Abstract
Methemoglobin concentration is an important pathophysio-logical biomarker, reflecting the oxygen-carrying and oxygen-releasing capabilities of hemoglobin (Hb). Raman spectroscopy is used to develop a novel technique for determining the methemoglobin concentration. Raman activity combined with two-dimensional correlation analysis is an attractive method for investigating Hb oxidation, exhibiting several relevant peaks in the range of 1200–1650 cm− 1. Methemoglobin concentration is estimated by measuring the intensity of Raman peaks in the ranges of 1210–1230 cm− 1 and 1340–1380 cm− 1 with 785-nm excitation. The correlation between Raman-based methemoglobin concentration estimations and the methemoglobin concentration measured using spectrophotometry was highly significant. These results suggest the potential of Raman spectroscopy as a new quantitative approach to determine the methemoglobin concentration.
Introduction
Methemoglobin concentration is a significant clinical biomarker that plays vital roles in pathophysiology, such as the detection of adverse effects of oxidative stress, inflammation, and vascular regulation (Mohorovic Citation2007, Umbreit Citation2007). The oxidation of the ferrous iron in hemoglobin (Hb) to the ferric form produces methemoglobin, which is unable to combine reversibly with oxygen and transport it in the body (Power et al. Citation2007). The physiological concentration of methemoglobin is 1% due to the activity of a special methemoglobin reductase system and may increase when erythrocytes are affected by a variety of genetic, dietary, idiopathic, toxic, xenobiotic, pharmaceutical, and environmental compounds (Mohorovic Citation2007). Furthermore, shortly after the blood is drawn from an organism, the methemoglobin enzymatic reduction systems become disrupted and the methemoglobin concentration is spontaneously elevated. Ideally, the measurement of methemoglobin concentration should reflect the oxygen-carrying and oxygen-releasing capabilities of Hb (Rieders and Macherone Citation2001).
The cyanide and blood gas analysis methods, based on the spectrophotometry, are the current methemoglobin concentration testing methods; however, the determination of methemoglobin concentration remains a challenge (Kan et al. Citation2010). Presently, the ability to accurately measure the methemoglobin concentration using these methods is limited to a narrow time window after sample collection via venipuncture or other collection methods due to the spontaneous oxidation of Hb to methemoglobin, which increases the actual methemoglobin concentration at the time of collection (Cruz-Landeira et al. Citation2002, Evelyn and Malloy Citation1938, Fleisch Citation1959, Leahy and Smith Citation1960). Additionally, current optical detection techniques based on the absorption are also limited for use in methemoglobin measurements because methemoglobin can be easily obscured by competing background signals from myoglobin in muscle tissue or other absorbing chromophores (Beilman et al. Citation2001, Chance et al. Citation1992, Feiner and Bickler Citation2010, Feiner et al. Citation2010, Nighswander-Rempel et al. Citation2005). Thus, it is imperative to develop a suitable quantitative methemoglobin analysis method without the risk of spontaneous methemoglobin production in the blood sample (Rieders and Macherone Citation2001). Moreover, the potential clinical use of Hb-based oxygen carriers (HBOCs) introduces additional complexity in measuring methemoglobin concentrations after chemical modifications to Hb, including polymerization, cross- linking, conjugation with macromolecules, and encapsulation into phospholipids vesicles (Ali Citation2001, Hughes et al. Citation1996, Linberg et al. Citation1998, O’Hara et al. Citation2001).
