Abstract
The success of cell microencapsulation technology in tissue engineering and protein delivery applications depends on the viability and functionality of the encapsulated cells, which in turn are dependent upon cell/matrix interactions. In this work, we compared the viability of cord blood-derived mesenchymal stromal cells (CB MSCs), engineered to secrete factor IX (FIX) for hemophilia treatment, and encapsulated in arginine-glycine-aspartate (RGD)-alginate versus fibrinogen-alginate microcapsules. We evaluated the effect of the biomimetic matrix on cell attachment, proliferation, and secretion of FIX. Compared with nonsupplemented alginate matrix, RGD-alginate significantly enhanced the viability of the encapsulated MSCs. Further, cells in RGD-alginate displayed distinct attachment morphology, thus suggesting that RGD-alginate can potentially be used for the encapsulation of MSCs in tissue engineering applications that require enhanced cell attachment and viability. However, our data also showed that RGD-alginate microcapsules, in contrast to fibrinogen-alginate microcapsules, did not significantly improve cell proliferation of or FIX secretion by encapsulated MSCs. Our findings suggest that evidence of cell attachment alone may not accurately predict the functionality of cells in biomimetic microcapsules.
Introduction
A deficiency in factor IX (FIX) leads to hemophilia B, an X-linked bleeding disorder which occurs in 1 in 30,000 males (Sadler and Davie Citation1987, Bolton-Maggs and Pasi Citation2003). Because current treatment for severe hemophilia B (patients with < 1% active FIX) involves the costly and life-long infusion of plasma derived or recombinant FIX (Gater et al. Citation2011), gene therapy has been explored as a potential alternative treatment. The most successful gene therapy method to date is the direct injection of viral vectors to deliver FIX (Nathwani et al. Citation2011). However, because of the potential safety concerns, there is a critical need for developing safer alternative gene therapy protocols (Marshall Citation2001, Check Citation2003).
Encapsulation of recombinant cells is a strategy employed for the continuous delivery of therapeutic proteins with the potential to treat a wide variety of protein deficiency diseases like hemophilia (Chang Citation2007, Citation1972, Orive et al. Citation2003, Citation2004, Lim and Sun Citation1980, Koo and Chang Citation1993, Liu and Chang Citation2012, Chang et al. Citation1993, Cieslinski and David Humes Citation1994, Wong and Chang Citation1986, Aebischer et al. Citation1994, Aebischer et al. Citation1986, Orive et al. Citation2006, Citation2009a, Thakur et al. Citation2010, Hortelano et al. Citation2001, Citation1996, Citation1999, Wen et al. Citation2006, Citation2007). FIX-engineered cells can be immuno-protected in semi-permeable, biocompatible microcapsules that are permeable to the secreted FIX transgene, thus allowing safe transplantation of allogeneic cells.
A judicious choice of cells is critical for the efficacy of this therapeutic strategy (Orive et al. Citation2003). Mesenchymal stromal cells (MSC) offer great clinical promise since they are easy to isolate, can be genetically manipulated to secrete bioactive molecules, are susceptible to molecules that modify their natural behavior, and, most importantly, have an immunosuppressive effect by modulating the immune function of the cell populations involved in alloantigen recognition and elimination (Ren et al. Citation2012).
The biomaterials used for cell encapsulation play a critical role in determining the viability and functionality of the encapsulated cells. Alginate hydrogels are natural biomaterials used extensively in cell encapsulation and transplantation because they are biocompatible, biodegradable, and gel rapidly within a solution of divalent ions. While alginates have many favorable properties, they do not specifically interact with mammalian cells, making alginate gel relatively inert. One potential way to improve performance of alginate biomaterials is to supplement the matrix with adhesion molecules like native full-length extracellular matrix (ECM) proteins such as fibronectin, fibrinogen, laminin, and collagen. However, coupling whole molecules can lead to nonspecific and difficult-to-control interactions. Alternatively, a variety of short peptides derived from ECM molecules can be readily coupled to alginate to mediate cell adhesion (Shin et al. Citation2003).
