Abstract
Objective: To study the feasibility of therapy using nano-bone gelatin to comminuted fracture by animal experiment. Methods: The animal models of transverse fracture were made on bilateral ulnas of 45 New Zealand white rabbits, which were divided into experimental group (repair with nano-bone gelatin), control group (repair with traditional medical glue), and blank group (unrepaired) randomly. The reconstruction effect in each group was evaluated using X-ray examination, MicroCT scanning, histopathology observation, and biomechanical test postoperation regularly. Result: On 12th week, the fractures in experimental and blank group get the marrow cavity completely unobstructed and good bone union; however, in control group, the fracture line slightly blurred with the marrow cavity not fully unobstructed; 6 weeks later, observation of bony calluses through MircoCT: experimental group, 68.5 ± 2.71%; blank group, 69.19 ± 2.3%; and control group, 49.35 ± 3.56%, there were no significant difference between the two groups (P > 0.05). The control group obviously showed worse bone union than the former two (P < 0.05). The histopathological examination shows that the bony calluses of experimental group are similar to those of the blank group; however, gelatin degraded slowly in control group with delayed union; on the 12th week, biomechanical test shows that the blank and experiment groups had basically same average bending strength values which had no significant difference (P > 0.05) and obviously were higher than those of the control group (P < 0.01). Conclusion: The nano-bone gelatin won’t lead to delayed union of fractures and may be beneficial to it, and so may be an ideal gelatin for fixing small fractures.
Keywords::
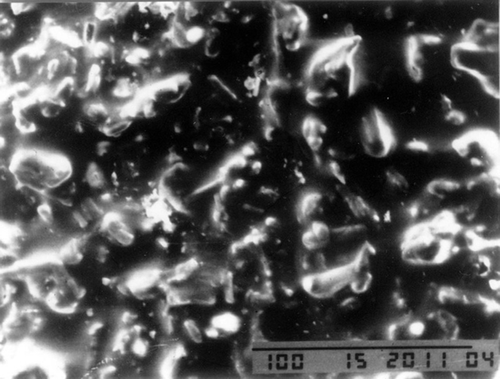
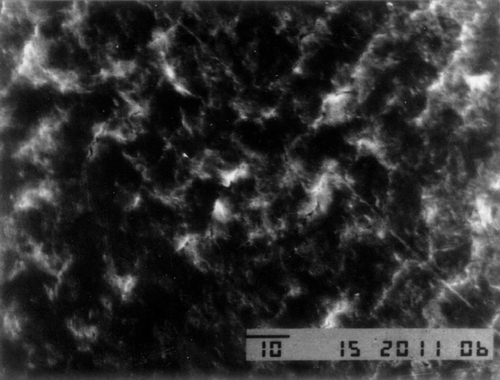
For serious comminuted fracture of limbs, failed fixation of broken bone blocks often leads to loss of small bone ones and therefore bone nonunion. In order to solve this problem and reduce the loss of bone mass and the incidence of bone nonunion, we have added nano-catalyst and nano-calcium sulfate hardener to a kind of medical adhesive (n-butyl-2-cyanoacrylate), of which the clinical use (Tian and Lu Citation2006, Singer and Thode Citation2004) has already been approved by FDA, to modify its viscosity, toughness, and degradation time in hope of improving its viscosity and decreasing its degradation time before having it compounded with icaritin that has osteoinductive activity so as to gain absorbable macromolecule nano-biological bone gelatin that has both osteoinduction and fracture fixation effects (Zhao et al. Citation2010). On the basis of previous in vitro experiments (Zhao et al. Citation2010), we have found that the cytotoxicity of modified nano-bone adhesive is not higher than that of the medical glue, but the strength and consolidation are more superiority. The former also can stimulate the ossification. We also demonstrated the nanostructure within nano-bone gelatin, and the nano-calcium sulfate did not change its structure when added to the adhesive by scanning electron microscope ( and ) (Zhao et al. Citation2010). So, this experiment will be performed inside animal body to verify its union effect on bone fracture and also to pave the way for the application of such gelatin in clinical use.
