Abstract
This study was conducted to evaluate the antimutagenic and antimicrobial activities of Schiff bases attached L-glutamine and L-asparagine. Antibacterial activities of the compounds against S. aureus, Sh. dys. typ 7, L. monocytogenes 4b, E. coli, S. typhi H, S. epidermis, Br. abortus, M. luteus, B. cereus, P. putida, and antifungal activity against Candida albicans were studied. These compounds were investigated for antimutagenic properties against Aflatoxin Bı (AFBı) using micronuclei (MN) assay in human lymphocyte cell culture in vitro. The protective role of these compounds against AFBı-induced MN is probably related to its doses.
Introduction
The chemistry of molecule-attached amino acid has been receiving significant current attention, because of their contribution to developments in medicinal chemistry (Sarı et al. Citation2013, Kuhl et al. Citation2005). Amino acids have promise as ideal targets for tumor imaging. They are required for sustenance of continuous uncontrolled growth of tumor cells. Numerous studies have demonstrated that malignant tumors can be detected with high sensitivity and specificity by imaging their increased metabolic rates of amino acids. Therefore, many natural and artificial amino acids have been radiolabeled for positron emission tomography (PET) imaging of tumor (McConathy et al. Citation2002).
Schiff bases attached amino acid is remarkable due to the imine group. Schiff bases are involved in many different biological processes: decarboxylation, transamination, electron transfer, etc. CitationAbram and Alberto investigated on amino acid–Schiff as novel inhibitors (Citation2006). They proposed that they may be used as lung scintigraphic agent of amino acid–Schiff bases. Complexes of attached amino acid Schiff base are used as non-enzymatic models for the metal-pyridoxal (vitamin B6) amino acid Schiff base systems, which are the key intermediates in many metabolic reactions of amino acids catalyzed by enzymes which require pyridoxal as a cofactor. So, the chemistry of transition metal complexes of amino acid-Schiff bases has received special attention due to their importance in variety of pharmaceutical and biological process. Liping Lu et al. studied the inhibitory activity against human tyrosine phosphatase 1B in vitro of oxovanadium (IV) complexes with amino acid–Schiff base (Lu et al. Citation2011). Zasukhina et al. have studied the antimutagenetic activity of compounds including nitrogen (2003). Many researches demonstrated that the genotoxicity of some metal salts (e.g., Cd(II), Ni(II), and Pt(II)) might depend on the phase of the cell cycle in human lymphocytes (Hartman and Hartwig Citation1998, Snow Citation1992, Coluccia et al. Citation1984).
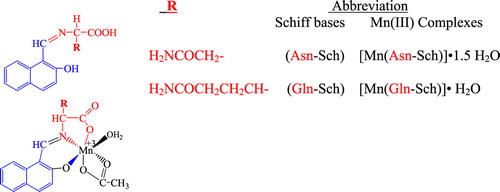
Recently, several studies have demonstrated that certain amino acids (such as cysteine, glycine, tryptophan, lysine, arginine, glutamine and alanine) provide antimutagenic effect by different test systems (Roy et al. Citation2002, Tavares et al. Citation1998, Handique and Aprem Citation1997). However, so far, no report has shown a protective effect of these compounds against AFBı genotoxicty. AFBı is the most potent of the naturally occurring mycotoxins. We know that AFBı causes various health effects on chickens in a dose–response pattern (Zhou et al. Citation2006, Pokharel et al. Citation2006). Therefore, it represents a serious risk to health in human populations (International Agency for Research on Cancer Citation1993). One of the major challenges in medical and drug delivery is to develop new antimutagens due to good prospects of their practical use for the prevention of delayed negative effects of induced mutagens in human, the main of which are high prevalence to hereditary diseases and cancer (Zasukhina et al. Citation2003). Therefore, this study was carried out to evaluate the antimicrobial and antimutagenic effects of Schiff bases attached L-glutamine and L-asparagine and their Mn (III) complexes on human peripheral blood in vitro, using micronucleus assay. First of all, amino acid–Schiff bases were synthesized using the condensation methods. Then, theirs Mn (III) complexes were synthesized by means of template method ().