Raman spectroscopy is a form of vibrational spectroscopy; the energy transitions arise from molecular vibrations, enabling the technique to selectively examine molecular bonding in and around the heme, the iron, and its ligands (Spiro Citation1975, Torres Filho et al. Citation2008). Raman spectroscopy has a wide range of applications and is an attractive technique for providing direct access to the state of Hb for non-invasive measurements (Buschman et al. Citation2000). For methemoglobin concentration analysis, Raman spectroscopy may be able to provide the clinical chemist with a simple, reagentless, quantitative method that is capable of simultaneously measuring other analytes. Relative to visible, UV and mid-IR light, tissue absorbs less near-infrared (NIR, 700–1300 nm) light; thus, NIR Raman spectroscopy produces stronger signals and probes more deeply (Berger et al. Citation1997, Wood et al. Citation2007). In this paper, Raman spectra of oxygenated Hb and oxidated Hb recorded at 785 nm are compared with those recorded at 514.5 nm, and Raman spectroscopy with 785-nm excitation is applied to methemoglobin concentration determination. We use two-dimensional (2D) Raman correlation spectroscopy, a powerful new analytical method that has been found to be advantageous especially for monitoring the methemoglobin concentration (Sasic et al. Citation2000, Xu et al. Citation2002). Raman scattering from Hb and methemoglobin occurs only at their prosthetic groups, without interference from surrounding globins or other parts of the red blood cell or of the suspending medium (Torres Filho et al. Citation2005); therefore, it is possible to investigate the vibrations of the heme groups of Hb solution exclusively, thus demonstrating the potential of Raman spectroscopy as a novel and convenient technique for determining the methemoglobin concentration.
Materials and methods
Sample preparation
Blood was extracted from healthy cattle. Hb solutions were prepared by osmotic rupture of the red blood cell membrane followed by centrifugation. The sample was used either undiluted or diluted with normal saline. The tested Hb concentration of the samples used for the Raman spectroscopic measurements of methemoglobin concentration ranged 6–8 g/dl. The sample oxygen saturation, measured using a blood gas analyzer (Radiometer ABL80COOX), was above 90%. Different methemoglobin concentrations were prepared by making the Hb reacting with the different volumes of potassium ferricyanide. The methemoglobin concentrations were measured using spectrophotometry (Helios β, Thermo), which is based on the measurements of light absorption at an isosbestic wavelength (590 nm) of the methemoglobin absorption spectrum and at a wavelength (630 nm) exhibiting the greatest variation for different methemoglobin concentrations. This method is a convenient and accurate reference approach for the determination of methemoglobin concentration, which was modified from the cyanide method of Evelyn and Malloy (Evelyn and Malloy Citation1938).
Raman instrumentation
A Renishaw inVia Raman microscope with a 785-nm laser input was used to collect data. The laser beam was focused on a 3 × 30 spot using a 50 × long-working-distance objective lens. The laser power is approximately 10 mW, and the exposure time is 50 s. The blood samples were placed in 96-well plates. To obtain consistent results, the focused laser spot was set to 200 μm underneath the liquid surface of samples.
Two-dimensional correlation analysis
In this study, spectra for the different methemoglobin concentrations were processed using 2D Raman spectroscopy using 2D Shige software, and the contour level was set as 8. The calculation of 2D correlation maps was performed after baseline correction and smoothing to remove the cosmic ray spikes caused by experimental conditions and to optimize the interpretation of the correlation plots (Ashton et al. Citation2006). The interpretation of synchronous 2D correlation plots has been used to determine the spectral changes associated with the change of the methemoglobin concentrations, based on the numerical method developed by Noda (Citation2004).
Data analysis
To estimate the methemoglobin concentration, the peak ratio (PR) of the intensities of the Raman bands for different methemoglobin concentrations were calculated using Torres Filho's method (Torres Filho et al. Citation2007):
Iinc is the absolute intensity increasing with increasing methemoglobin concentration, whereas Idec is the absolute intensity decreasing with increasing methemoglobin concentration. For each spectrum, the Raman bands of Iinc and Idec were in the same vibrational mode or characterized as the same monitor of heme structure, which can be inferred from the 2D correlation Raman spectra. These spectral ranges were chosen to maximize the signal-to-noise ratio and to minimize the contribution of the overlap between the two Hb Raman bands.
MetHb% (methemoglobin concentration) was estimated using the PR according to the formula
MetHb% = PR× a+ b. (a)
The coefficients a and b were obtained from calibration experiments in which MetHb% was independently measured using spectrophotometry. For correlation analysis, linear regressions were performed, and the correlation coefficients and their significance were tested and analyzed using Origin 8.0 and SAS. All P values correspond to two-tailed tests, with significance set at 0.01.