Arginine glycine aspartic acid (RGD) is an adhesion tri-peptide found in ECM proteins such as fibronectin, fibrin, and vitronectin (Ruoslahti and Pierschbacher Citation1986). It binds directly to several integrin receptors, including αVβ3 and α5β1, and is commonly coupled to biomaterials to control cell adhesion (Boudreau and Jones Citation1999, Lee and Mooney Citation2012). Previous studies showed that bioactive, RGD-coupled alginate matrix prolonged the long-term in vitro and in vivo viability of the seeded cells (Orive et al. Citation2009b, Bidarra et al. Citation2010, Markusen et al. Citation2006, Duggal et al. Citation2009). We recently designed and constructed fibrinogen-alginate microcapsules for enhanced viability and FIX secretion from the encapsulated MSCs (Sayyar et al. Citation2012). In this study, we constructed RGD-coupled alginate microcapsules for human MSCs and compared their effect on viability and FIX secretion to that of fibrinogen-alginate microcapsules.
The main aim and challenge of this work was to design and construct bioactive alginate-based microcapsules that preserve the long-term viability and functionality of encapsulated FIX-engineered MSCs. The specific objectives of this study were to: i) investigate whether the incorporation of RGD peptide can provide the required cell matrix signaling to increase cell viability; ii) assess whether the RGD-alginate matrix affects FIX secretion from the encapsulated MSCs; and iii) compare the effect of RGD-coupled alginate to the effect of fibrinogen-alginate microcapsules on the viability, attachment, proliferation, and FIX secretion of the encapsulated MSCs.
Materials and methods
MSC culture
Human cord blood was obtained after delivery with the mother's informed consent in accordance with the guidelines set out by the University of Alberta Health Research Ethics Board. Light density mononuclear cells were separated by Percoll density gradient centrifugation (Amersham Biosciences, Uppsala, Sweden) and cultured in Iscove's-modified Dulbecco's media (IMDM; Invitrogen, Burlington, ON, Canada) supplemented with 10% fetal bovine serum (FBS) and streptomycin (100 μg/ml) at 37°C in 5% CO2. After 24 h, nonadherent cells were removed and complete medium was replaced. MSCs were passaged when they reached about 60% confluency, and cells from Passages 4 to 6 were used in the experiments. Flow cytometric analysis showed positive expression for the nonhematopoietic markers CD73, CD90, and CD105, but not for the hematopoietic markers CD34 and CD45 (data not shown).
Engineering of MSCs
The PLVX-Puro vector DNA (Clontech, Mountain View, CA, USA) was engineered with traditional restriction enzyme techniques to generate a FIX-expressing lentiviral DNA construct with CMV promoter. Viral particles were generated with the Lenti-X Expression System (Clontech) according to the manufacturer's protocol. Briefly, LentiX 293T cells were transfected with the 4th generation VSV-G packaging DNA and PLVX-FIXI expression plasmid using Xfect transfection reagent to generate viable virus particles. Two rounds of freshly produced viral supernatant were used to transduce CB MSCs for 24 h at an MOI of approximately 20. At the time of transduction, MSCs were at Passage 4 and cultured in six-well plates at 40% confluency in IMDM supplemented with 10% FBS. Centrifugation at 1500 rpm for 90 min at 32°C greatly promoted viral transduction efficiency. Transduced cells were selected using puromycin (3 μg/ml) beginning 24 h after the second round of transduction. Cell media and selection antibiotics were changed every 2–3 days for 10–14 days.
Incorporation of fibrinogen into alginate microcapsules
RGD-alginate was purchased from FMC Biopolymer (Philadelphia, PA, USA). The GRGDSP peptide was cross-linked to MVG alginate with a molecular weight of about 280 kDa with 7–8 peptides per alginate molecule. Fibrinogen was added to the alginate solution at a concentration of 750 μg/ml. To verify that fibrinogen remained on the alginate microcapsules following the encapsulation process, samples were taken over time from the media in which the microcapsules were incubated and analyzed using UV spectroscopy (Beckman Coulter TM, Mississauga, ON, Canada) at 280 nm.