Materials and methods
Chemicals and physical measurements
All chemicals investigated in the study were of reagent grade, and were purified when it was necessary. All organic solvents used in this study were purified according to the standard methods. The amino acids (L-Glutamine and L- Asparagine), 2-hydroxy-1-naphthaldehyde, methanol, and n-heptane were purchased from Sigma-Aldrich. Manganese (III) acetate was prepared using the method of Gunduz et al. (Gunduz et al. Citation1994). Elemental analyses were performed with a LECO-CHNS-9320 instrument. Metal contents were determined using a Philips PU 9285 atomic absorption instrument. 1H NMR spectra were recorded with a Bruker DPX-300 MHz and 100 MHz using TMS as an internal standard and CDCl3 as a solvent. Electronic spectra were recorded on a UV-1800 ENG240V spectrophotometer in ethanol. IR spectra were recorded on a Mattson-5000 FTIR instrument in KBr pellets. Melting points were determined with a Barnstead-Electrothermal-9200 melting point apparatus. Magnetic measurements were performed with a Sherwood Scientific magnetic susceptibility balance (Model No: MK 1) at 21°C with Hg[Co(NCS)4] as a calibration.
Test microorganisms and medium
The bacterial subcultures chosen were as follows: Listeria monocytogenes 4b ATCC19115, Staphylococcus aureus ATCC25923, Escherichia coli ATCC1280, Salmonella typhi H NCTC901.8394, Brucella abortus RSKK03026, Staphylococcus epidermis sp., Micrococcus luteusATCC9341, Shigella dysenteria type 7 NCTC 9363, Pseudomonas putida sp., Bacillus cereus RSKK863. An antifungal susceptibility test was carried out using Candida albicans Y-1200-NIH, Tokyo.
Synthesis
Schiff bases: A solution of 2-hydroxy-1-naphthaldehyde (5 mmol, 0.61 g) in methanol (50 ml) was added to amino acid (L-glutamine and L-asparagine, 5 mmol) MeOH solution (50 ml), and the synthesis method was performed according to an our previous article (Sakıyan et al. Citation2004).
Mn(III) complexes (template method): Mn(III) complexes were prepared using the template method (Sakiyan Citation2007). First, Mn(CH3COO)3.2H2O was synthesized according to our previous article (Gunduz et al. Citation1994). Then, the amino acid (L-glutamine and L-aspartic acid), 2-hydroxy-1- naphthaldehyde, and Mn(CH3COO)3 were dissolved in methanol and was synthesized according to the procedure (Sakiyan Citation2007).
Detection of antimicrobial activity
The ligands and complexes were tested for their antimicrobial activity using well-diffusion method. Each ligand and complex was kept dry at room temperature and dissolved (10 − 3 M) in DMF. DMF was used as a solvent and also as a control. It was found to have no antimicrobial activity against any of the tested organisms. A volume of 1% (v/v) of 24-h broth culture containing 106 CFU/ml was placed in sterile Petri dishes. Mueller-Hinton agar (MHA) (15 ml) kept at 45°C was then poured into the Petri dishes and allowed to solidify. Then, 6-mm-diameter wells were punched carefully using a sterile cork borer and were entirely filled with the test solutions. The plates were incubated for 24 h at 37°C. On completion of the incubation period, the mean value obtained for the two holes was used to calculate the zone of growth inhibition of each sample. Bacterial subcultures and yeast were tested for resistance to five antibiotics (produced by Oxoid Lt., Basingstoke, UK): ampicillin (preventing the growth of gram-negative bacteria), nystatin (binding to sterols in the fungal cellular membrane, altering the permeability and allowing leakage of the cellular contents), kanamycin (used in molecular biology as an agent to isolate bacteria), sulfamethoxazol (bacteriostatic antibacterial agent that interferes with folic acid synthesis in susceptible bacteria), amoxycillin (b-lactam antibiotic used to treat bacterial infections caused by susceptible microorganisms).