Materials and methods
Preparation of nano-bone gelatin
In this experiment, we have chosen nano-calcium sulfate powder (Peters et al. Citation2005, Thomas and Puleo Citation2008) which forms even suspension in gelatin and can significantly improve the adhesive strength within a certain proportional range; we therefore have found the best mixture ratio between nano-calcium sulfate and n-butyl-2-cyanoacrylate to achieve a special effect (Zhao et al. Citation2010) similar to that of sanded cement. Icaritin is currently the focus of bone tissue engineering researches, which plays an osteogenic induction role similar to BMP-2 and can have the best osteogenic stimulating effect (Huang et al. Citation2007, Chen et al. Citation2005) at the concentration ranging from 10 − 5 to 10 − 7mol/L. More importantly, icaritin at such concentrations can be completely soluble in gelatin and then serve as a special additive. We therefore can modify n-butyl-2-cyanoacrylate by proportionally adding nano-calcium sulfate and icaritin into the same to work out orthopedically dedicated nano-bone gelatin with greater adhesive strength and osteogenic induction effect.
Model of bone gelatin repairing rabbit's ulna fracture
Animals and groups: Select 45 healthy male adult purebred New Zealand white rabbits (provided by Guangdong Animal Experiment Center), each weighing (2.5 ± 0.5) in kilograms, and randomly divide them into the experiment group, control group, and blank group, 15 for each, fixing each's fracture, respectively, with nano-bone gelatin, ordinary medical adhesive (n-butyl-2-cyanoacrylate, supplied by Shenzhen Xinhailing Biotechnology Co., Ltd.), and nothing.
Select ketalar injection (10 mg/kg) combined with 3.0% pentobarbital (1.0 ml/kg) for anesthesia by intramuscular injection. Let rabbits lie face up and make a 2-cm-long incision along ulna to expose ulnae; a miniature electric saw is used to make transverse fractures of ulnae’ middle segments; periostea around fracture lines are damaged; a liquid transfer gun is used to apply 20 ul of nano-bone gelatin to the experiment group for bone fixation, 20 ul of ordinary medical adhesive for the control group, and nothing for the blank group; the wounds are sewn up by layer. Neither is internal and external fixations made locally, nor are the wounds bandaged. After the anesthesia effect disappears, the rabbits are put into the cages and raised as usual and receive penicillin via intramuscular injection, 800,000 U/day for 3 days.
Observation indexes and methods
General condition and gross specimen: Observe rabbits’ diet, activities, and wound reaction after operation; obtain specimen and observe the condition of fracture union.
X-ray examination: Perform the examination on experimental limbs on set time after operation.
Histopathological examination: After the X-ray examination, the air embolization method is used to kill rabbits for ulnae specimens; a piece of 5-mm bone tissue crossing the fracture line is cut off, fixed using 10% formalin, treated by normal decalcification and dehydration, embedded by paraffin, sliced continuously on a 5 μm-size basis, and stained by HE before put under light microscope for observation.
MicroCT-based observation of fracture union condition (Hayakawa et al. Citation2010, Toben et al. Citation2011): Perform the examination on experimental limbs on set time after operation under MicroCT (scanning layer resolution, 30 μm; peak voltage, 40 KV; and current, 114 μA) to regenerate 3D image of bone fracture; a 3D image software is used to calculate total volume of broken ends of fractured bone (TV), volume of bony callus (BV), and its ratio (BV/TV).
Biomechanical test: Obtain fresh specimens of bilateral ulnae on 12th week and perform the three-point bending test on them. The test shall have a 1 mm/min of acceleration and 40 mm of interval between two fulcrums; the maximum bending point of ulna centers, put downward and record maximum of specimen when it fractures. The White formula is used to determine its bending strength Sf (MPa) = 3 PL/2 bd2 [P: maximum failure load (N), L: test span, namely distance between two fulcrums, b: width of specimen (mm), and d: thickness of specimen (mm)]. Know biomechanical performance of each group and compare their fracture unions.
