Abstract
We report that adenovirus mediated TNF-related apoptosis-inducing ligand (TRAIL) influenced the cell growth and cell cycle in the glioma cells in vitro. After being infected with the Ad-sTRAIL, U251 cell growth was inhibited. The expression of sTRAIL was detected using immunofluorescence. The higher rate of apoptosis was demonstrated using short-term microculture tetrazoliun (MTT) assay and flow cytometry. The rate of Ad-sTRAIL-inducing U251 cell apoptosis was increased depending on the dosage and the time. The apoptosis of G0/G1 and S phase cells was more significant than that of the control groups. The growth and proliferation of U251 cell line was inhibited after the infection of Ad-sTRAIL. It is dose- and time dependent.
Keywords::
Introduction
Gliomas are the most ordinary primary brain tumors in adults. Despite advances in surgical and medical therapy over the last 20 years, no significant increase in survival has been achieved for patients with this disease (Den et al. Citation2013). There is an urgent need for new treatments based on a better molecular understanding of gliomagenesis. The concepts of molecular therapies for malignant glioma are currently being studied in preclinical and clinical settings, including small molecules targeting specific receptor-mediated signaling pathways and gene therapies (Zhang et al. Citation2011).
Early in 1995, Wiley SR reported that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a new member of the TNF family and described its characterization in inducing cell apoptosis (Wiley et al. Citation1995). After this, studies on TRAIL were carried out in an imposing array. The research, concentrating on TRAIL, was included two main directions, signal transduction mechanism and therapeutic alliance with other strategies.
Based on the recent studies in cell cycle and apoptosis, we designed the experiment on the inhibition of glioma with sTRAIL in vitro. Replication-defective adenovirus vector expressing soluble TRAIL gene was constructed in our preceding research (Wang et al. Citation2011). Human U251 glioma cell line was used to estimate the effect of sTRAIL. A series of methods were used to assay the Ad-sTRAIL's effectiveness.
Materials and methods
Cell culture and reagents
Human U251 glioma cell line was purchased from the Cell Culture Center of Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Shanghai, China) and cultured in DMEM medium (Gibco BRL, Grand Island, NY) with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) purchased from China Center of Type Culture Collection (Wuhan University), and cultured in modified Eagle's medium with 2 mM L-glutamine and 0.1 mM non-essential amino acids (MEM-NEAA) (Cell Culture Centre of Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing), 1.0 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 10% FBS. All cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2.
Detection of the expression of sTRAIL by immunofluorescence analysis
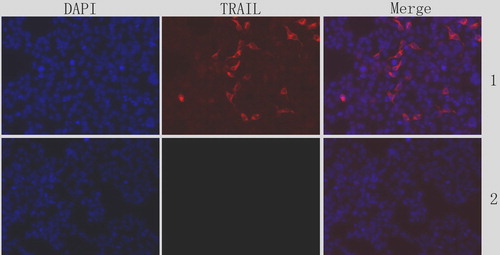
U251 cells were cultured in 24-well plates and permitted to adhere for 24 h before adding adenovirus. Before infection, the cells were washed with PBS, and then the vectors were added at the indicated number of multiplicity of infection (MOI)/cell in PBS (MOI = 100). The control group was added with 1 × PBS in the same volume. After the 24-h treatment of vectors, we carried out immunofluorescence analysis with a monoclonal antibody for mouse TRAIL as a capture antibody and FITC-marked goat anti-mouse IgG. The morphology of the cells’ nuclei was observed and captured using a fluorescence microscope after DAPI staining at an excitation wavelength of 350 nm.
