Abstract
Polyphenol oxidase (PPO, EC.1.14.18.1) isolated from artichoke (Cynara scolymus) was entrapped within alginate and alginate+ carrageenan beads, and the catecholase and cresolase activities of both entrapped enzymes were determined. Some properties of these immobilized enzymes such as optimum pH and temperature, kinetic parameters (Km and Vmax), thermal, and storage stability were determined and compared to each other.
The highest catecholase activity was observed in alginate gel (370 U/g bead) while the highest cresolase activity was in alginate+ carrageenan gel (90 U/g bead). For catecholase and cresolase activities, optimum pHs of alginate and alginate+ carrageenan beads were determined to be 7.0 and 4.0, respectively. Optimum temperatures for catecholase activity were determined to be 40°C for both entrapped enzymes. These values for cresolase activity were 30°C and 20°C, respectively. Immobilized artichoke PPOs greatly preserved their thermal stability which exists anyway. The catalytic efficiency value (Vmax/Km) of the alginate beads is approximately high as two-and-a-half folds of that of alginate+κ-carrageenan beads for cresolase activity. These values were very close for catecholase activity. Immobilized beads saved their both activities after 30 days of storage at 4°C.
Introduction
Polyphenol oxidase (PPO, tyrosinase, EC. 1.14.18.1) exhibits both a cresolase activity and a catecholase activity, because it has two active sites: oxidation of monophenolic substrates (l-tyrosine) via a chemical reductor (AH2), such as ascorbat, NADH, NH2OH, and H2O or/and the oxidoreduction of o-diphenolic substrates, respectively. PPO also catalyzes other intermediate reactions in the pathway to melanin production, whereas tyrosine hydroxylase catalyzes only the production of l-DOPA. To prevent the subsequent conversion of l-DOPA into melanin in the presence of PPO, ascorbate is added to the medium, leaving l-DOPA as the final product (Seetharam and Saville Citation2002).
In this study, artichoke PPO was immobilized in cupper-alginate and cupper alginate-κ-carrageenan gel beads by entrapment method. Entrapment is one of the simplest methods of immobilization, and consists of the inclusion of enzymes or cells within polymeric matrices (Sahin et al. Citation2005, Ates et al. Citation2006). The immobilization by entrapment has been accepted as an appropriate technique to fix enzymes. Both substrate (catechol, l-tyrosine) and the product (benzoquinon, l-DOPA) are of low molecular size with high diffusion rates on several supporting matrixes (Mammeralla and Rubiolo Citation2005).
Alginate, a naturally occurring polysaccharide that forms gels by ionotropic gelation, is the most popular of all supports. The hardness and mechanical strength of alginate gel increased with the increased affinity for the divalent cation. Copper has been used as the cross-linking agent in PPO immobilizations because of the higher affinity of alginate for Cu2+ with respect to Ca2+ (Palmieri et al. Citation1994). κ-carrageenan, another naturally occurring polysaccharide which forms gel with K+ ions, increased the enzyme activity. Unfortunately, carrageenan gels are very weak and will break easily, reducing the application of the polymer as supporting matrix for enzyme immobilizations (Mammeralla and Rubiolo Citation2005).
The main purpose of this study is to prepare a new matrix for the immobilization of artichoke PPO, because biological material immobilization is one of the most important steps for the industrial treatments. Immobilization of PPO by using various materials has been reported by many researchers in the literature (Seetharam and Saville Citation2002, Ates et al. Citation2006, Palmieri et al. Citation1994, Pialis et al. Citation1996, Carvalho et al. Citation2000, Munjal and Sawhney Citation2002, Pialis and Saville Citation1998, Ho et al. Citation2003, Vilanova et al. Citation1984, Yahsi et al. Citation2005, Yıldız et al. Citation2006).
