Abstract
The aim of this study was to produce a chitosan-crosslinked nanofibrous biodegradable poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nerve conduit. The artificial scaffold was designed by electrospinning method, and cross-linked with chitosan by chemical method. The scaffolds were evaluated by microscopic, physical, and mechanical analyses, and cell culture assays with Schwann cells. Results of analyses showed a good resilience and compliance with movement as a neural graft. Cellular experiments showed a better cell adhesion and growth inside the crosslinked nanofibrous scaffolds compared with un-crosslinked ones. This neural conduit appears to have the right organization for testing in vivo nerve tissue engineering studies.
Introduction
Peripheral nerves form an extensive network that links the brain and spinal cord to all other parts of the body (Hughes Citation2002, Burnett and Zager Citation2004, Yin et al. Citation1998). A nerve injury can interfere with communication between the brain and the muscles controlled by a nerve, affecting a person's ability to move certain muscles or have normal sensations (Biazar et al. Citation2010a). Reconstruction of damaged nerves results from different factors that have been investigated by different methods. A number of methods, such as utilizing allograft techniques; cell therapy including Schwann cells (SCs), stem cells, fibroblasts, and olfactory cells; or drug therapy using biologic tubes, designed scaffolds with synthetic and natural materials and oriented channels, and absorbable and nonabsorbable synthetic and natural polymers with unique features benefiting from new nanotechnology were studied for improving the performance of strategies to repair damaged nerve tissue (Ghaemmaghami et al. Citation2009, Firouzi et al. Citation2006, Ducker and Hayes Citation1970). Common grafting in surgery was autograft or nerve removal from elsewhere of the body. Unfortunately, autografts had limitations such as body injury, repeated surgery and disproportion of grafted nerve tissue in terms of size and structure of nerve tissue (Evans Citation2000, Heath and Rutkowski Citation1998) also; transplantation of allograft or xenograft had similar problems in addition to the stimulation of the immune system (Brunelli et al. Citation1993, Tong et al. Citation1994, Fansa et al. Citation2002, Barcelos et al. Citation2003, Godard et al. Citation2009). Therefore the studies were conducted on the application of artificial neural tubes to form neural cords (Archibald et al. Citation1995, Fields et al. Citation1989, Keeley et al. Citation1993). Clinical investigations showed functional improvement and regeneration of peripheral nerve tissue with a gap of 3–5 mm by silicone tube (Lundborg et al. Citation1997). One of the scaffolds for nerve regeneration was poly L-lactic acid (PLA) hollow tubes that was successful for rebuilding of neural fibers with gaps of 14 and 18 mm. Biodegradable sutures composed of polyamide fibers showed similar results for nerve regeneration with gaps of 7 and 15 mm, but were not suitable for longer gaps (Lundborg et al. Citation1997). Among the synthetic polymers, poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) microbial polyester can be noted as biocompatible and biodegradable copolymer. PHBV has suitable properties for cellular growth and adhesion and benefits from controllable degradation (Williams et al. Citation1999, Hromadka et al. Citation2008). The ability of being prepared as nanofibers has improved the performance of biomaterials. Electro-spinning is one of the most important methods for fabrication of nanofibrous scaffolds (Venugopal et al. Citation2008, Biazar et al. Citation2010b, Ai et al. Citation2011, Majdi et al. Citation2011, Montazeri et al. Citation2011, Rezaei-Tavirani et al. Citation2011, Biazar and Heidari Citation2013, CitationBiazar et al. in press (a), CitationHeidari et al. in press, CitationBiazar et al. in press (b)). In this study, nanofibrous PHBV conduit were fabricated by electrospinning method and cross-linked using chitosan by chemical method. The polymeric film or tube was evaluated by scanning electron microscope (SEM), physical and mechanical analyses, and cellular assays.