Immunofluorescence microscope
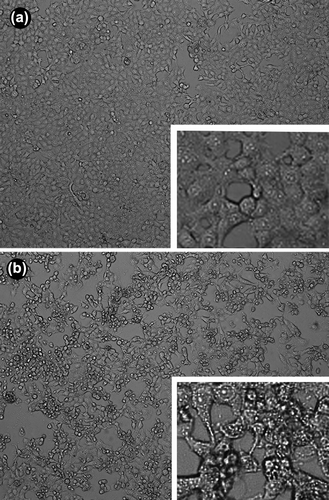
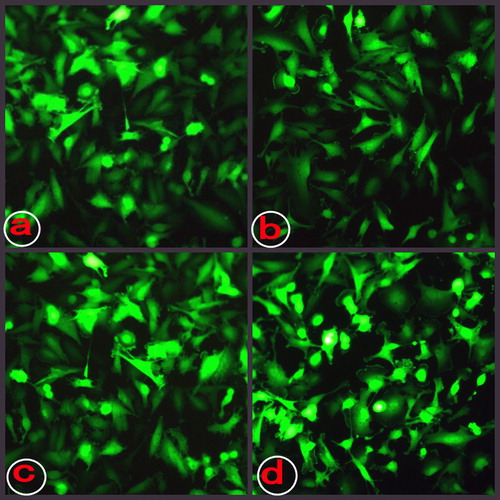
U251 cells were cultured in 24-well plates and permitted to adhere for 24 h before adding adenovirus. Before infection, the cells were washed with PBS, and then the vectors were added at the indicated number (MOI = 100). The control group was added with 1 × PBS in the same volume. After 24- and 48-h treatment of vectors, the expression of sTRAIL was detected using light microscope and fluorescence microscope.
Cell viability assay
Cell viability was quantified using a short-term microculture tetrazolium (MTT) assay. In a 96-well microplate, 1 × 104 cells per well were exposed to the treatments with Ad-sTRAIL, Ad-eGFP for 24, 48, 72, and 96 h, respectively. Cells were infected with 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, and 100 MOI of virus vectors, respectively; 10% (volume/volume) of stock MTT (3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) solution dissolved in medium at a concentration of 5 mg/mL was added to the medium directly, and cells were incubated for 30 min at 37°C, to allow cell-mediated reduction of MTT and to assay cell viability. The medium was aspirated, and cells were washed with phosphate-buffered saline. DMSO (0.5 mL, equal to the volume of medium) was added to solubilize the dye. Absorbance was measured at 570 nm.
Cell apoptosis detected using flow cytometry
U251 cells were seeded (25,000 cells/well) in 24-well plates and infected with the Ad-sTRAIL, Ad-eGFP (100 PFU/cell). After 48 h, cell death was determined using PI-Annexin V staining.
Cell cycle analysis
U251 cells were seeded (25,000 cells/well) in 24-well plates and infected with the Ad-sTRAIL, Ad-eGFP (100 pfu/cell) for 48 h. At the end of incubation, the cells were fixed in 70% ethanol at 4°C for 30 min. Cells were washed twice with PBS, resuspended in PBS containing 1 mg/ml RNase and 40 μg/ml propidium iodide, and kept at 37°C in the dark for 30 min. Red fluorescence was analyzed with FACS Calibur flow cytometer (BD, Heidelberg, Germany), using a peak fluorescence gate to discriminate aggregates. Cell distribution among cell cycle phases was determined using Cell Quest Pro software (BD, Heidelberg, Germany).
Statistical analysis
Data are presented as mean ± SD. Statistical analysis was conducted using the software package SPSS 17.0. Significance was assessed using Student's t-test and one- way ANOVA followed by Bonferroni t-test for the comparisons of multiple means, or using Kruskal–Wallis test and subsequent pairwise comparisons. Significance was set at p < 0.05.
Results
Expression of sTRAIL gene in U251 cells
The expression of sTRAIL gene in U251 cells was detected using immunofluorescence after 24 h of the infection of Ad-sTRAIL. Ad-EGFP group was detected as a control. We can see the significant expression of sTRAIL in .
Morphological changes of TRAIL-induced apoptosis in U251 cells
The morphological changes under light and immunofluorescence microscope are shown in and .