Cell encapsulation
MVG ultrapure alginate (MW 291 kDa) and custom-made, GRGDSP-coupled alginate (peptide/alginate molecular ratio of approximately 10/1) made from MVG were purchased from FMC BioPolymer. Human plasma fibrinogen (Sigma Aldrich, Oakville, ON, Canada) was incorporated into the MVG alginate solution as described above. FIX-engineered CB MSCs were suspended in a RGD-alginate, fibrinogen-alginate, and nonsupplemented alginate solution (control) at a concentration of 3 × 106 cells/ml (1.56% alginate). Microencapsulation was performed with an encapsulator (Nisco Engineering Inc., Zurich, Switzerland) as previously described (Chang et al. Citation1994). After extrusion, alginate microcapsules were coated with 0.05% (w/v) poly-L-lysine (PLL) and an outer layer of alginate 0.03% (w/v). Encapsulated cells were cultured under regular tissue culture conditions.
Assessment of viability and proliferation of encapsulated cells
Viability of encapsulated MSCs was assessed using trypan blue exclusion assay (Invitrogen) as previously described (Wen et al. Citation2007, García-Martín et al. Citation2002, Thakur et al. Citation2010, Sayyar et al. Citation2012). Briefly, a sample of microcapsules was placed on a microscope slide and crushed with a glass cover slip to release the encapsulated cells. The cells were then stained with trypan blue; dead (blue) and live (transparent) cells were quantified by inverted light microscope (Leica DM IL, Leica Microsystems, Richmond Hill, ON, Canada).
Proliferation of encapsulated MSCs was assessed by MTT assay according to the manufacturer's instructions, with modification for encapsulated cells (Thakur et al. Citation2010, Sayyar et al. Citation2012). Briefly, MTT reagent (Sigma-Aldrich) was prepared as a 5 mg/ml solution in PBS. One hundred microliters of microcapsules were incubated with 10 μl MTT reagent in a 96-well plate for 3 hours at 37°C. The encapsulated cells were periodically monitored under an inverted microscope for the presence of intracellular punctate (purple precipitate). After 3 hours, when the purple precipitate was clearly visible, the MTT conversion was stopped by removing the MTT-containing medium and the resultant formazan was dissolved in 100 μl dimethyl sulphoxide (BDH Inc. Toronto, Ont., Canada). The plate was incubated in the dark for 3 hours at room temperature and the absorbance of each well was measured at 562 nm in a microtiter plate reader (EL 808, BIO-TEK Instruments Inc., Winooski, VT, USA).
F-actin cytoskeleton staining of encapsulated cells
Encapsulated cells (100 μl of capsules) were fixed in 4% paraformaldehyde. Cells were then washed once in pre-warmed PBS and permeabilized by incubation in 0.1% Triton X-100 in PBS at room temperature for 10 minutes followed by two washes in PBS. Cells were stained with Alexa Fluor 633 phalloidin (15 μl methanolic stock solution in 200 μl PBS) (Molecular Probes, Invitrogen) for 30 min in the dark. To reduce nonspecific background staining with these conjugates, 1% bovine serum albumin was added to the staining solution. A small portion of each stained sample was placed onto a glass slide and analyzed by an inverted confocal microscopy (Zeiss 510, Carl Zeiss Inc., Toronto, ON, Canada).
Transmission electron microscopy
All transmission electron microscopic (TEM) studies were done in collaboration with the electron microscopy facility at the McMaster Children's Hospital (Hamilton, ON, Canada).
Alginate microcapsules containing cells were fixed with 2% glutaraldehyde (v/v) in 0.1 M sodium cacodylate buffer (pH 7.4). The samples were rinsed twice with buffer solution then postfixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 hour. The samples were dehydrated through a graded ethanol series (50%, 70% (2X), 95% (2X), 100% (2X)).
The final dehydration for the TEM samples was done in 100% propylene oxide (PO). Infiltration with Spurr's resin was done using a graded series of PO:Spurr's (2:1, 1:1, 1:2, 0:1 (3X)) with rotation of the samples in between solution changes. The samples were transferred to embedding molds which were then filled with fresh 100% Spurr's resin and polymerized overnight in a 60°C oven. Thin sections were cut on a Leica UCT ultramicrotome (Leica Microsystems) and picked up onto copper grids. The sections were poststained with uranyl acetate and lead citrate and then viewed in a JEOL JEM 1200 EX TEMSCAN transmission electron microscope (JEOL, Peabody, MA, USA) operating at an accelerating voltage of 80 kV.
FIX ELISA assay
Human FIX antigen was detected by ELISA assay as previously described (Wen et al. Citation2006, Citation2007, Sheffield et al. Citation2004, Sayyar et al. Citation2012) using the reagents and protocol from Affinity Biologicals Inc. (Ancaster, ON, Canada).