In this study, new matrix, Cu-alginate-K-κ-carrageenan gel, for immobilization of artichoke PPO was prepared by using the blend of alginate and κ-carrageenan gels as well as using only alginate gel as support. That kind of blends may exhibit better activity and mechanical stability. Since the gel structure obtained with the hydrocolloids can be modified, the efficiency of the enzyme entrapment can be different due to the modification of the carrier structure (Mammeralla and Rubiolo Citation2005). As far as we know, the immobilization of artichoke PPO by these matrixes has not been reported in the literature except for our study which used alginate gels (Kocaturk and Yagar Citation2010). These biopolymer matrixes have certain advantages over other materials such as low cost, ease of enzyme accessibility, hydrophilic character, and the presence of hydroxyl groups on the surface capable of interaction with proteins (Sahin et al. Citation2005).
In the present study, some properties of PPOs entrapped within alginate gel beads and alginate+κ-carrageenan gel beads such as optimum pH, temperature, Michaelis–Menten kinetic constants (Km and Vmax), thermal and storage stability were determined, and these entrapped enzymes were compared to each other.
Experimental
Materials
Sodium alginate and κ-carrageenan were purchased from Fluka Biochemika (Germany). All the remaining reagents were of analytical grade and used without further purification.
Enzyme isolation and assays of PPO activity
The isolation of PPO from artichoke heads and determination of both activities of artichoke PPO were carried out as stated in our previous study (Kocaturk and Yagar Citation2010). Cresolase activity of artichoke PPO was determined by following the production of l-DOPA utilizing a modification of procedure reported by Arnow (Citation1937). Cresolase activity was determined by using the slope of the calibration curve [12].
Catecholase activity was determined by measuring the increase in absorbance at 420 nm for catechol by in the presence of air oxygen. One unit of catecholase activity was defined as the amount of enzyme leading to an increase in absorbance of 0.001 per min (Yagar and Sagiroglu Citation2002).
Protein concentration was determined by the method of Lowry with bovine serum albumin as standard (Lowry et al. Citation1951).
All activities and protein assays were expressed as the mean of at least three different samples.
Enzyme immobilization
The procedure of Palmieri et al. was employed (1994). 5 mL of the enzyme preparation (9.12 mg/mL protein) and 100 mg of alginate were added so as to obtain a 2% solution of sodium alginate. The mixture obtained was extruded dropwise through a syringe (0.8 mm diameter) in to a gently stirred 2% (w/v) CuCl2 solution at 4°C. After 2 h the formed beads containing enzyme were separated from the CuCl2 solution by filtration. They were washed on a filter twice with cold distilled water and stored in 0.1 M Tris-HCl buffer (pH 7.0) for further use (Munjal and Sawhney Citation2002).
In the second part, artichoke PPO was immobilized in alginate/κ-carrageenan polymer blends. Sodium alginate (100 mg) and κ-carrageenan (100 mg) were dissolved in 10 mL enzyme solution (9.12 mg/mL protein). This mixture was stirred and dropped into 2% CuCl2 and KCl solution using a syringe (0.8 mm diameter). The obtained beads containing PPO enzyme were filtered and washed with cold distilled water. These beads were stored in 0.1 M Tris-HCl (pH 7.0) at 4°C for further use. The filtered solutions and two washings were collected for protein determination.
Characterization of immobilized PPO
The effect of pH on the cresolase and catecholase activities of artichoke PPOs immobilized in alginate gel and alginate+κ-carrageenan gels was investigated by using buffer solutions of different pH (glycine-HCl buffer for pHs 2.0 and 3.0, acetate buffer for pHs 4.0 and 5.0; malate buffer for pHs 6.0 and 7.0, and borate buffer for pHs 8.0 and 9.0). 2.5 mM L-tyrosine and 20 mM catechol solutions were used as substrate in cresolase and catecholase activity assays, respectively.
The influence of temperature on the cresolase activities of both immobilized enzymes was determined by incubating the reaction mixture at different temperatures (10, 20, 30, 40, 50, and 60°C) whereas catecholase activities were determined at the temperature range of 20–60°C.
The effect of substrate concentration on the cresolase and catecholase activities of both immobilized PPO was studied by using different concentration of tyrosine (0.001, 0.0025, 0.005, 0.0075, and 0.01 M) and different concentration of catechol (0.002, 0.004, 0.006, 0.008, and 0.01 M), respectively. Km and Vmax values were calculated from the Lineweaver–Burk plots.