Statistical analysis
The acquired experimental data are expressed by ![]() ± s and processed statistically by the software package SPSS ver. 13.0; the data of X-ray, histological rating, MicroCT bone mass analysis, and biomechanical bending strength undergo the variance analysis of specimens’ mean and the Q test on a pair basis; P < 0.05 indicates significant differences.
± s and processed statistically by the software package SPSS ver. 13.0; the data of X-ray, histological rating, MicroCT bone mass analysis, and biomechanical bending strength undergo the variance analysis of specimens’ mean and the Q test on a pair basis; P < 0.05 indicates significant differences.
Results
General condition
After the operation, all experimental animals showed normal diet and activities; no deaths and infected wounds were found; and no significant inflammatory reactions were spotted in each group of gross specimens
X-ray performance
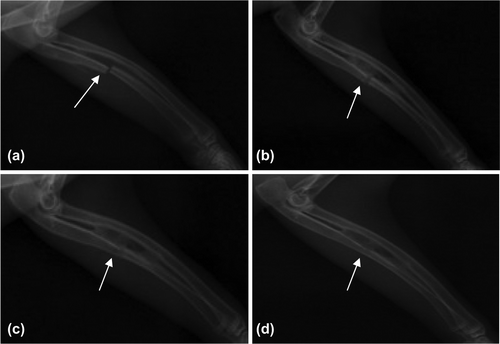
Experiment group: 2 weeks later, bony callus formed around the fracture line, obvious bony callus forming between broken ends of fracture (a); 4 weeks later, the fracture line blurred slightly, bony callus passing between the broken ends and more forming around the line, the marrow cavity not unobstructed (b); 8 weeks later, the fracture line basically disappeared, the marrow cavity not fully unobstructed (c); 12 weeks later, the fracture line disappeared with the marrow cavity completely unobstructed and good bone union (d) ().
Figure 3. Bone union of experiment group (denoted by arrows). a. 2 weeks later, bony callus formed; b. 4 weeks later, bony callus passing between the broken ends; c. 8 weeks later, the fracture line basically disappeared; and d. 12 weeks later, marrow cavity completely unobstructed.
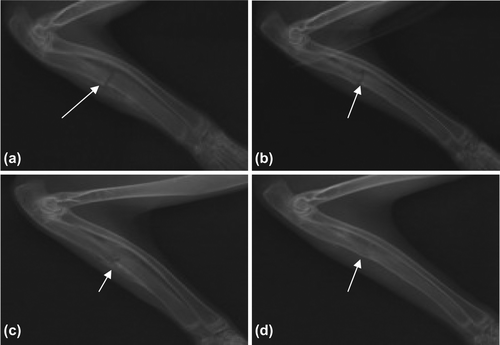
Control group: 2 weeks later, no obvious bony callus formed with well-aligned broken ends (a); 4 weeks later, a little bone callus formed with no obvious callus passing between the broken ends which reasonably aligned with each other (b); 8 weeks later, bony callus formed with a little callus passing between the broken ends, the marrow cavity obstructed, and the fracture line staying clear (c); 12 weeks later, the fracture line slightly blurred with a little callus passing between the broken ends, the marrow cavity not fully unobstructed (d) ().
Figure 4. Bone Union of Control Group (denoted by arrows). a. 2 weeks later, no obvious bony callus formed; b. 4 weeks later, a little bone callus formed; c. 8 weeks later, the fracture line staying clear; and d. 12 weeks later, the marrow cavity not fully unobstructed.