Results
Raman spectra were obtained with cattle Hb solutions with different methemoglobin concentrations. For intermediate methemoglobin concentrations, the Raman spectra of Hb solutions showed numerous peaks in the range of 400–1700 cm− 1. depicts representative spectra for low, medium, and high methemoglobin concentrations. A number of changes are apparent in the range of 1200–1700 cm− 1. The amplitude of each peak varied according to the methemoglobin concentration. The spectra show a number of features that distinguish between the oxyhemoglobin solutions and methemoglobin solutions. In particular, the band at 1645 cm− 1 appears in the spectra of oxyhemoglobin solutions but not in those of methemoglobin solutions. The 1550 cm− 1 band is the most intense for high methemoglobin concentrations. The band at 1375 cm− 1 is more pronounced for high-methemoglobin-concentration Hb solutions than for highly oxygenated Hb solutions, and the 1215 cm− 1 band shifts to 1228 cm− 1. The peaks in the low wavenumber region (range, 400–700 cm− 1) are attenuated from the low to the high methemoglobin concentrations. The spectrum of the oxygenated Hb solutions (low methemoglobin concentration) shows a relatively strong band at 418 cm− 1, which was assigned to the δ (Fe-O-O) bending mode based on an early study (Wood et al. Citation2007). Another band appearing at 570 cm− 1 for the oxygenated Hb solutions but absent for oxidated Hb solutions (high methemoglobin concentration) correlates well with the previously assigned υ (Fe-O2) mode (Abe et al. Citation1978). compares the spectra for the Hb solutions (low methemoglobin concentration () and high methemoglobin concentration ()) at excitation wavelengths between 514.5 nm and 785 nm. In comparison with the 514.5-nm spectrum, the spectrum recorded at 785 nm shows greater enhancement in the range of 1500–1700 cm− 1. The 785-nm spectrum exhibits apparently enhanced bands in this region, such as the 1550, 1565, and 1621 cm− 1 bands and the amide I band at 1660 cm− 1. The protein bands at 1660 cm− 1 and 1005 cm− 1 are not observed in the spectra recorded at any methemoglobin concentration at 514.5 nm, but they do appear at 785 nm, which is similar to the results of Bayden et al. (Wood et al. Citation2007). The position of the oxidation state marker band υ4, which appears at 1378 cm− 1, is enhanced by 514.5-nm excitation but not by 785-nm excitation for low methemoglobin concentration. For high methemoglobin concentration, the υ4 band in the spectra with both 514.5-nm and 785-nm excitation increases in intensity.
Figure 1. Representative 785-nm spectra after baseline correction and smoothing for low, medium, and high methemoglobin concentrations extracted from the oxidation experiment with different volumes of 10% potassium ferricyanide (power at the sample, 10 mW).
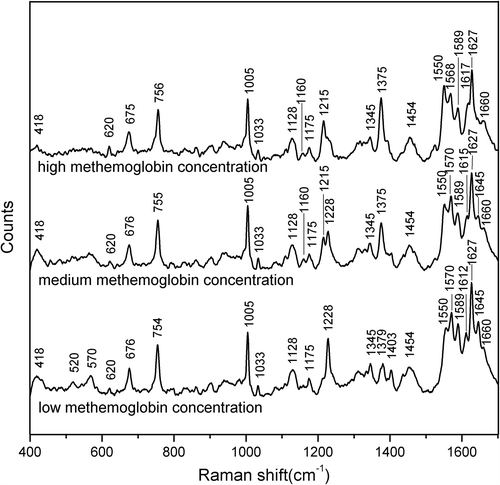
Figure 2. Comparison of the spectra recorded for the Hb solutions with (a) low methemoglobin concentration and (b) high methemoglobin concentration using 514.5-nm and 785-nm laser excitation, showing the major band assignments in the range of 500–2000 cm− 1.