The artichoke PPOs immobilized in alginate gel and alginate+κ-carrageenan gels were incubated for 15, 30, 45, and 60 min at different temperatures between 50 and 70°C to study the thermal stabilities of the enzymes. The obtained results were compared with that of free enzyme.
Both the immobilized enzymes were stored in malate buffer (pH 7.0) and acetate buffer (pH 4.0) at 4°C for catecholase and cresolase activity assays, respectively. Their enzyme activities were measured at regular time intervals.
Results and discussion
In this study, alginate and alginate+κ-carrageenan polymer blends were used as support material for immobilization of PPO isolated from artichoke head. Alginate is a natural polymer that can be extracted mainly from brown seaweeds and various bacteria and has been used immobilization material. It consists of mannuronic and gluronic acids and could be converted into hydrogels via cross-linking with divalent cations (Onal et al. Citation2007). Copper chloride solution was used as the cross-linking agent in this study, which used Cu2+ for PPO immobilization widely. In our previous study, artichoke PPO was entrapped within cupper alginate beads, and immobilization conditions were determined (Kocaturk and Yagar Citation2010). In the present study, some biochemical properties of PPOs entrapped in alginate beads and alginate+κ-carrageenan beads were determined, and immobilized enzymes were compared in terms of these biochemical properties.
Alginate is preferred over other materials because of its various advantages such as biodegradability, hydrophilicity, natural origin (Onal et al. Citation2007). The carrageenans are alternating copolymers of 1,3-linked β-D-galactose and 1,4-linked 3,6-anhydro-α-D-galactose. The three major types are designated, by kappa (κ), lambda (λ), and iota (ι), according to the relative number and position of sulfate ester substituent on these sugars. Because of ionic nature of polymer, gelation is strongly influenced by the presence of electrolytes. κ-Carrageenan produces firm, thermally reversible gels with potassium ions. The gelling and melting temperatures of κ-carrageenan are dependent almost solely on the concentration of potassium ions. The hardness of carrageenan gel is dependent on calcium ions (Bickerstaff Citation1997).
In this study, a new matrix which consists of Cu-alginate and K-κ-carrageenan polymers was prepared as well as copper-alginate gels as immobilization support, and was used 2% CuCl2 and KCl solution as cross-linking reagent. Artichoke PPO entrapped in a three-dimensional lattice of ionically cross-linked alginate and carrageenan.
Artichoke head has high PPO activity (Espin et al. Citation1997, López-Molina et al. Citation2003, Aydemir Citation2004, Tuncay and Yagar Citation2011). It has been thought that immobilized PPO could be used in the production of l-DOPA. l-DOPA is the drug of choice for treatment of Parkinson's disease, and currently it has produced chemically. Recent research has centered upon microbiological production of l-DOPA from Escherichia coli and Erwinia herbicola and upon feasibility of l-DOPA production from tyrosinase (Pialis et al. Citation1996). Therefore, cresolase activity of immobilized artichoke PPO was also investigated as well as its catecholase activity in this study.
Effects of pH on artichoke PPO entrapped in alginate and alginate+κ-carrageenan gels
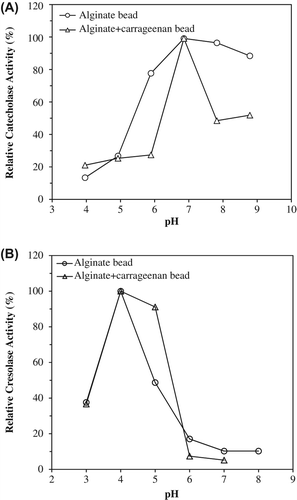
Enzymes exhibit their maximum activities under certain conditions. The pH is one of the important parameters capable of altering enzymatic activities in aqueous solutions. The effect of pH on the catecholase and cresolase activities of immobilized PPOs was studied within pH 3.0 and 9.0 (). As shown in , optimum pHs were determined to be 7.0 and 4.0 for catecholase and cresolase activities of immobilized PPO into alginate and alginate+κ-carrageenan gels, respectively. Aydemir and Tuncay reported that pH optimum of free artichoke PPO was observed in the range of pH 5.0–7.0, when used catechol as substrate (Aydemir Citation2004, Tuncay and Yagar Citation2011). After immobilizations, optimum pHs for PPO did not change for catecholase activities.