Cytogenetic analysis
Peripheral blood lymphocytes were taken from four (two men and two women) non-smoking healthy individuals. Lymphocyte cultures were set up by adding 0.5 mL of heparinized whole blood to RPMI-1640 chromosome medium supplemented with 15% heat-inactivated fetal calf serum, 100 IU/mL streptomycine, 100 IU/mL penicillin, and 1% L-glutamine. Lymphocytes were stimulated to divide by 1% phytohaemaglutinin.
The experiments were performed in 18 groups as follows:
Group 1:Control;
Group 2: 5 μM AFB1;
Group 3: (Asn-Sch) 40 μM;
Group 4: 5 μM AFB1 + (Asn-Sch) (5 μg/ml);
Group 5: 5 μM AFB1 + (Asn-Sch) (10 μg/ml);
Group 6: 5 μM AFB1 + (Asn-Sch) (20 μg/ml);
Group 7: [Mn(Asn-Sch)(OAc 40 μM;
Group 8: 5 μM AFB1 + [Mn(Asn-Sch)(OAc)(H2O)] (5 μg/ml)
Group 9: 5 μM AFB1 + [Mn(Asn-Sch)(OAc)(H2O)] (10 μg/ml);
Group 10: 5 μM AFB1 + [Mn(Asn-Sch)(OAc)(H2O)] (20 μg/ml)
Group 11: Gln-Sch 40 μM;
Group 12: 5 μM AFB1 + Gln-Sch (5 μg/ml);
Group 13: 5 μM AFB1 + Gln-Sch (10 μg/ml);
Group 14: 5 μM AFB1 + Gln-Sch (20 μg/ml);
Group 15: [Mn(Gln-Sch)OAc] 40 μM;
Group 16: 5 μM AFB1 + [Mn(Gln-Sch)OAc)(H2O)] (5 μg/ml);
Group 17: 5 μM AFB1 + [Mn(Gln-Sch)OAc)(H2O)] (10 μg/ml);
Group 18: 5 μM AFB1 + [Mn(Gln-Sch)OAc)(H2O)] (20 μg/ml)
On micronuclei (MN) analysis, Cytochalasin B was added 44 h after phytohemagglutinin (PHA) stimulation to a final concentration of 3 g/ml. Twenty-eight hours later (after 72-h cultivation), the cells were harvested by centrifugation (1000 g × 10 min). The supernatant was removed, the cells were mixed thoroughly, and 5 ml of cold hypotonic solution (0.05 M KCl) was added. The cells were subsequently incubated at 37°C for 20 min and centrifuged again (1000 g × 10 min). The pellet was mixed thoroughly, and 5-ml fresh fixative (1:3, aceticacid/methanol) was added dropwise. This fix ation procedure was repeated three times, and the tube was centrifuged again. The cell pellet was then resuspended in 1 ml of fresh fixative, dropped onto a clean microscopic slide, incubated at 37°C or at room temperature overnight, and stained with Giemsadye. Coded slides were scored blind by two independent individuals. Only binucleated cells were scored for MN analysis. For each subject, at least 2000 binucleated cells were analyzed for the presence of MN. For the MN scoring, the micronucleus criteria described by Countryman and Heddle were used: a diameter less than 1/3 of the main nucleus, non-refractility, not touching, and with the same color as the nucleus or lighter (for MN analysis, SPSS 15.0 program was used).
Results and discussion
Analytical data and some of the physical properties of the Schiff bases and their complexes are summarized in . The complexes are only soluble in DMF and DMSO, but insoluble in organic solvents like C2H5OH, CCl4 and benzene.
Table I. Analytical data, important IR vibration frequencies (cm−1), UV-Visible spectra values (nm) of all synthesized molecules, and 1H-NMR spectral data of Schiff bases attached L-Glutamine, L-Asparagine.