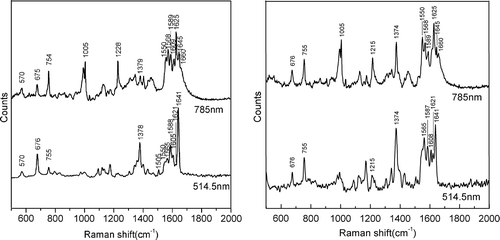
To select the most plausible band for methemoglobin formation from Hb, the synchronous 2D Raman correlation method was used to produce 2D Raman contour maps. Pretreatment methods are necessary to generate clear and consistent 2D correlation plots to exclude the numerous ridges often present between the correlation peaks. The diagonal peaks referred to as autopeaks in synchronous correlation spectra, pretreated with baseline and smoothing, are present on the diagonal, mainly in the range of 1200–1700 cm− 1, as shown in . According to the 2D correlation rules, strong autopeaks appear when any peaks in the region of interest exhibit a strong change in intensity under a given perturbation (Yu et al. Citation2003). The observation of few or no autopeaks indicates a lack of susceptibility of the applied perturbation for that spectral region. Therefore, the presence of autopeaks at 1215, 1228, 1375, 1550, 1568, 1589, 1617, 1627, and 1645 cm− 1 in indicates that spectral changes were induced by the increase in the methemoglobin content. Positive cross peaks are observed at the ranges of 1228 1570, 1589, 1612, 1627 and 1645 cm− 1, showing that these bands change in the same direction. Except for 1228 cm− 1, these bands were not easily identifiable in the 1D spectra for increasing methemoglobin concentration presented in but are easily confirmed with this 2D correlation map. Negative cross peaks positioned at 1215 and 1228 cm− 1 are assigned to the C-H methine bending vibration with changing methemoglobin content. In brief, the positive cross peaks positioned at 1215, 1375 cm− 1; 1215, 1550 cm− 1; 1375, 1550 cm− 1; and 1570, 1589, 1615, 1627 and 1645 cm− 1 change in the same direction. The negative cross peaks at 1215, 1228 cm− 1; 1228, 1375 cm− 1; 1228, 1550 cm− 1; 1215, 1570–1645 cm− 1; 1375, 1570–1645 cm− 1; and 1550, 1570–1645 cm− 1 exhibit anticorrelation.
Figure 3. Two-dimensional Raman correlation synchronous spectra in the range of 1200–1700 cm− 1 with the perturbation of oxidation by potassium ferricyanide.
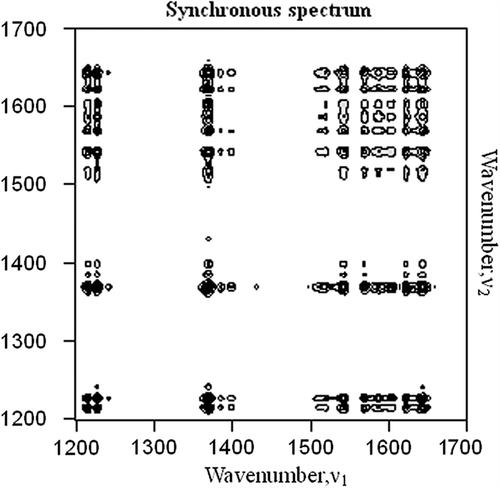
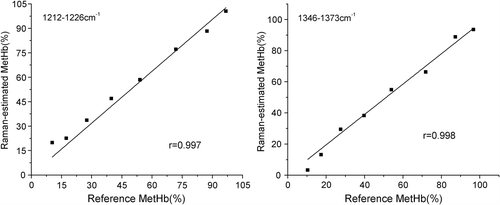
The PR was calculated for each set of measurements at the bands observed from the 2D Raman spectra and related to the methemoglobin concentration measured by spectrophotometry using a least squares linear regression. Each Raman peak was in the same position in each spectrum, independent of the Hb concentration (3.5–12 g/dl) and path length (0–400 μm). The coefficients obtained from the regressions between PR and the methemoglobin concentration (measured spectrophotometrically) were used to estimate the methemoglobin concentrations from the Raman spectra. illustrates that the correlation between Raman-based methemoglobin concentration estimations and methemoglobin concentration measured using spectrophotometry was highly significant for the ranges of 1345–1375 cm− 1 and 1215–1228 cm− 1 (r > 0.99, P < 0.01) but not for 1550–1570 cm− 1, 1550–1589 cm− 1, 1550–1615 cm− 1, 1550–1627 cm− 1 or 1550–1645 cm− 1. Pretreatment methods, such as baseline fluctuation and smoothing, likely affect the peak intensity selection from Raman spectra, and the use of PR is ideal for reducing this influence.