Figure 1. The effect of pH on catecholase (A) and cresolase (B) activities of entrapped polyphenol oxidase in alginate gel and alginate+κ-carrageenan polymer blends.
Entrapment is physical enclosure of the enzymes in spaces formed in the matrix structures. Since enzymes do not chemically bond to carrier, the three-dimensional structure of the enzyme may not affected by immobilization procedure, optimum pH, and temperature of immobilized enzyme can be observed to be similar that of free enzyme.
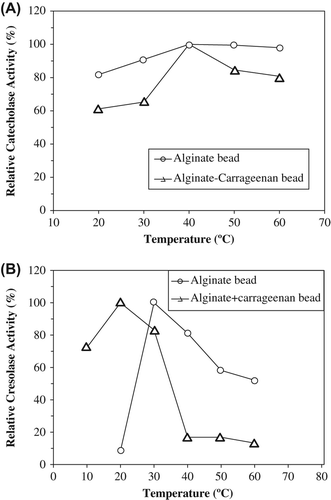
For the determination of optimum temperatures of the immobilized PPOs, catalytic activities were studied at their optimum pHs in the temperature range 10–60°C (). The optimum temperature of entrapped PPO was 40°C for into alginate and alginate+κ-carrageenan gels (). For cresolase activities, optimum temperatures immobilized PPO into alginate and alginate+κ-carrageenan gels were determined to be 30 and 20°C, respectively ().
Figure 2. The effect of temperature on catecholase (A) and cresolase (B) activities entrapped polyphenol oxidase in alginate gel and alginate+κ-carrageenan polymer blends.
The effect of the substrate concentration on the kinetics of the reaction catalyzed by immobilized PPO into alginate and alginate+κ-carrageenan gels was studied using catechol and tyrosine as substrate at their optimal temperature and pH for catecholase and cresolase activity assays, respectively. From Lineweaver–Burk plot of 1/V versus 1/[S] (), Km and Vmax of the immobilized enzymes calculated and results are given . As seen as in , Km values of entrapped enzyme alginate and alginate+κ-carrageenan beads are 7.0 and 7.5 mM × 10 − 3 for catecholase activity while 1.96 and 5.15 mM × 10 − 3 for cresolase activity, respectively. Vmax values of all enzymes were observed to be very close. Vmax values of entrapped enzyme alginate and alginate+κ-carrageenan beads are 1000 and 1250 U/g bead for catecholase activity, while 98 and 104 U/g bead for cresolase activity. The catalytic efficiency value (Vmax/Km) of the alginate beads is approximately high as two-and-a-half folds of that of alginate+κ-carrageenan beads for cresolase activity, both enzymes come close for catecholase activity.
Figure 3. Kinetic parameters of entrapped polyphenol oxidase in alginate gel and alginate+κ-carrageenan polymer blends for catecholase (A) and cresolase (B) activities.
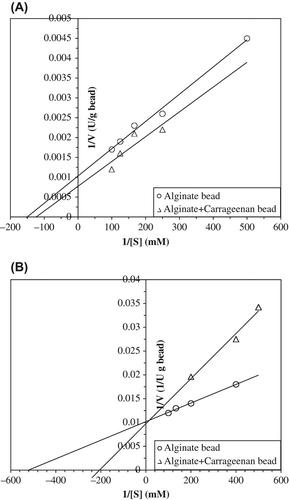
Table I. Km and Vmax values of entrapped artichoke polyphenol oxidase into alginate and alginate+κ-carrageenan gel beads for catecholase and cresolase activities.
