellan K, Wiseman L, Markham A. 1999. Terbinafine: an update of its use in superficial mycoses. Drugs. 58:179–202.
- Mitkari BV, Korde SA, Mahadik KR, Kokare CK. 2010. Formulation and evaluation of topical liposomal gel for fluconazole. Ind J Pharm Edu Res. 44:324–333.
- Mohanraj VJ, Chen Y. 2006. Nanoparticles – a review. Trop J Pharm Res. 5:561–573.
- Moossavi M, Bagheri B, Scher R. 2001. Systemic antifungal therapy. Dermatol Clin. 19:35–52.
- Muller RH, Radtke M, Wissing SA. 2002a. Solid lipid nanoparticles (SLN) nanostrucured lipid carrier (NLC) in cosmetic and dermatological preparation. Adv Drug Deliv Rev. 54:S131–S155.
- Muller RH, Mäder K, Gohla S. 2000. Solid lipid nanoparticles (SLN) for controlled drug delivery-review of the state of the art. Eur J Pharm Biopharm. 50:161–177.
- Müller RH, Radtke M, Souto EB. 2005. Nanostructured lipid carriers: a novel generation of solid lipid carriers. Pharm Tech Europe. 17:45–50.
- Müller RH, Radtke M, Wissing SA. 2002b. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 242:121–128.
- Nagarsenker MS, Londhe VY, Nadkarni GD. 1999. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int J Pharm. 190:63–70.
- Nair R, Sevukarajan M, Mohammed B, Kumar J. 2010. Formulation of microemulsion based vaginal gel-in vitro and in vivo evaluation. Der Pharm Let. 2:99–105.
- Ning M, Yingzhi G, Huaizhong P, Xianli C, Zhongwei G. 2005. Preparation, in vitro and in vivo evaluation of liposomal/niosomal gel delivery systems for clotrimazole. Drug Dev Ind Pharm. 31:375–383.
- Norbert M, David BF, Pieter RC. 2001. Developments in liposomal drug delivery systems. Expert Opin Biol Ther. 1:1–25.
- Okada H, Toguchi H. 1995. Biodegradable microspheres in drug delivery. Crit Rev Ther Drug Carrier Syst. 12:1–99.
- Ola HE, Soad AY, Omaima NE. 2010. Effect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomes. Saudi Pharm J. 18:217–224.
- Paolicellia P, Correntea F, Serricchioa D, Cerretoa F, Cesaa S, Titab B, et al. 2011. The system SLN-Dextran hydrogel: An application for the topical delivery of Ketoconazole. J Chem Pharm Res. 3:410–421.
- Patel MR, Patel BR, Parikh RJ, Bhatt KK, Solanki BA. 2010. Investigating the effect of vehicle on in-vitro skin permeation of ketoconazole applied in O/W microemulsions. Acta Pharm Sci. 52:65–87.
- Patel PR, Patel HH, Baria HA. 2009. Formulation and evaluation of carbopol gel containing liposomes of ketoconazole. Int J Drug Del Tech. 1:42–45.
- Paul BK, Moulik SP. 1997. Microemulsions: over view. J Disp Sci Technol. 18:301–367.
- Perveze Z, Johnson MW, Rublin RA. 2007. Terbinafine-induced hepatic failure requiring liver trans-plantation. Liver Transpl. 13:162–164.
- Pfaller MA, Messer SA, Boyken L. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS Antifungal Surveillance Program conducted in 2001 and 2002. J Clin Microbiol. 42:3142–3146.
- Piemi MPY, Korner D, Benita S, Marty JP. 1999. Positively and negatively charged submicron emulsions for enhanced topical delivery of antifungal drugs. J Control Rel. 58:177–187.
- Prausnitz MR, Noonan JS. 1998. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 87:1479–1488.
- Rangel TR. 1999. Fungal keratitis, ocular immunology and uveitis foundation: massachusetts Eye Research and Surgery Institution.
- Rivera PA, Martinez-oharriz MC, Rubio M, Irache JM, Espuelas S. 2004. Fluconazole encapsulation in PLGA microspheres by spray-drying. J Microencapsul. 21:203–211.
- Robert EM, Kalia YN. 2006. New developments in topical antifungal therapy. Am J Drug Deliv. 4:231–247.
- Roberts BJ, Friedlander SF. 2005. Tinea capitis: a treatment update. Pediatr Ann. 34:191–200.