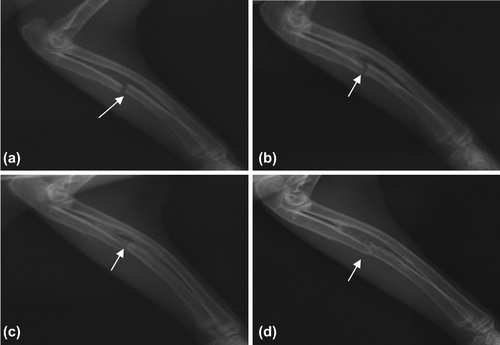
Blank group: 2 weeks later, a little bony callus formed (a); 4 weeks later, some bony calluses formed with callus passing between the broken ends (b); 8 weeks later, much more bony calluses formed, the fracture line blurring with good bone union and not thoroughly unobstructed marrow cavity (c); and 12 weeks later, the fracture line disappeared with the unobstructed marrow cavity and complete bone union (d) ().
Figure 5. Bone Union of Blank Group (denoted by arrows). a. 2 weeks later, a little bony callus formed; b. 4 weeks later, some bony calluses formed with callus passing between the broken ends; c. 8 weeks later, the fracture line blurring with good bone union; and d. 12 weeks later, the unobstructed marrow cavity and complete bone union.
Histopathological examination
Experiment group: 2 weeks later, the gelatin began degrading, a little bony callus forming (a); 8 weeks later, the gelatin basically degraded, a large quantity of bony callus forming (b); 12 weeks later, the gelatin completely degraded, the osteocytes growing actively, and bony union being good (c) ().
Figure 6. Histological Slice of Experiment Group. a. 2 weeks later, the gelatin began degrading, a little bony callus forming; b. 8 weeks later, the gelatin basically degraded, a large quantity of bony callus forming; and c. 12 weeks later, the gelatin completely degraded, the osteocytes growing actively, bony union being good.
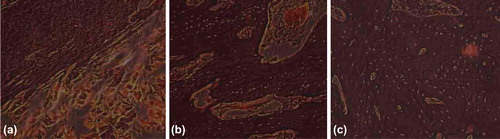
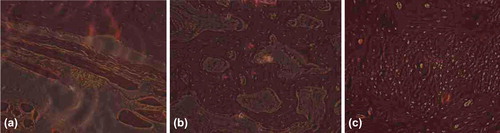
Control group: 2 weeks later, lots of gelatin can be seen between the broken ends (a); 8 weeks later, few gelatin degraded, not many bony calluses forming (b); and 12 weeks later, a little residual gelatin remained, much bony callus forming (c) ().
Figure 7. Histological Slice of Control Group (→denote bony callus, [FX] denote gelatin). a. 2 weeks later, lots of gelatin can be seen between the broken ends; b. 8 weeks later, few gelatin degraded, not many bony calluses forming; and c. 12 weeks later, a little residual gelatin remained, much bony callus forming.
![Figure 7. Histological Slice of Control Group (→denote bony callus, [FX] denote gelatin). a. 2 weeks later, lots of gelatin can be seen between the broken ends; b. 8 weeks later, few gelatin degraded, not many bony calluses forming; and c. 12 weeks later, a little residual gelatin remained, much bony callus forming.](/cms/asset/f75304a8-f09c-40eb-bc84-769088088c26/ianb_a_821411_f0007_b.jpg)
Blank group: 2 weeks later, no obvious bony callus formed (a); 8 weeks later, a great deal of bony calluses formed (b); and 12 weeks later, bone union was good, the osteocytes growing actively (c) ().
Observation of bone fracture through MircoCT (6 weeks)
Scanning of broken ends of bone fracture
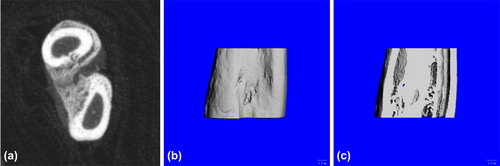
Experiment group: good formation of bony callus at fracture which basically united (a); the three-dimensional reconstruction showed no obvious fracture line, uneven surface (b); and the coronal section showed good bone union and not fully unobstructed marrow cavity (c) ().