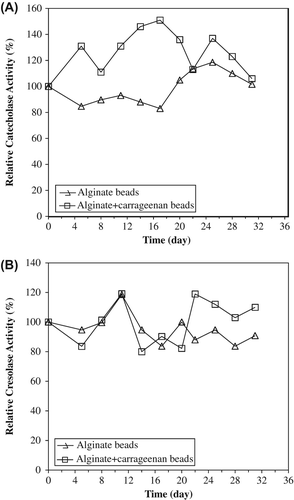
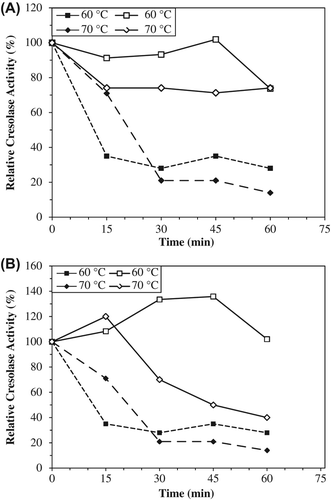
Thermal stability experiments were carried out with free and immobilized enzymes, which were incubated in the absence of substrate at various temperatures. The alginate+κ-carrageenan beads preserved about 74% of its initial catecholase activities at 60°C while alginate beads preserved all activity and free PPO retained about 34% of its initial activity. At 70°C, the alginate and alginate+κ-carrageenan beads retained their cresolase activities about to level of 40% and 74%, respectively. Free PPO only preserved 14% of its cresolase activity ( and ). It was observed that immobilization enhanced both enzymatic activities of artichoke PPO. The immobilized PPOs within alginate and alginate+κ-carrageenan beads are more stable than the free one at temperatures 60°C and greater.
Figure 4. The thermal stabilities of entrapped PPOs in alginate+κ-carrageenan beads (A) and alginate beads (B) for catecholase activities. The free enzyme and beads were incubated for various time intervals (15–60 min) at the specified temperature (50–60°C) and rapidly cooled. The activity was measured at 25°C, was taken as 100% and activities measured (50–60°C) were compared with the activity measured at 25°C. Empty symbols show immobilized enzyme (solid line), filled symbols free enzyme (dotted line). Each value is a mean of duplicate experiments.
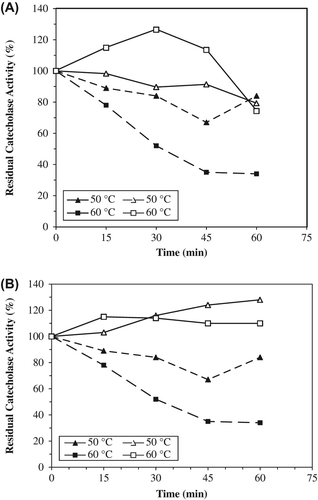
Figure 5. The thermal stabilities of entrapped PPOs in alginate+κ-carrageenan (A) and alginate gels (B) for cresolase activities. Empty symbols show immobilized enzyme (solid line), filled symbols free enzyme (dotted line). The free enzyme and beads were incubated for various time intervals (15–60 min) at the specified temperature (60–70°C) and rapidly cooled. The activity was measured at 25°C, was taken as 100% and activities measured (60–70°C) were compared with the activity measured at 25°C. Each value is a mean of duplicate experiments.
presents a comparison of storage stability for both immobilized lipase forms at 4°C. Storage stability is one of the most important parameters to be considered in immobilized enzyme products. At the end of 30 days of storage, the entrapped artichoke PPOs, alginate and alginate+κ-carrageenan beads, retained about 86% and 70% of their original catecholase activities, respectively. Both beads preserved about 76% and 92% of its initial cresolase activities, respectively ().
Conclusion
Polymer blends give an opportunity to create supports which give desired properties for enzyme immobilization. This study aimed mainly to prepare a new matrix for the immobilization of artichoke PPO. PPO was entrapped within Cu-alginate-K-κ-carrageenan gel beads as well as Cu-alginate beads, and some of their biochemical properties were determined. The entrapped PPOs had shown higher catecholase activity than cresolase activity. Both immobilization methods enhanced thermal stabilities of PPO for either cresolase or catecholase activities. Both entrapped PPOs exhibit high storage stability for 30 days. This kind of alginate-carrageenan blends may be used in various industrial treatments and biotechnological applications for PPOs.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This research was funded by Research Fund of Trakya University (TUBAP-821).
References
- Arnow LE. 1937. Colorimetric determination of components of 3-dihydroxyphenylalanine-tyrosine mixtures. J Biol Chem. 118: 531–537.
- Ates S, Cortenlioglu E, Bayraktar E, Mehmetoglu U. 2006. Production of L-DOPA using Cu-alginate gel immobilized tyrosinase in a batch and packed bed reactor. Enzyme Microb Technol. 40:683–687.