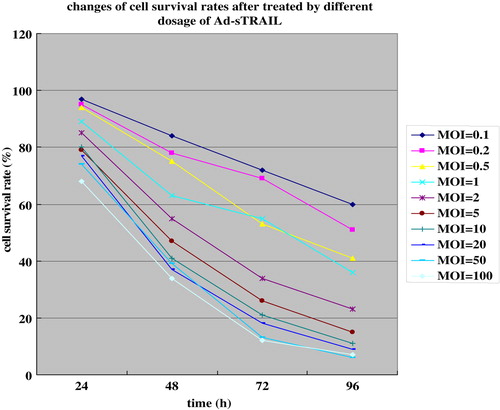
Effect of sTRAIL on the cell proliferation
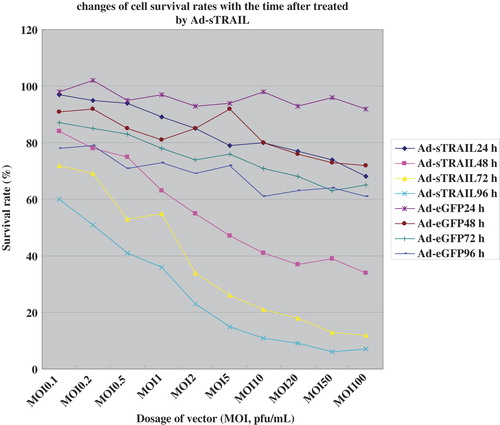
The cell-killing effect of the Ad-sTRAIL was analyzed by quantifying apoptotic U251 cells by measuring cell viability via MTT assay ( and ). The results show a significant difference in cell killing between groups in response to treatment with sTRAIL-expressing vectors versus control vectors. The survival rate of U251 cells in the Ad-sTRAIL group was shown to be dosage- and time dependent.
Cell apoptosis detected by flow cytometry
Cell death was determined using PI-Annexin V staining. WinMDI software was used to analyze the results. Results are shown in . The upper-right quadrant (UR) represents the apoptosis of advanced stage. The lower-right quadrant (LR) represents the apoptosis of prophase. Each of the apoptosis rate of Ad-sTRAIL group was more obvious than that of the control group (p < 0.05).
Table I. Apoptosis rate of U251 cells after 48 h treated with virus vector (MOI = 100) (%, ![]() ± SD).
± SD).
DNA content detected using flow cytometry
PI staining for nuclei DNA content analysis was used to characterize the cell cycle in U251 cells treated with Ad-sTRAIL for 48 h. The percentage of G0/G1 and S phase cells of Ad-sTRAIL group was decreased significantly compared with that of control groups (, p < 0.05) showing a G0/G1 and S phase cytotoxicity of sTRAIL specifically. Proportionality of G2/M phase in the Ad-sTRAIL group increased significantly when compared with that of control groups (, p < 0.05), suggesting that sTRAIL could induce the cell cycle arrest at G2/M phase.
Table II. Cell cycle of U251 cells after 48 h treated with virus vector (MOI = 100) (%, ![]() ± SD).
± SD).
Discussion
The available preliminary data indicate that activation of apoptotic TRAIL receptor signaling using sTRAIL may indeed prove beneficial to cancer patients and certainly warrant further evaluation of this reagent in clinical trials. Knowledge on the mechanisms of TRAIL promoted the utilization of TRAIL. However, intrinsic and/or acquired resistance to TRAIL receptor signaling is likely to pose a significant hurdle to clinical efficiency. Intrinsic or acquired resistance to TRAIL can often be overcome by a combination of TRAIL-based agents with chemotherapeutics, radiations, or other novel therapeutic drugs (Jeong et al. Citation2009, Hamai et al. Citation2006). Thus, the presence of in vitro synergy may be a useful indicator for potential clinical benefit in combinatorial strategies.