IR, UV–visible, and NMR spectra of Schiff bases and their Mn(III) complexes
summarizes the main IR and UV–visible bands of the azomethine (Schiff bases) and their Mn(III) complexes. In the IR spectra of the Schiff bases, the most characteristic bands appear at 1656–1644 cm− 1 as an overlap which is attributable to v(C = O) and v(C = N) stretching of the keto and imine forms (Sakiyan Citation2007). These bands are shifted to 1627–1617 cm− 1 in the complexes, which means that the imino nitrogen and phenolic oxygen of the ligands are coordinated to the Mn(III) ion (Sakiyan Citation2007). The IR spectra of the complexes show medium-to-intense broad bands at 586–575 cm− 1 assigned to v(M–N) stretching, and bands at 510–459 assigned to v(M-O) vibration (Sakıyan et al. Citation2004, Sarı et al. Citation2006). The 1H NMR spectrum of amino acid–Schiff bases, recorded in DMSO-d6, showed the following signals: phenolic -OH proton at 14.33–13.96 ppm (1H), -COOH protons at 10.74–10.92 ppm (1H), aromatic-H proton at 6.59–8.05 ppm (2H) phenyl as a multiplet and -CH = N- at 9.10–8.93 ppm (1H). The three signals are at 4.50 ppm and 2.00–2.90 ppm -CH and -CH2 protons. In the spectra of Schiff bases, two medium-intensity bands exhibit at ˜ 300 nm and ˜400–420 nm. It follows from the literature that these bands can be assigned to the phenol-imine and keto-amine forms (Sakiyan Citation2007). They may be attributed to n→ π*, and π→π* type transitions, respectively. Although in the spectra of all complexes the band at ˜ 300 nm exists, the other bands at ˜ 400 and 420 nm are shifted to shoulder bands. This means that in the complex Schiff bases the bands exist only in the phenol-imine form and coordinated Mn(III) ion with phenolic oxygen and imine nitrogen (Sakiyan Citation2007). For manganese (III) complexes a rather broad band appears at 475–510 nm in the visible region, and this may be attributed to the 5Eg → 5T2g transition in the octahedral complexes (Sakiyan Citation2007).
Biological activity and antimutagenic activity of amino acid–Schiff bases and their Mn(III) complexes
Amino acid–Schiff bases and their Mn(III) complexes were screened for antimicrobial activity in DMF solvent as a control substance. The compounds were tested with the same concentrations in DMF solution (0.25 μg/ml). All the synthesized compounds and antibiotic exhibited varying degree of inhibitory effects on the growth of different tested strains (, .). Synthesized amino acid–Schiff bases were inactive against L.monocytogenes, whereas their complexes were active. In general, [Mn(Asn-Sch)(OAc)(H2O)] complexes are more potent bactericides than the (Asn-Sch). This enhancement in activity may be explained on the basis of chelation theory (Sari et al. 2013, Sakiyan and Yilmaz Citation2003). Chelation reduces the polarity of the metal ion. Hence, a complex has lipophilic character, and increases the interaction between metal ion and the lipid. This lead to the breakdown of the permeability barrier of the cell wall, resulting in interference with the normal cellular processes (Sarı et al. Citation2006; Lemonnier et al. Citation2012). (Gln-Sch) was observed to be the most active against M. luteus. Furthermore, (Gln-Sch) was shown to be the most active against studied bacteria. This result may be due to differences in the structure. Electron-donating effect of glutamine group is higher than that of asparagine group. So, -NH2 molecule of glutamine group has a stronger electron-donating effect than asparagine group. This amine (-NH2) group has a strong interaction with protein or DNA chain of bacteria. In additon, the antibacterial activity of these compounds was also compared with five commercial antibiotics, namely Kanamycin, Sulfamethoxazol, Ampicillin, Amoxycillin and Nystatin. It was seen that the synthesized compounds were effective as the antibiotics mentioned.