- Salerno C, Adriana MC, Carlos B. 2011. Study of in-vitro release and percutaneous absorption of fluconazole from topical dosage form. AAPS Pharm Sci Tech. 11;986–993.
- Sathali AAH, Rajalakhmi G. 2010. Evaluation of transdermal targeted niosomal drug delivery of terbinafine hydrochloride. Int J Pharm Tech Res. 2:2081–2089.
- Scher RK, Tavakkol A, Sigurgeirsson B. 2007. Onychomycosis: diagnosis and definition of cure. JAAD. 56:939–944.
- Schulman JH, Stoeckenius W, Prince LM. 1959. Mechanism of formation and structure of micro emulsions by electron microscopy. J Phys Chem. 63:1677–1680.
- Sessa G, Weissmann C. 1968. Phospholipid spherules (liposomes) as a model for biological membranes. J Lipid Res. 9:310–318.
- Shah RR, Magdum CS, Wadkar AK, Naikwade NS. 2009. Fluconazole topical microemulsion: preparation and evaluation. Res J Pharm Tech. 2:353–357.
- Shinoda K, Friberg S. 1975. Microemulsions- colloidal aspects. Adv Colloid Interface Sci. 4:281–300.
- Singh P, Prakash D, Ramesh B, Singh N, Mani TT. 2011. Biodegradable polymeric microspheres as drug carriers; A review. Ind J Nov Drug Del. 3:70–82.
- Souto EB, Muller RH. 2005. SLN and NLC for topical delivery of ketoconazole. J Microencapsul. 22:501–510.
- Souto EB, Wissing SA, Barbosa CM, Müller RH. 2004. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 278:71–77.
- Stary A, Sarnow E. 1998. Fluconazole in the treatment of tinea corporis and tinea cruris. Dermatology. 196:237–241.
- Sutton D, Nasongkla N, Blanco E, Gao J. 2007. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 24:1029–1045.
- Svejgaard E. 1997. Recalcitrant dermatophyte infection. Dermatol Ther. 3:75–78.
- Szepietowski JC, Salomon J. 2007. Do fungi play a role in psoriatic nails?. Mycoses. 50:437–442.
- Thomas PA, Geraldine P, Kaliamurthy J. 1997. Current perspectives in mycotic keratitis: diagnosis, management and pathogenesis. Adv Med Myco. 2:111–131.
- Thomas PA. 1994. Mycotic keratitis: an underestimated mycosis. J Med Vet Mycol. 32:235–254.
- Touitou E, Dayan N, Bergelson L, Godi B, Eliaz M. 2000. Ethosomes- novel vesicular carrier for enhanced delivery, chracterization and skin permeation properties. J Control Rel. 65:403–418.
- Utreja S, Jain NK. 2001. Solid lipid nanoparticles. In: Utreja S, Jain NK, Eds. Advances in Controlled and Novel Drug Delivery. New Delhi: CBS publishers, pp. 408–425.
- Verma DD, Fahr A. 2004. Synergistic penetration effect of ethanol and phospholipids on the topical delivery of Cyclosporin A. J Control Rel. 97:55–66.
- Verma MLA, Palani S. 2010. Development and in-vitro evaluation of liposomal gel of ciclopirox olamine. Int J Pharm Bio Sci. 1:1–6.
- Verma P, Pathak K. 2012. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine. 8:489–496.
- Whitcher JP, Srinivasan M, Upadhyay MP. 2001. Corneal blindness: a global perspective. Bull World Health Organ. 79:214–222.
- Williamson J, Gordon AM, Wood R. 1968. Fungal flora of the conjunctival sac in health and disease. Br J Ophthalmol, 52, 127–133.
- Yeun CMW, Kan CW, Cheuk KL, Cheung HC, Cheng SY, Yip J, Lam PL. 2011. Development of miconazole nitrate containing chitosan microcapsules and their anti-Aspergillus niger activity. J Microencapsul. doi:10.3109/02652048.2011.642017.
- Zakir F, Vaidya B, Goyal AK, Malik B, Vyas SP. 2010. Development and characterization of oleic acid vesicle for topical delivery of Fluconazole. Drug Del. 17:238–248.
- Zuber T, Baddam K. 2001. Superficial fungal infection of the skin: where and how it appears help determine therapy. Postgrad Med. 109:117–132.