Research discovered that the signal transduction mechanism includes the extrinsic apoptosis pathway and the intrinsic apoptosis pathway (Kim et al. Citation2004, Kimberley and Screaton Citation2004, Ashkenazi et al. Citation2008). The extrinsic apoptosis pathway triggers apoptosis independently of p53 in response to pro-apoptotic ligands. These ligands activate specific pro-apoptotic receptors, such as TRAIL-R1 and TRAIL-R2. Death receptor binding leads to the recruitment of the adaptor Fas-associated death domain (FADD) and initiators procaspase-8 and procaspase-10 to rapidly form the death-inducing signaling complex (DISC). Procaspase-8 and procaspase-10 are cleaved into its activated configuration caspase-8 and caspase-10. Caspase-8 and caspase-10 in turn activate the effector caspase-3, caspase 6 and caspase-7, so triggering apoptosis. The intrinsic apoptosis pathway is triggered in response to DNA damage and other types of severe cell stress and involves the release of pro-apoptotic factors from the mitochondria. The p53 oncogene protein activates the pro-apoptotic B-cell chronic lymphocytic leukemia/lymphoma 2(Bcl-2) family proteins Bcl-2-associated protein (BAX) and Bcl-2 homologous antagonist/killer (BAK), which leads to the release of cytochrome c. Cytochrome c activates the apoptotic protease caspase-9. Caspase-9, in turn, activates downstream caspases, including caspase-3, caspase-6 and caspase-7, leading to apoptosis. SMAC/DIABLO (DIABLO: direct IAP-binding protein with low pI; IAP, inhibitor of apoptosis protein; SMAC: second mitochondria-derived activator of caspase), directly interacts with an inhibitor of apoptosis protein (IAP), preventing them from attenuating apoptosis. Anti-apoptotic members of the Bcl-2 family regulate the mitochondria-initiated caspase activation pathway by preventing the release of cytochrome c.
From the date we can see that most of the studies on the mechanism of TRAIL are about apoptosis. However, apoptosis and cell cycle are interwined in cell Iife and death, but how the apoptosis programs in cell cycle progression is not clear yet. Arsenic trioxide (As2O3) has been found to induce apoptosis in leukemia cell lines and clinical remissions in patients with acute promyelocytic leukemia. Ling et al. investigated the cytotoxic effect and mechanisms of action of As2O3 in human tumor cell lines (Ling et al. Citation2002). G2/M cell cycle checkpoint is a key regulator in regulating the cell proliferation and apoptosis. Kandel et al. discovered that Akt activation via overexpression of a constitutively active form or via the loss of PTEN can overcome a G2/M cell cycle checkpoint that is induced by DNA damage and suggested that this new activity of Akt in conjunction with its anti-apoptotic activity may contribute to genetic instability and could explain its frequent activation in human cancers (Kandel et al. Citation2002). It has been reported that some of the TRAIL receptors, which is regulated the cell cycle progression, can activate both caspase (through Fas-associated death domain protein [FADD]/TNF receptor 1–associated death domain protein [TRADD]) and nuclear factor NF-κB pathways in tumor cells (Degli-Esposti et al. Citation1997). An important question is whether TRAIL has any effect on the cell cycle of gliomas. Or whether there is any synergistic effect between TRAIL and cell cycle regulating factors? No definite reports were discovered till now.
In this experiment, we transfected the Ad-sTRAIL into U251 cells in vitro to investigate the Ad-sTRAIL effect on malignant glioma cells and the linkage between TRAIL- induced cell apoptosis and cell cycle. U251 cells expressed low levels of TRAIL (Chen et al. Citation2010). Results show the evidence that TRAIL can inhibit U251 cell growth by inducing apoptosis. Cell cycle analysis found that cells in G0/G1and S phase declined more significantly than those in control groups, while cell survival rate of G2/M phase increased than that of control group. We concluded that sTRAIL could kill G0/G1 and S phase U251 cells specifically, or sTRAIL could induce the cell cycle arrest at G2/M phase. The mechanism lies in the expression of interrelated genes and interaction of these factors.
Xu et.al. discovered that the APCA-induced cell cycle arrest at G2/M phase correlated with cyclinB1 and cyclin-dependent kinase 1 expression downregulation in a p53- independent manner, and also caused an increase in apoptosis, which was confirmed by characteristic morphological changes and increased apoptotic sub-G1 population (Xu et al. Citation2011). Furthermore, translocation inhibition of nuclear factor-κB, upregulation of Bax, and downregulation of Bcl-2, activation of caspase-3 and caspase-9, and cleavage of poly-(ADP-ribose) polymerase were observed in HeLa cells treated with APCA, which indicated that the mitochondrial pathway was involved in the apoptosis signal pathway. Aghaei et al. reported that A3 adenosine receptor could suppressed cell proliferation and induced G1 cell cycle arrest by downregulating the expression of CDK4, cyclin D1 and upregulating p53 expression (Aghaei et al. Citation2011). Simultaneously, loss of MMP, activation of caspase-3 and downregulation of Bcl-2 expression indicated mitochondrial signaling pathway that involved in the apoptosis. BAX protein is regarded as a key initiator of apoptosis by triggering the release of cytochrome c from mitochondria.