Statistical analysis
Analysis of variance (ANOVA) was carried out to determine whether significant differences existed in the groups of data. Student's t-test was conducted as a post-hoc test to compare pairs of data. Differences were considered significant for P < 0.05. Data are expressed as means ± SD.
Results
Assessment of viability, proliferation and FIX secretion of MSCs in RGD-alginate microcapsules
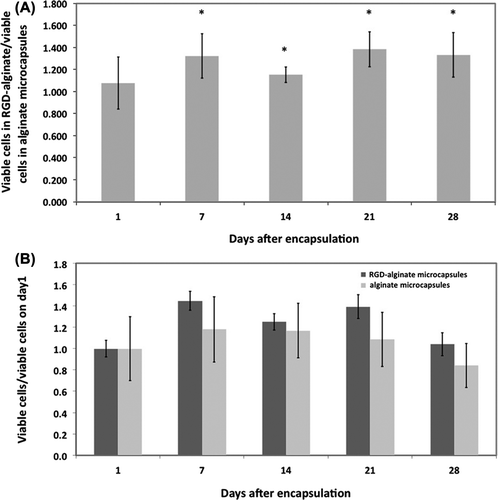
We first evaluated the viability and proliferation of FIX- engineered MSCs in three-dimensional RGD-alginate matrices in vitro for 28 days. Trypan blue exclusion assay showed high cell viability on Day 1 in both samples (82% in alginate and 89% in RGD-alginate). By Day 28, viability dropped to 51% in alginate microcapsules but remained statistically higher at 72% (p < 0.05) in RGD-alginate microcapsules. MTT assay results also confirmed more proliferating cells in RGD-alginate than in alginate microcapsules on Day 28 (). However, and despite the enhanced viability, RGD-alginate did not result in a statistically significant increase in cell proliferation during the 4-week in vitro culture ().
Figure 1. Viability and proliferation of human CB MSCs in RGD-alginate. (A) The effect of RGD-alginate on viability of encapsulated cells. The ratio of viable cells in RGD-alginate to the viable cells in alginate was calculated using the MTT assay. (B) Comparison of MSC proliferation in RGD-alginate and alginate (no significant difference). The ratio of viable cells at different days per viable cells at Day 1 was calculated using the MTT assay. Data are means ± SD, n = 3, Student's t-test. *Significant difference from a value of 1.0, P < 0.05.
Next, FIX ELISA assay was used to evaluate the effect of RGD-alginate encapsulation on FIX secretion by MSC during the 28 days of in vitro culture. The presence of RGD peptides in the alginate matrix resulted in slightly higher, albeit non-statistically significant, increase in FIX secretion from the encapsulated MSC during most of the culture period ().
Comparison of RGD-alginate vs. fibrinogen-alginate on cell viability and FIX secretion
We have previously reported significantly enhanced FIX secretion from MSC encapsulated in fibrinogen- supplemented alginate microcapsules (Sayyar et al. Citation2012). Here, the viability and FIX secretion of MSCs in RGD-alginate microcapsules versus fibrinogen-alginate microcapsules were compared. Both RGD-alginate and fibrinogen-alginate microcapsules significantly enhanced the viability of the encapsulated cells compared with nonsupplemented microcapsules during 28 days of in vitro culture ().
Figure 3. Effect of RGD or fibrinogen modification on viability and FIX secretion of encapsulated cells. (A) Effect of RGD modification versus fibrinogen modification on viability of the encapsulated MSC. (B) Effect of RGD modification versus fibrinogen modification on FIX secretion from the encapsulated MSCs. *Significant difference from a value of 1.0, P < 0.05.