Figure 4. Methemoglobin concentration values estimated from Raman spectroscopy and measured using spectrophotometry. Each point represents one measurement at a given methemoglobin concentration (MetHb(%)). Each panel presents the 8 measurements used to calculate each least squares regression line. The correlation coefficients (r) were all statistically significant. Raman-estimated methemoglobin concentration was calculated using the regression coefficients obtained from the formula (a) in the ranges of 1210–1230 cm− 1 and the 1340–1380 cm− 1. The spectrophotometrically measured methemoglobin concentration is shown on the horizontal axis.
Discussion
These experiments indicate the potential for NIR Raman spectroscopy to measure the methemoglobin concentration for ultimate clinical use. Our results show, for the first time, the application of Raman spectroscopy with 785-nm excitation to evaluate the different methemoglobin concentrations in vitro. Photodissociation and hemolysis are minimized at longer excitation wavelengths (Wood et al. Citation2009, Citation2005, Wood and McNaughton Citation2002). A comparison of the spectra at 514.5-nm and 785-nm excitation supports the hypothesis that, for shorter excitation wavelengths, the resonance enhancement mechanism of the heme group dominates the spectrum, compared to which the protein bands are negligible (Wood et al. Citation2007). Similar to nearly all the micro-Raman methods currently available, we employed a single wide-aperture objective in the backscattering configuration. The laser beam coaxially illuminated the sample through the objective, which transmitted the backscattered radiation toward the spectrometer. In addition, water immersion objectives were used to increase the numerical aperture and, thereby, the resolution of the system. Importantly, previous studies demonstrated that Hb spectra from red blood cells did not differ from purified Hb spectra (Torres Filho et al. Citation2005, Citation2007, Wood et al. Citation2007, Wood and McNaughton 2002, Wood et al. Citation2001). This fact is advantageous because it allows the study of methemoglobin in its natural environment, allowing the chemical characteristics obtained from previous spectroscopic studies of Hb to be applied to in vivo studies (Polakovs et al. Citation2008, Torres Filho et al. Citation2005).
In the present study, Raman spectra obtained for different methemoglobin concentrations showed several clear peaks in the range of 1200–1700 cm− 1, including a variety of oxidation states, spin states, and coordination numbers. In particular, the oxidation state marker band, known as υ4, appeared at 1375 cm− 1, consistent with the iron atoms in the ferric states. Another difference was observed in the methine deformation region of the range between 1215 cm− 1 and 1228 cm− 1, which assigned to the C-H methine in-plane bending vibration (Abe et al. Citation1978, Wood et al. Citation2007). A number of changes were also observed in the range of 1500–1650 cm− 1. The position of these Hb Raman bands in this range agrees with the literature values (Spiro and Strekas Citation1972). We have assumed that the peak heights are proportional to the methemoglobin concentrations over a wide range. However, the absolute signal intensity of the Raman signal is influenced by many factors, such as laser intensity, fluorescence background, container materials, focal plane, and exposure time. Therefore, it is necessary to apply correct pretreatment methods to improve the quality of the Raman signals. The application of 2D correlation spectroscopy to Raman scattering is an innovative method to determine the spectral changes associated with methemoglobin concentrations. Baseline correction and smoothing can reduce the noise, resulting in clear and reliable 2D Raman spectra of Hb with oxidation perturbation. Analysis of the 2D Raman plots shows that the spin state is enhanced and the redox state is oxidized with increasing methemoglobin concentration. The band at 1228 cm− 1, assigned to the C-H methine in-plane bending vibration, loses intensity as the Hb solution becomes oxidized, whereas the band at 1215 cm− 1 appears to gain intensity, which mirrors the deoxygenation of Hb (Wood et al. Citation2007). This observation provides evidence that the methemoglobin concentration is directly proportional to the strength of the methemoglobin Raman signal. This in vitro calibration showed an excellent correlation between the methemoglobin values measured using spectrophotometry and those estimated from the Raman spectra. Successive determinations of methemoglobin concentrations (from Raman signals) showed good reproducibility.