Figure 9. Postoperative 6-week Bone Union of Experiment Group. a. cross section showed good formation of bony callus at fracture; b. three-dimensional reconstruction showed no obvious fracture line; and c. coronal section showed good bone union and not fully unobstructed marrow cavity.
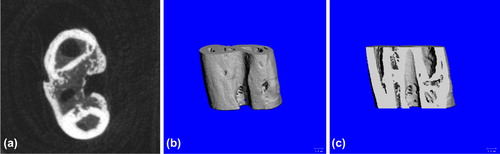
Control group: Slow bone union with few bony callus (a); the 3D reconstruction showed clear fracture line with few bony callus formed between broken ends (b); and the coronal section showed clear fracture line (c) ().
Figure 10. Postoperative 6-week Bone Union of Control Group. a. cross section showed slow bone union with few bony callus; b. three-dimensional reconstruction showed clear fracture line with few bony callus formed between broken ends; and c. coronal section showed clear fracture line.
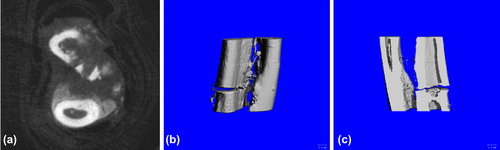
Blank group: Good bone union with a great quantity of calluses formed (a); the 3D reconstruction showed no obvious fracture line, uneven surface caused by formed calluses (b); the tomography of the coronal section showed plenty of bony calluses and obstructed marrow cavity (c) ().
Bone mass analysis of broken ends (Chappard et al. Citation2010)
Six weeks later, both blank group and experiment group showed much more bony calluses and good bone union; there were no significant differences between the two groups (P > 0.05). The control group obviously showed worse bone union than the former two (P < 0.05).
The bone mass at fracture of each group is shown in .
Table I. The bone mass at fracture of each group.
Biomechanical test
On the 12th week, the statistical analysis of bending strength data measured from the three-point bending test performed on the specimens of each of the three groups showed that the blank and experiment groups had basically same average bending strength values which had no significant difference (P > 0.05) and obviously were higher than that of the control group (P < 0.01). The bending strength of each group is shown in .
Table II. The bending strength of each group.
Discussion
The main purpose of the nano-bone gelatin production is to treat serious comminuted fracture. However, for this gelatin may lead a delayed bone union, to observe the process as soon as possible and eliminate much interferential factor, we have chosen the simple fractures as objects. Such type of fracture could union by itself even without any internal fixation. So it is easy to demonstrate that the bone gelatin lead a delayed union or not. If not, this experiment will make a solid foundation for further experiment.
For in vivo application of nano-bone gelatin, the toxicity of such gelatin is the primary issue to be discussed. Only if the toxicity is acceptable, it has practical significance (Azevedo et al. Citation2003). The medical adhesive (n-butyl-2- cyanoacrylate) has already been approved by FDA for clinical use; the additives, nano-calcium sulfate and icaritin, used are nontoxic substance; the former is a kind of inert matter which has been made into an injectable bone cement (Lee et al. Citation2002) clinically serving as a filler for treating bone defect; the latter is a traditional Chinese medicine used to treat postmenopausal osteoporosis. Theoretically, the two additives, if added into the adhesive, will therefore not increase its toxicity. In appearance, there are no chemical reactions between the additives and the adhesive because neither the former nor the latter coagulate and the mixture of both simply forms even suspension. Our cell experiment verified that the toxicity of nano-bone gelatin does not significantly differ from that of the ordinary gelatins.