Figure 2. Imaging of antimicrobial affectivities of [Mn(Gln-Sch) (OAc)(H2O)] and (Gln-Sch) against C. albicans, M. luteus and P. putida (2: [Mn (Gln-Sch) (OAc)(H2O)], 1: (Gln-Sch)).
![Figure 2. Imaging of antimicrobial affectivities of [Mn(Gln-Sch) (OAc)(H2O)] and (Gln-Sch) against C. albicans, M. luteus and P. putida (2: [Mn (Gln-Sch) (OAc)(H2O)], 1: (Gln-Sch)).](/cms/asset/771cbd24-6334-4208-bf3e-05a49650aff0/ianb_a_11116920_f0002_b.jpg)
Table II. Antimicrobial activity of studied compounds (0.25 μg/ml) and standard reagents (diameter of zone inhibition (mm)).
Antimutagene activity
AFBı caused significant MN formations on peripheral lymphocytes, as seen in . This increase was found to be statistically significant (P < 0.001 and P < 0.05). On the other hand, these effects of AFBı on MN were reduced after treatment with [Mn(Asn-Sch)(OAc)(H2O)], (Asn-Sch), (Gln-Sch), and [Mn(Gln-Sch)(OAc)(H2O)] (P < 0.001 and < 0.05). Especially the treatment with glutamines groups was more effective than the treatment with asparagine groups. Mutagenic results of MN assay showed that any concentration of these compounds did not show mutagenic activity. Previous researches have reported antimutagenic activity of some free amino acids (Roy et al. Citation2002, Tavares et al. Citation1998, Handique and Aprem Citation1997). Consequently, in the present study, it has been revealed that these compounds are the active inhibitors of mutagenic activity of AFBı. Antimutagenic effects of these compounds are probably related to their action on the enzymatic activation system. This effect of studied compounds can be attributed primarily to their antioxidant action or cofactor for enzymes system, which is known to protect DNA and other cellular components from damage by oxygen radicals.
Table III. Comparison the effects on the number of MN different concentrations of (Asn-Sch), [Mn(Asn-Sch)(OAc], (Gln-Sch), [Mn(Gln-Sch)OAc] together with AFB1 in human peripheral lymphocytes.
Conclusion
In summary, compounds attached to amino acid have been prepared for preliminary screening as antimicrobial and antimutagenic agents. They exhibited a very good antimicrobial activity against a wide range of microorganisms. Results from this study showed that antimutagenic activity and antimicrobial affectivity are compatible with each other.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This research was supported by the Gazi University Research Fund (Project number: 05/2010-03).
References
- Abram U, Alberto R. 2006. Technetium and rhenium-coordination chemistry and nuclear medical applications. J Braz Chem Soc. 17:1486–1500.
- Coluccia M, Correale M, Fanizzi FP, Giordano D, Maresca L, Mariggiò MA, et al. 1984. Mutagenic activity of some platinum complexes: chemical properties and biological activity. Toxicol Environ Chem. 8:1–8.
- Gunduz T, Gunduz N, Sakiyan I. 1994. A new method for synthesis of manganese(III) acetate dihydrate. Synth React Inorg Met Org Chem. 24:519–524.
- Handique AK, Aprem H. 1997. Antimutagenic activity of tryptophan and alanine. Curr Sci72:578–580.
- Hartman M, Hartwig A. 1998. Disturbance of DNA damage recognition after UV-irradiation by nickel(II) and cadmium(II) in mammalian cells. Carcinogenesis. 19:617–621.
- International Agency for Research on Cancer, World Health Organization, 1993. Aflatoxins. Pages 245–395 in: Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans 56. IACR, Lyon, France.
- Kuhl A, Hahn MG, Dumić M, Mittendorf J. 2005. Alicyclic beta-amino acids in Medicinal Chemistry. Amino Acids. 29:89–100.