In addition, the position of υ4, which appears at 1375 cm− 1, is consistent with Yamamoto et al.'s oxidation state marker band hypothesis for ferric hemes (Yammoto and Palmer Citation1973). In their study, the strongest band of oxyhemoglobin occurred at 1375 cm− 1 with resonance Raman spectroscopy, which supports the assignment of a ferric structure to the iron ion in oxyhemoglobin; this is consistent with the structure proposed by Weiss but not the structures proposed by Pauling and Griffith (Weiss Citation1964). In our Hb solution sample, the primary oxyhemoglobin concentration is above 90%, which indicates that the iron in both oxyhemoglobin and methemoglobin is ferric, and the band related to the redox state of these samples occurs between 1370 and 1378 cm− 1. In this research, the 785-nm Raman spectra showed that as the Hb solution is oxidized, the oxyhemoglobin transforms into methemoglobin, increasing the height of the peak at 1375 cm− 1. From this result, we came to the same conclusion as Johjima, namely that the characteristics of this peak are different from the oxidation marker band assigned by resonance Raman spectroscopy. Instead, the intensity is strongly related to the sixth ligand field strength, which may also reflect the distance between heme iron and the sixth ligand (Johjima et al. Citation1996).
The laser used in this study had an average power in the range of 0.1–100 mW and 50-s exposures. An improved signal-to-noise ratio could also be achieved by averaging a larger number of measurements. However, the repetition of the measurement on a single sample might cause light and/or heat damage. A recent study demonstrated that prolonged laser exposure causes denatured proteins and heme stacking, enhancing numerous bands in the Raman spectra (Wood et al. Citation2007). Two-dimensional Raman spectroscopy experiments confirmed an increase in the signal-to-noise ratio with increasing laser power and collection times; however, prolonged exposure to a laser power of 100 mW or less did not cause protein photooxidation.
The type of oxidizing agent used in the treatment of ferrous Hb may lead to different properties for the corresponding ferric Hb. Potassium ferricyanide and sodium nitrite are frequently used for preparing methemoglobin, but studies (Iorio Citation1981) have indicated that reaction with nitrite, rather than with potassium ferricyanide, leads to a more pronounced formation of hemichromes, which may influence the Raman measurements. When potassium ferricyanide is added to Hb at neutral pH and in very small excess over the heme, oxidation essentially occurs only at the iron, and side reactions can be ignored. However, a large excess of the reagent will induce oxidative processes at other sites of the protein, such as the –SH groups.
In summary, NIR Raman spectroscopy is a promising technique for measuring methemoglobin concentrations. Because the spectrum of Hb could be easily distinguished from any other Hb-free compartment, Raman spectroscopy can be used to determine methemoglobin concentrations in vivo and in situ. In principle, extending the NIR Raman technique application of methemoglobin concentration measurement to forensic areas, blood research, clinical diagnoses, and studies on blood substitutes may offer distinct advantages over other techniques by providing non-invasive, real-time, environmentally friendly, and reliable in vivo determinations, which are unsuitable for techniques requiring transillumination (Boyd et al. Citation2011, Brazhe et al. Citation2009, Chen et al. Citation2011, Joseph and Fratantoni Citation1991). Further research is required to make this technique practical in clinical laboratories and to develop in vivo methemoglobin concentration determination schemes.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by grants from the National Natural Science Foundation of China (No. 31271001), the National key scientific research projects of China (No. 2012CB933001) and the National High Technology Research and Development Program of China (No. 2012AA021902).
References
- Abe M, Kitagawa T, Kyogoku Y. 1978. Resonance Raman spectra of octaethylporphyrinato-Ni(lI) and meso-deuterated and 15N substituted derivatives. II. Anormal coordinate analysis. J Chem Phys. 69:4526–4534.
- Ali A. 2001. Co-oximetry interference by hemoglobin-based blood substitutes. Anesth Analg. 92:863–869.
- Ashton L, Boguslawa CMB, Blanch EW. 2006. Application of two-dimensional correlation analysis to Raman optical activity. J Mol Struct. 799:61–71.
- Beilman GJ, Myers D, Cerra FB, Lazaron V, Dahms RA, Conroy MJ, Hammer BE. 2001. Near-infrared and nuclear magnetic resonance spectroscopic assessment of tissue energetics in an isolated, perfused canine hind limb model of dysoxia. Shock. 15:392–397.
- Berger AJ, Itzkan I, Feld MS. 1997. Feasibility of measuring blood glucose concentration by near-infrared Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 53A:287–292.
- Boyd S, Bertino MF, Seashols SJ. 2011. Raman spectroscopy of blood samples for forensic applications. Forensic Sci Int. 208:124–128.