From the result, we concluded that the growth and proliferation of U251 cell line was inhibited after infection of Ad-sTRAIL. Ad-sTRAIL's cytotoxicity is dose- and time dependent. The rate of Ad-sTRAIL-induced U251 cell apoptosis increased with the increase in dosage and time, suggesting a G0/G1 and S phase-specific cytotoxicity of sTRAIL. The apoptosis of G0/G1 and S phase cells were more significant than that of the control groups. Maybe that sTRAIL could induce the cell cycle arrest at G2/weM phase. Apoptosis of U251 cells induced by sTRAIL is a cell cycle event.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aghaei M, Panjehpour M, Karami-Tehrani F, Salami S. 2011. Molecular mechanisms of A3 adenosine receptor-induced G1 cell cycle arrest and apoptosis in androgen-dependent and independent prostate cancer cell lines: involvement of intrinsic pathway. J Cancer Res Clin Oncol. 137:1511–1523.
- Ashkenazi A, Holland P, Eckhardt SG. 2008. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol. 26:3621–3630.
- Chen J, Sun X, Yang W, Jiang G, Li X. 2010. Cisplatin-enhanced sensitivity of glioblastoma multiforme U251 cells to adenovirus-delivered TRAIL in vitro. Tumour Biol. 31:613–622.
- Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. 1997. The novel receptor TRAIL-R4 induces NF- kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 7:813–820.
- Den RB, Kamrava M, Sheng Z, Werner-Wasik M, Dougherty E, Marinucchi M, et al. 2013. A phase I study of the combination of sorafenib with temozolomide and radiation therapy for the treatment of primary and recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys. 85:321–328.
- Hamai A, Richon C, Meslin F, Faure F, Kauffmann A, Lecluse Y, et al. 2006. Imatinib enhances human melanoma cell susceptibility to TRAIL-induced cell death: Relationship to Bcl-2 family and caspase activation. Oncogene. 25:7618–7634.
- Jeong M, Kwon YS, Park SH, Kim CY, Jeun SS, Song KW, et al. 2009. Possible novel therapy for malignant gliomas with secretable trimeric TRAIL. PLoS One. 4:e4545.
- Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, et al. 2002. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 22:7831–7841.
- Kim MH, Billiar TR, Seol DW. 2004. The secretable form of trimeric TRAIL, a potent inducer of apoptosis. Biochem Biophys Res Commun. 321:930–935.
- Kimberley FC, Screaton GR. 2004. Following a TRAIL: update on a ligand and its five receptors. Cell Res. 14:359–372.
- Ling YH, Jiang JD, Holland JF, Perez-Soler R. 2002. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol Pharmacol. 62:529–538.
- Wang Y, Dou Y, Liu S, Yao R, Sui A, Liu XP. 2011. Construction and identification of secreting recombinant adenoviral vector with human TRAIL gene. Med J Qilu. 26(6): 494–498.
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. 1995. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 3:673–682.
- Xu K, Liang X, Wang F, Xie L, Xu Y, Liu J, Qian X. 2011. Induction of G2/M phase arrest and apoptosis by potent antitumor APCA in human cervix carcinoma cells. Anticancer Drugs. 22:875–885.
- Zhang C, Wu R, Zhu H, Hu YZ, Jiang H, Lin NM, et al. 2011. Enhanced anti-tumor activity by the combination of TRAIL/ Apo-2L and combretastatin A-4 against human colon cancer cells via induction of apoptosis in vitro and in vivo. Cancer Lett. 302: 11–19.