- Lemonnier G, Lion C, Quirion JC, Pin JP, Goudet C, Jubault P. 2012. α-Amino-β- fluorocyclopropanecarboxylic acids as a new tool for drug development: synthesis of glutamic acid analogs and agonist activity towards metabotropic glutamate receptor 4. Bioorg Med Chem. 20:4716–4726.
- Lu L, Yue J, Yuan C, Zhu M, Han H, Liu Z, Guo M. 2011. Ternary oxovanadium(IV) complexes with amino acid-Schiffbase and polypyridyl derivatives: synthesis, characterization, and protein tyrosine phosphatase 1B inhibition. J Inorg Biochem. 105: 1323–1328.
- McConathy J, Martarello L, Malveaux EJ, Camp VM, Simpson NE, Simpson CP, et al. 2002. Radiolabeled amino acids for tumor imaging with PET: radiosynthesis and biological evaluation of 2-amino-3-[18F]fluoro-2-methylpropanoic acid and 3-[18F] fluoro-2-methyl-2-(methylamino)propanoic acid. J Med Chem. 23:2240–2249.
- Pokharel YR, Han EH, Kim JY, Oh SJ, Kim SK, Woo ER, et al. 2006. Potent protective effect of isoimperatorin against aflatoxin B1-inducible cytotoxicity in H4IIE cells: bifunctional effects on glutathione S-transferase and CYP1A. Carcinogenesis. 27: 2483–2490.
- Roy MK, Kuwabara Y, Hara K, Watanabe Y, Tamai Y. 2002. Antimutagenic effect of amino acids on the mutagenicity of N-methyl- N ’-nitro-N-nitrosoguanidine (MNNG). Biosci Biotechnol Biochem. 66:1400–1402.
- Tavares DC, Cecchi AO, Antunes LM, Takahash CS. 1998. Protective effects of the amino acid glutamine and of ascorbic acid against chromosomal damage induced by doxorubicin in mammalian cells. Teratog Carcinog Mutagen. 18:153–161.
- Sarı N, Gürkan P, Çete S, Şakiyan İ. 2006. Synthesis, potentiometric and antimicrobial activity studies on DL-amino acids–schiff bases and their complexes. Russ J Coord Chem. 32:511–517.
- Sarı N, Pişkin N, Öğütcü H, Kurnaz YN. 2013. Spectroscopic characterization of novel D-aminoacid-Schiff bases and their Cr(III) and Ni(II) complexes as antimicrobial agents. Med Chem Res. 22:580–587.
- Sakiyan I. 2007. Synthesis and characterization of four new manganese(III) complexes and amino acid (L-aspartic acid, L- asparagine, L-glutamic acid, L-glutamine) Schiff bases. Trans Met Chem. 32:131–135.
- Sakıyan İ, Logoglu E, Arslan S, Sari N, Sakıyan N. 2004. Antimicrobial activities of N-(2-hydroxy-1-naphthalidene)-amino acid(glycine, alanine, phenylalanine, histidine, tryptophane) Schiff bases and their manganese(III) complexes. BioMetals. 17:115–120.
- Sakiyan I, Yilmaz H. 2003. Manganese(III) complexes of some amino acid (L-serine, L-methionine,L-cystein) Schiff bases derived from 2-hydroxy-1-naphthaldehyde. Synth React Inorg Met Org Chem. 33:971–983.
- Snow ET. 1992. Metal carcinogenesis: mechanistic implications. Pharmacol Therapeut. 53:31–65.
- Zasukhina GD, Vasi'eva IM, Mikhal'chik ES, Durnev AD, Gromov SP, Fedorova OA, Alfimov MV. 2003. Antimutagenic and antioxidant activities of crown compounds in comparison with the effects of garlic extract. Bull Exp Biol Med. 135:261–264.
- Zhou Q, Xie H, Zhang L, Stewart JK, Gu XX, Ryan JJ. 2006. cis- Terpenones as an effective chemopreventive agent against aflatoxin B1-induced cytotoxicity and TCDD-induced P450 1A/B activity in HepG2 cells. Chem Res Toxicol. 19:1415–1419.