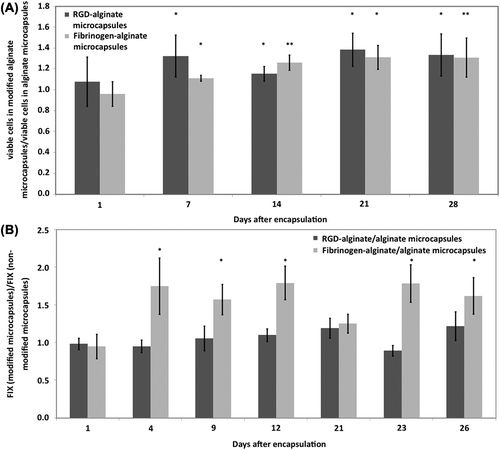
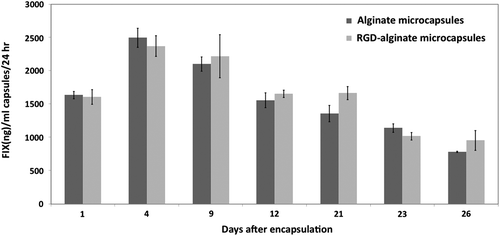
FIX secreted (measured as ng FIX/ml capsules/ml media/24 hr) from RGD-alginate or fibrinogen-alginate microcapsules were normalized to the secretion from nonsupplemented alginate microcapsules (). The incorporation of fibrinogen resulted in significantly higher FIX secretion from the encapsulated cells compared to RGD-alginate microcapsules.
Cell–matrix analysis
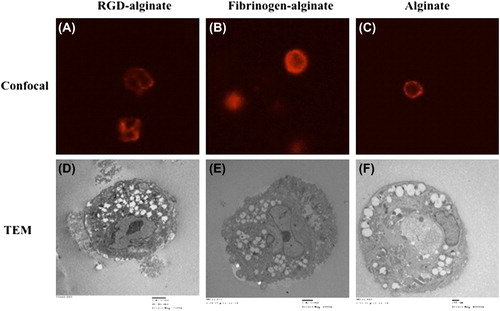
To analyze cell–matrix interactions within microcapsules, we assessed evidence of cell attachment in encapsulated cells within RGD-alginate, fibrinogen-alginate, and nonsupplemented alginate using a confocal microscopy and a transmission electron microscopy (TEM) (). Confocal microscopy revealed that cells encapsulated in nonsupplemented alginate were completely rounded with strong F-actin filament expression close to the cell membrane (). Additionally, there was no significant difference between the cells encapsulated in fibrinogen-alginate and nonsupplemented alginate microcapsules in terms of morphology. There were no distinct patterns or alignment of the filaments in these cells (Figures 4B and 4C), but cells encapsulated in RGD-alginate microcapsules acquired a more compact shape while depicting a more intricate pattern of F-actin filaments with more defined structure and distinct pattern ().
Under TEM, cells encapsulated in RGD-alginate microcapsules clearly demonstrated the presence of filopodia-like membrane extensions into the surrounding matrix (). Membrane extensions were less clearly demonstrated by the cells encapsulated in fibrinogen-alginate matrix () and were not observed in cells encapsulated in nonsupplemented alginate microcapsules ().
Discussion
The presence of cross-linked RGD peptides in alginate gels has been previously shown to enhance the viability of interacting C2C12 murine myoblasts (Orive et al. Citation2009b), chondrocytes (Degala et al. Citation2011), and osteoblasts (Evangelista et al. Citation2007). It was also previously observed that GRGDY- and GRGDSP-coupled alginate beads substantially retained the viability of encapsulated MSCs due to the enhanced cell–matrix interactions (Markusen et al. Citation2006, Duggal et al. Citation2009). Alginate is an inert biomaterial with low capacity to support cell attachment or interaction due to the lack of suitable mammalian cell adhesion molecules and the low protein adsorption capacity (Lee and Mooney Citation2012, Augst et al. Citation2006). Integrin receptors on the cell surface have the ability to interact with the biomimetic cues provided with RGD peptides in RGD-alginate matrix. Additionally, since the RGD peptides are cross-linked to the alginate matrix, this interaction leads to the attachment of cells to the inner core of the microcapsules while in suspension. Also, it was shown that the RGD-alginate matrix provides additional mechanical integrity to cells in alginate hydrogels via binding interactions between cells and the adhesion ligands coupled to the alginate chains (Lee and Mooney Citation2012, Orive et al. Citation2009b, Drury et al. Citation2005).
In this study, analysis of cell–matrix interactions within microcapsules using confocal microscopy demonstrates distinct patterns or alignment of the filaments, and TEM analysis confirms the presence of filopodia-like membrane extensions into the surrounding matrix in the cells enclosed in RGD-alginate. On the other hand, despite providing adhesion sites using RGD peptides that yield enhanced cell viability, encapsulated MSCs demonstrate no additional proliferation advantage. This is consistent with previous studies that report the inability of MSCs to proliferate within either 1% or 1.8% RGD-alginate microcapsules (Duggal et al. Citation2009). It is possible that other concentrations of alginate or different hydrogel/peptide properties are required to increase the proliferation of the encapsulated MSCs.