- Brazhe NA, Abdali S, Brazhe AR, Luneva OG, Bryzgalova NY, Parshina EY, et al. 2009. New insight into erythrocyte through in vivo surface-enhanced Raman spectroscopy. Biophys J. 97:3206–3214.
- Buschman HP, Marple ET, Wach ML, Bennett B, Schut TC, Bruining HA, et al. 2000. In vivo determination of the molecular composition of artery wall by intravascular Raman spectroscopy. Anal Chem. 72:3771–3775.
- Chance B, Wang NG, Maris M, Nioka S, Sevick E. 1992. Quantitation of tissue optical characteristics and hemoglobin desaturation by time- and frequency-resolved multi-wavelength spectrophotometry. Adv Exp Med Biol. 317:297–304.
- Chen ZP, Lovett D, Morris J. 2011. Process analytical technologies and real time process control a review of some spectroscopic issues and challenges. J Process Control. 21:1467–1482.
- Cruz-Landeira A, Bal MJ, Quintela O, Lopez-Rivadulla M. 2002. Determination of methemoglobin and total hemoglobin in toxicological studies by derivative spectrophotometry. J Anal Toxicol. 26:67–72.
- Evelyn KA, Malloy HT. 1938. Microdetermination of oxyhemoglobin, methemoglobin, methemoglobin, and sulfhemoglobin in single sample of blood. J Biol Chem. 126:655–662.
- Feiner JR, Bickler PE. 2010. Improved accuracy of methemoglobin detection by pulse CO-oximetry during hypoxia. Anesth Analg. 111:1160–1167.
- Feiner JR, Bickler PE, Mannheimer PD. 2010. Accuracy of methemoglobin detection by pulse CO-oximetry during Hypoxia. Anesth Analg. 111:143–148.
- Fleisch H. 1959. Quantitative determination of methemoglobin and of methemalbumin in the blood. Helvetica Physiol Et Pharmacol Acta. 17:318–328.
- Hughes GS Jr, Francom SF, Antal EJ, Adams WJ, Locker PK, Yancey EP, Jacobs EE. 1996. Effects of a novel hemoglobin-based oxygen carrier on percent oxygen saturation as determined with arterial blood gas analysis and pulse oximetry. Ann Emerg Med. 27:164–169.
- Iorio EED. 1981. Preparation and Characterization of Hemoglobin Derivatives. In: Antonini E, Rossi-Bernardi L, Chiancone E, Eds. Hemoglobins. Preparation and Characterization of Hemoglobin Derivatives. New York: Academic Press, p. 65.
- Johjima T, Wariishi H, Tanaka H. 1996. The effect of ligand field strength on nonresonance Raman characteristics of hemoproteins. Biochem Biophys Res Commun. 226:601–606.
- Joseph C, Fratantoni MD. 1991. Points to consider in the safety evaluation of hemoglobin-based oxygen carriers. Center for biologics evaluation and research. Transfusion. 31:369–371.
- Kan X, You G, Zhao L, Zhou H. 2010. Advances in research on the method for methemoglobin determination (in Chinese). Bull Acad Mil Med Sci. 34:385–388.
- Leahy T, Smith R. 1960. Notes on methemoglobin determination. Clin Chem. 6:148–152.
- Linberg R, Conover CD, Shum KL, Shorr RGL. 1998. Hemoglobin based oxygen carriers: how much methemoglobin is too much?Artif Cells Blood Substit Immobil Biotechnol. 26:133–148.
- Mohorovic L. 2007. The role of methemoglobinemia in early and late complicated pregnancy. Med Hypotheses. 68:1114–1119.
- Nighswander-Rempel SP, Kupriyanov VV, Shaw RA. 2005. Relative contributions of hemoglobin and myoglobin to near-infrared spectroscopic images of cardiac tissue. Appl Spectrosc. 59:190–193.
- Noda I. 2004. Advances in two-dimensional correlation spectroscopy. Vib Spectrosc36:143–165.
- O’Hara JF Jr, Colburn WA, Tetzlaff JE, Novick AC, Angermeier KW, Schubert A. 2001. Hemoglobin and methemoglobin concentrations after large-dose infusions of diaspirin cross-linked hemoglobin. Anesth Analg. 92:44–48.