- Aydemir T. 2004. Partial purification and characterization of polyphenol oxidase from artichoke (Cynara Scolymus L.) heads. Food Chem. 87:59–67.
- Bickerstaff GF. 1997. Immobilization of Enzymes and Cells, in Methods in Enzymology, vol. 1. New Jersey: Humana Press.
- Carvalho GMJ, Alves TLM, Freire DMG. 2000. L-DOPA production by immobilized tyrosinase. Appl Biochem Biotechnol. 84–86: 791–800.
- Espin JC, Tudela J, Canovas FG. 1997. Monophenolase activity of polyphenol oxidase from artichoke (Cynara Scolymus L.) heads. Lebensm Wiss UTechnol. 30:819–825.
- Ho PY, Chiou MS, Chao AC. 2003. Production of L-DOPA by tyrosinase immobilized on modified polystyrene. Appl Biochem Biotechnol. 111:139–152.
- Kocaturk S, Yagar H. 2010. Optimization of polyphenol oxidase immobilization in copper alginate beads. Artif Cell Blood Subtit Biotechnol. 38:157–163.
- López-Molina D, Hiner ANP, Tudela J, García-Cánovas F, Rodríguez-López JN. 2003. Enzymatic removal of phenols from aqueous solution by artichoke (Cynara Scolymus L.) extracts. Enzyme Microb Technol. 33:738–742.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Mammeralla EJ, Rubiolo AM. 2005. Study of the deactivation of β- galactosidase entrapped in alginate-carrageenan gels. J Mol Catal B Enzym. 34:7–13.
- Munjal N, Sawhney SK. 2002. Stability and properties of mushroom tyrosinase entrapped in alginate, polyacrylamid, and gelatin gels. Enzyme Microb Technol. 30:613–619.
- Onal S, Hamarat Baysal S, Ozdemir G. 2007. Studies on the applicability of alginate-entrapped Chrysemonas luteola TEM 05 for heavy metal biosorption. J Hazard Mater. 146:417–420.
- Palmieri G, Giardina P, Desiderio B, Marzullo L, Giamberini M, Sonnia G. 1994. A new enzyme immobilization procedure using cupper alginate gel: Application to a fungal phenol oxidase. Enzyme Microb Technol. 16:151–158.
- Pialis P, Jimenez Hamann MC, Saville BA. 1996. L-DOPA production from Tyrosinase immobilized on Nylon 6,6. Biotech Bioeng. 51: 141–147.
- Pialis P, Saville BA. 1998. Production of L-DOPA from tyrosinase immobilized on Nylon 6,6: enzyme stability and scaleup. Enzyme Microb Technol. 22:261–268.
- Sahin F, Demira G, Tümtürk H. 2005. A novel matrix for the immobilization of acetylcholinesterase. Int J Biol Macromol. 37:148–153.
- Seetharam G, Saville BA. 2002. L-DOPA production from tyrosinase immobilized on zeolite. Enzyme Microb Technol. 1:747–753.
- Tuncay D, Yagar H. 2011. Comparison of polyphenol oxidases prepared from different parts of artichoke (Cynara Scolymus L.). Int J Food Prop. 14:809–821.
- Vilanova E, Manjon A, Iborra J. 1984. Tyrosine hydroxylase activity of immobilized tyrosinase on enzacryl-AA and CP-AA supports: stabilization and properties. Biotechl Bioeng. 26:1306–1312.
- Yagar H, Sagiroglu A. 2002. Partially purification and characterization of polyphenol oxidase of quince. Turk J Chem. 26:97–103.
- Yahsi A, Sahin F, Demirel G, Tümtürk H. 2005. Binary immobilization of tyrosinase by using alginate gel beads and poly(acrylamide- co-acrylic acid) hydrogels. Int J Biol Macromol. 36:253–258.
- Yıldız HB, Toppare L, Hepuzer Gursel Y, Yagci Y. 2006. Immobilization of polyphenol oxidase in conducting graft copolymers and determination of phenolic amount in red wines with enzyme electrodes. Enzyme Microb Technol. 39:945–948.