The increase in FIX secretion from encapsulated MSCs in RGD-alginate microcapsules was not statistically significant. We have previously reported significantly higher FIX secretion from MSCs encapsulated in fibrinogen-alginate microcapsules (Sayyar et al. Citation2012). To further understand such discrepancy, RGD-alginate and fibrinogen-alginate microcapsules were compared in more detail under microscopy.
Analysis of cell–matrix interactions within microcapsules using confocal microscopy revealed distinct patterns or alignment of the filaments in the cells enclosed in RGD-alginate, but not in fibrinogen-alginate or nonsupplemented alginate microcapsules. Such results support a previous study of MSCs in 1.8% RGD-alginate beads in which the cells acquired a compact shape with distinct pattern of actin filaments (Duggal et al. Citation2009).
As detected by TEM, cells encapsulated in RGD-alginate microcapsules clearly demonstrated the presence of filopodia-like membrane extensions into the surrounding matrix, which were less obvious in fibrinogen-alginate and were not observed at all in nonsupplemented alginate microcapsules. Such differences under microscopy might be related to the cross-linked chemistry of the RGD-alginate matrix, as opposed to the noncross-linked nature of the fibrinogen-alginate matrix. However, it is also possible that fibrinogen concentrations other than tested here could affect cell attachment and lead to different results.
We recently showed that fibrinogen-alginate micro- capsules significantly increased the proliferation of the encapsulated MSCs (Sayyar et al. Citation2012). In this study, the MTT assay confirmed that incorporation of RGD peptide into the alginate microcapsules did not result in significantly increased proliferation of encapsulated cells. Considering that both RGD-alginate and fibrinogen-alginate microcapsules improved the viability of the encapsulated cells to the same degree, it is likely that enhanced proliferation has a more significant effect on improving the FIX secretion from encapsulated cells. Therefore, evidence of MSCs attachment alone does not appear to be a direct predictor of better cellular function. Future studies are required to more precisely evaluate the biological mechanisms behind MSC–RGD or MSC–fibrinogen interactions that regulate cell behavior and protein secretion.
Conclusions
Cell encapsulation is an important strategy for cell-based therapies. Yet, these cell-based systems are not being clinically used due largely to their immunogenicity, as well as to the compromised viability and functionality of the encapsulated cells. This work involved design and construction of RGD-alginate microcapsules to encapsulate CB MSCs for enhanced viability and functionality. The main goals of this study was to study whether or not the viability of the encapsulated, FIX-engineered MSCs can be enhanced in three-dimensional RGD-alginate microcapsules and to evaluate RGD-alginate microcapsules as a method of FIX secretion for hemophilia B treatment. Moreover we compared RGD-alginate with our previously designed, fibrinogen- supplemented alginate microcapsules in terms of their effect on cell viability, proliferation, and FIX secretion. Compared with the nonsupplemented alginate microcapsules, RGD-alginate microcapsules significantly improved the viability of the encapsulated cells. The key advantage of RGD-alginate microcapsules is that while the hydrogel matrix encapsulates the cells and protects them from the host immune response, the RGD molecules cross-linked to the alginate matrix at the core of the microcapsule enhance cell viability by providing the cells with attachment site while in suspension, making them more useful for tissue engineering applications where improved cell attachment and viability are of great importance. However, given the insignificant effect of RGD-alginate matrix on cell proliferation, FIX secretion was not significantly improved. Similar to RGD-alginate, fibrinogen-supplemented alginate microcapsules significantly enhanced the viability of encapsulated MSCs but also increased cell proliferation and subsequent FIX secretion from the encapsulated MSC. This suggests that although both RGD-alginate and fibrinogen-supplemented alginate microcapsules provide biomimetic three-dimensional environment for encapsulated MSCs applicable in cell-therapy technologies, fibrinogen-supplemented alginate microcapsules may have a higher potential for cell-based gene therapy of hemophilia B and other inherited or acquired protein deficiencies.