- Polakovs M, Mironova-Ulmane N, Kurjane N, Reinholds E, Grube M. 2008. Micro-Raman scattering and infrared spectra of hemoglobin. Sixth International Conference on Advanced Optical Materials and Devices (Aomd-6), 7142.
- Power GG, Bragg SL, Oshiro BT, Dejam A, Hunter CJ, Blood AB. 2007. A novel method of measuring reduction of nitrite-induced methemoglobin applied to fetal and adult blood of humans and sheep. J Appl Physiol. 103:1359–1365.
- Rieders F, Macherone AJ. 2001. Blood methemoglobin analysis. The Fredric Rieders Family Renaissance Foundation: Willow Grove, PA, USA.
- Sasic S, Muszynski A, Ozaki Y. 2000. A new possibility of the generalized two-dimensional correlation spectroscopy. 1. Sample-sample correlation spectroscopy. J Phys Chem A. 104:6380–6387.
- Spiro TG. 1975. Resonance Raman spectroscopic studies of heme proteins. Biochim Biophys Acta. 416:169–189.
- Spiro TG, Strekas TC. 1972. Resonance Raman spectra of hemoglobin and cytochrome c: inverse polarization and vibronic scattering. Proc Natl Acad Sci U S A. 69:2622–2626.
- Torres Filho IP, Terner J, Pittman RN, Proffitt E, Ward KR. 2008. Measurement of hemoglobin oxygen saturation using Raman microspectroscopy and 532-nm excitation. J Appl Physiol. 104: 1809–1817.
- Torres Filho IP, Terner J, Pittman RN, Somera LG III, Ward KR. 2005. Hemoglobin oxygen saturation measurements using resonance Raman intravital microscopy. Am J Physiol Heart Circ Physiol. 289:H488–H495.
- Torres Filho IP, Filler R, Proffitt EK, Torres LN, Terner J, Pittman RN, Ward KR. 2007. Raman micro-spectroscopy measurement of hemoglobin oxygen saturation using 532 nm excitation. Proceedings of the 8th World Congress for Microcirculation, pp. 221–225.
- Umbreit J. 2007. Methemoglobin – It's not just blue: a concise review. Am J Hematol. 82:134–144.
- Weiss JJ. 1964. Nature of the iron-oxygen bond in oxyhaemoglobin. Nature. 203:182–183.
- Wood BR, Asghari-Khiavi M, Mechler A, Bambery KR, McNaughton D. 2009. A resonance Raman spectroscopic investigation into the effects of fixation and dehydration on heme environment of hemoglobin. J Raman Spectrosc. 40:1668–1674.
- Wood BR, Caspers P, Puppels GJ, Pandiancherri S, McNaughton D. 2007. Resonance Raman spectroscopy of red blood cells using near-infrared laser excitation. Anal Bioanal Chem. 387:1691–1703.
- Wood BR, Hammer L, Davis L, McNaughton D. 2005. Raman microspectroscopy and imaging provides insights into heme aggregation and denaturation within human erythrocytes. J Biomed Opt. 10:014005.
- Wood BR, McNaughton D. 2002. Micro-Raman characterization of high- and low-spin heme moieties within single living erythrocytes. Biopolymers67:259–262.
- Wood BR, Tait B, McNaughton D. 2001. Micro-Raman characterisation of the R to T state transition of haemoglobin within a single living erythrocyte. Biochim Biophys Acta. 1539:58–70.
- Xu JZ, Zhao Y, Zhao B, Xu W, Wu Y, Zhao D, Xi S. 2002. Application of 2D Raman correlation spectroscopy to studing the effect of rare-earth Eu3 + on hemoglobin. Chem J Chin Univ Chin. 23: 1110–1112.
- Yammoto T, Palmer G. 1973. The valence and spin state of iron in oxyhemoglobin as inferred from resonance Raman spectroscopy. J Biol Chem. 248:5211–5213.
- Yu KH, Yoo YH, Rhee JM, Lee MH, Yu SC. 2003. Two-dimensional Raman correlation spectroscopy study of the pathway for the thermal imidization of poly(amic acid). Bull Korean Chem Soc. 24:357–362.