The nano-bone gelatin developed by us is an improved n-butyl-2-cyanoacrylate. The latter is a medical all-purpose adhesive used. However, the application of adhesive in orthopedical treatment is limited (Zhao and Xiao Citation2010) due to some inherent defects, such as bad impact resistance, low viscosity leading to difficult or poor adhesion of parts with much tissue fluid and adipose tissue, and slow in vivo degradation leading to nonideal bone union effect and the danger of union to be hindered by thick gelatin layer. The application of medical all-purpose adhesive in orthopedics is limited by these defects. Therefore, two problems need to be resolved immediately. The first one is adhesive viscosity. The current biological adhesives falling into the cyanoacrylate category can only be used in adhesion of small bone blocks of parts bearing no weights. They cannot be used to fix fractured bone of weight-bearing parts; the second one is slow in vivo degradation. This kind of biological adhesive may form a barrier between broken ends to prevent newly formed bony calluses from passing through, which leads to bone nonunion.
Through the modification, the nano-bone gelatin has gained better mechanical strength and can stimulate osteogenesis and improve fracture union. Such results prove that the modification to the cyanoacrylate adhesive is successful. However, we should also clearly realize that nano-bone gelatin still cannot meet the requirements of bearing human body weight, especially its fatigue resistance cannot match that of steel plates and screws. The nano-bone gelatin developed by us is intended to be used with internal fixation to improve local stability, use internal fixation less frequently, and replace steel wire cerclage fixation.
The application of nano-bone gelatin in department of orthopedics is therapeutically significant as it can fix broken bone blocks that cannot be fixed in the traditional way and reduce bony defect and incidence of bone nonunion. The gelatin degrades as the bone grows and can locally promote the growth of osteocytes, indicating that it not only fixes fracture but also improves bone union. The combined use of improved bone adhesive and traditional internal fixation can be the therapeutic revolution of bone block fixation for patients who suffer from serious comminuted fractures and complex-part fractures.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Azevedo CL, Marques MM, Bombana AC. 2003. Cytotoxic effects of cyanoacrylates used as retrograde filling materials: an in vitro analysis. Pesq Odontol Bras. 17:113–118.
- Chappard D, Baslé MF, Legrand E, Audran M. 2010. New laboratory tools in the assessment of bone quality. Osteoporos Int. 6:107–124.
- Chen KM, Ge BF, Ma HP, Liu XY, Bai MH, Wang Y. 2005. Icariin, a flavonoid from the herb Epimedium, enhances the osteogenic differentiation of rat primary bone marrow stromal cells. Pharmazie. 60:939–942.
- Hayakawa T, Mochizuki C, Hara H, Yang F, Shen H, Wang S, Sato M. 2010. In vivo evaluation of composites of PLGA and apatite with two different levels of crystallinity. J Mater Sci Mater Med. 21:251–258.
- Huang J, Yuan L, Wang X, Zhang TL, Wang K. 2007. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 87:832–840.
- Lee GH, Khoury JG, Bell JE, Buckwalter JA. 2002. Adverse reactions to OsteoSet bone graft substitute the incidence in a consecutive series. Iowa Orthop J. 22:35–38.
- Peters CL, Hines JL, Bachus KN, Craig MA, Bloebaum RD. 2005. Biological effects of calcium sulfate as a bone graft substitute in ovine metaphyseal defects. J Biomed Mater Res A. 76:456–462.
- Singer AJ, Thode HC Jr. 2004. A review of the literature on octyl- cyanoacrylate tissue adhesive. Am J Surg. 187:238–248.
- Thomas MV, Puleo DA. 2008. Calcium sulfate: properties and clinical applications. J Biomed Mater Res B Appl Biomater. 7:597–610.
- Tian X, Lu Y. 2006. Fuaile medical adhesive and its application. Int Surg J. 33:145–149.
- Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann JE, Frisch JT, et al. 2011. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res. 26:113–124.
- Zhao Z, Lei M, Xiao D, Xiong J, Ma J, Xu Y, et al. 2010In-vitro experiment of nano bone gelatin modified from medical adhesive. Chin Tissue Eng Res Clin Rehabil. 14:8818–8823.
- Zhao Z, Xiao D. 2010. Progress of research on biological gelatin of cyanoacrylate category fixing fractured bones. Depart Orthop. 1:54–56.