Acknowledgements
Thanks to Dr. Frederick A. Ofosu for the generous gift of fibrinogen, and Marcia Reid and Marnie Timleck for processing the TEM and SEM samples.
Declaration of interest
The authors report no declaration of interest. The authors alone are responsible for the content and writing of the paper.
This work was funded in part by grants from Canadian Blood Services to G. H. and A. J. W. and NSERC IDEM-CREATE.
References
- Aebischer P, Russell PC, Christenson L, Panol G, Monchik JM, Galletti PM. 1986. A bioartificial parathyroid. ASAIO Trans. 32:134–137.
- Aebischer P, Goddard M, Signore AP, Timpson RL. 1994. Functional recovery in hemiparkinsonian primates transplanted with polymer-encapsulated PC12 cells. Exp Neurol. 126:151–158.
- Augst AD, Kong HJ, Mooney DJ. 2006. Alginate hydrogels as biomaterials. Macromol Biosci. 6:623–633.
- Bidarra SJ, Barrias CC, Barbosa MA, Soares R, Granja PL. 2010. Immobilization of human mesenchymal stem cells within RGD-grafted alginate microspheres and assessment of their angiogenic potential. Biomacromolecules. 11:1956–1964.
- Bolton-Maggs PHB, Pasi KJ. 2003. Haemophilias A and B. Lancet. 361:1801–1809.
- Boudreau NJ, Jones PL. 1999. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 339:481–488.
- Chang PL, Hortelano G, Awrey DE, Tse M. 1994. Growth of recombinant fibroblasts in alginate microcapsules. Biotechnol Bioeng. 43:925–933.
- Chang PL, Shen N, Westcott AJ. 1993. Delivery of recombinant gene products with microencapsulated cells in vivo. Hum Gene Ther. 4:433–440.
- Chang T. 1972. Arificial Cells. Illinois USA: Charles C Thomas Springfield.
- Chang T. 2007. ARTIFICIAL CELLS: biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation, cell/stem cell therapy. London: World Scientific Publisher/Imperial College Press.
- Check E. 2003. Harmful potential of viral vectors fuels doubts over gene therapy. Nature. 423:573–574.
- Cieslinski DA, David Humes H. 1994. Tissue engineering of a bioartificial kidney. Biotechnol Bioeng. 43:678–681.
- Degala S, Zipfel WR, Bonassar LJ. 2011. Chondrocyte calcium signaling in response to fluid flow is regulated by matrix adhesion in 3-D alginate scaffolds. Arch Biochem Biophys. 505:112–117.
- Drury JL, Boontheekul T, Mooney DJ. 2005. Cellular cross-linking of peptide modified hydrogels. J Biomech Eng. 127:220–228.
- Duggal S, Frønsdal KB, Szöke K, Shahdadfar A, Melvik JE, Brinchmann JE. 2009. Phenotype and gene expression of human mesenchymal stem cells in alginate scaffolds. Tissue Eng Part A. 15:1763–1773.
- Evangelista MB, Hsiong SX, Fernandes R, Sampaio P, Kong HJ, Barrias CC, et al. 2007. Upregulation of bone cell differentiation through immobilization within a synthetic extracellular matrix. Biomaterials. 28:3644–3655.
- García-Martín C, Chuah MK, Van Damme A, Robinson KE, Vanzieleghem B, Saint-Remy JM, et al. 2002. Therapeutic levels of human factor VIII in mice implanted with encapsulated cells: potential for gene therapy of haemophilia A. J Gene Med. 4:215–223.
- Gater A, Thomson TA, Strandberg-Larsen M. 2011. Haemophilia B: impact on patients and economic burden of disease. Thromb Haemost. 106:398–404.
- Hortelano G, Al-Hendy A, Ofosu FA, Chang PL. 1996. Delivery of human factor IX in mice by encapsulated recombinant myoblasts: a novel approach towards allogeneic gene therapy of hemophilia B. Blood. 87:5095–5103.
- Hortelano G, Xu N, Vandenberg A, Solera J, Chang PL, Ofosu FA. 1999. Persistent delivery of factor IX in mice: gene therapy for hemophilia using implantable microcapsules. Hum Gene Ther. 10:1281–1288.
- Hortelano G, Wang L, Xu N, Ofosu FA. 2001. Sustained and therapeutic delivery of factor IX in nude haemophilia B mice by encapsulated C2C12 myoblasts: concurrent tumourigenesis. Haemophilia. 7:207–214.
- Koo J, Chang TM. 1993. Secretion of erythropoietin from microencapsulated rat kidney cells: preliminary results. Int J Artif Organs. 16:557–560.
- Lee KY, Mooney DJ. 2012. Alginate: properties and biomedical applications. Prog Polym Sci. 37:106–126.
- Lim F, Sun AM. 1980. Microencapsulated islets as bioartificial endocrine pancreas. Science (New York, N.Y.). 210:908–910.
- Liu ZC, Chang, TMS. 2012. Intrasplenic transplantation of bioencapsulated mesenchymal stem cells improves the recovery rates of 90% partial hepatectomized rats. Stem Cells Int. 2012:1–6.
- Markusen JF, Mason C, Hull DA, Town MA, Tabor AB, Clements M, et al. 2006. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue Eng. 12:821–830.
- Marshall E. 2001. Gene therapy. Panel reviews risks of germ line changes. Science (New York, N.Y.). 294:2268–2269.
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. 2011. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 365:2357–2365.
- Orive G, De Castro M, Kong HJ, Hernández RM, Ponce S, Mooney DJ, Pedraz JL. 2009a. Bioactive cell-hydrogel microcapsules for cell-based drug delivery. J Control Release. 135:203–210.
- Orive G, De Castro M, Kong HJ, Hernández RM, Ponce S, Mooney DJ, Pedraz JL. 2009b. Bioactive cell-hydrogel microcapsules for cell-based drug delivery. J Control Release. 135:203–210.
- Orive G, Tam SK, Pedraz JL, Hallé JP. 2006. Biocompatibility of alginate-poly-l-lysine microcapsules for cell therapy. Biomaterials. 27:3691–3700.
- Orive G, Hernández RM, Gascón AR, Calafiore R, Chang TM, De Vos P. 2003. Cell encapsulation: promise and progress. Nat Med. 9:104–107.
- Orive G, Hernández RM, Rodríguez Gascón A, Calafiore R, Chang TM, de Vos P, et al. 2004. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 22:87–92.
- Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, Shi Y. 2012. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 1:51–58.
- Ruoslahti E, Pierschbacher MD. 1986. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 44:517–518.
- Sadler J, Davie E. 1987. The metabolic basis of inherited disease. In: Hemophilia A, hemophilia B, and von Willebrand's disease. New York: W.B. Saunders, pp. 575–598.
- Sayyar B, Dodd M, Wen J, Ma S, Marquez-Curtis L, Janowska- Wieczorek A, Hortelano G. 2012. Encapsulation of factor IX–engineered mesenchymal stem cells in fibrinogen–alginate microcapsules enhances their viability and transgene secretion. J Tissue Eng. Available at: http://tej.sagepub.com/content/early/2012/11/02/2041731412462018 [Accessed November 20, 2012].
- Sheffield WP, Mamdani A, Hortelano G, Gataiance S, Eltringham-Smith L, Begbie ME, et al. 2004. Effects of genetic fusion of factor IX to albumin on in vivo clearance in mice and rabbits. Br J Haematol. 126:565–573.
- Shin H, Jo S, Mikos AG. 2003. Biomimetic materials for tissue engineering. Biomaterials. 24:4353–4364.
- Thakur A, Sengupta R, Matsui H, Lillicrap D, Jones K, Hortelano G. 2010. Characterization of viability and proliferation of alginate-poly-L-lysine-alginate encapsulated myoblasts using flow cytometry. J Biomed Mater Res B Appl Biomater. 94:296–304.
- Wen J, Xu N, Li A, Bourgeois J, Ofosu FA, Hortelano G. 2007. Encapsulated human primary myoblasts deliver functional hFIX in hemophilic mice. J Gene Med. 9:1002–1010.
- Wen J, Vargas AG, Ofosu FA, Hortelano G. 2006. Sustained and therapeutic levels of human factor IX in hemophilia B mice implanted with microcapsules: key role of encapsulated cells. J Gene Med. 8:362–369.
- Wong H, Chang TM. 1986. Bioartificial liver: implanted artificial cells microencapsulated living hepatocytes increases survival of liver failure rats. Int J Artif Organs. 9:335–336.