Materials and methods
Fabrication of nanofibrous scaffold
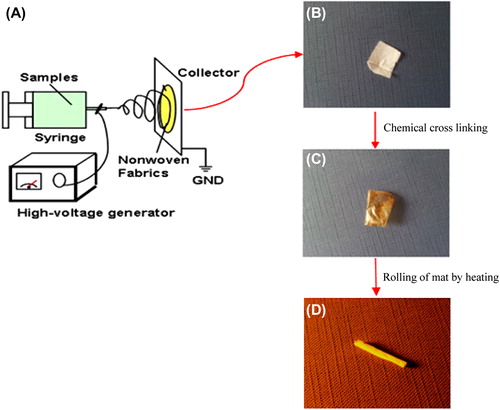
PHBV (molecular weight of 680 KDa) was purchased from Sigma–Aldrich (USA). 2, 2, 2-trifluoroethanol (TFE) was also purchased from Sigma–Aldrich and was used as solvent to prepare PHBV solutions. Both polymer and solvent were used without further purification. Electro-spinning apparatus used in this study was prepared from Fanavaran Nano-Meghyas Company (Tehran, Iran). The PHBV was dissolved in TFE at a concentration of 2%w/v and loaded into a glass syringe controlled by syringe pump. A positive high voltage source through a wire was applied at the tip of a syringe needle and a strong electric field (20 Kv) was applied between the PHBV solution and the collector. As soon as the electric field reached a critical value with increasing voltage, mutual charge repulsion overcame the surface tension of the polymer solution and an electrically charged jet was ejected from the tip of a conical shape as the Taylor cone. Ultrafine fibers are formed by narrowing the ejected jet fluid as it undergoes increasing surface charge density due to the evaporation of the solvent. The nanofibers fabricated with a pre-determined variables of electrospinning (Syringe Size: 17 mm, Collector speed: 1000 rpm, Injected speed: 2 ml/min, Syringe tip distance to collector: 75 mm, Voltage: 20 Kv, Temperature: 30°C, Time: 7 h). The electrospinning set has been shown in . The electrospun PHBV nanofibrous mat was carefully detached from the collector and dried in vacuum for 2 days at room temperature to remove the solvent molecules completely.
Crosslinking of nanofibers
Chitosan (DA: 75–85%; Medium molecular weight: Sigma–Aldrich) was immobilized onto the nanofiber surfaces based on the following protocol. Chitosan was rinsed in acetic acid buffer solution (50 mM, pH = 5.0). Then, the nanofibrous mat was submerged into the 6 M NaOH solution for 15 min. The hydrolyzed nanofibrous mats were rinsed into MES buffer (pH = 6.0) containing 10 mM EDC and 10 mM sulfo-NHS to activate the carboxyl groups on the surfaces. The nanofibrous mats were rinsed into chitosan solution (15 mg/ml in acetic acid buffer solution, 50 mM, pH = 5.0) and was shaken gently for 24 h at 4°C. The obtained samples were placed inside a vacuum oven to fully lose the humidity. The chitosan-crosslinked electrospun mat () was rolled around the cylindrical rod to form a 3D tubular structure and was maintained in this form using a thermal agent ().
Structural characterization
The surface characteristics of crosslinked and un-crosslinked nanofibers were studied by a SEM (Cambridge Stereo-scan, S-360, Wetzlar, Germany) to analyze the changes in the surface morphology. The films were first gold sputtered for 2 h (Joel fine coat) to provide surface conduction before scanning. The sample surface's static contact angles were investigated by a contact angle measuring apparatus (Krüss G10, Matthews, NC) according to the sessile drop method. For mechanical investigations, the nanofibers were subjected to stress–strain analysis using a universal testing machine under an extension rate of 5 mm/min and 100 N load cell. The specific surface area of nanofibrous mats were determined by surface area and pore size analyzer.
Cell culture
SCs were obtained from sciatic nerves of 8-day-old rats according to earlier described methods (Wei et al. Citation2009). In brief, the nerve segments were washed in DMEM/F-12 twice, then re-suspended in 0.3% collagenase Type II solution (100 μl per segment) and incubated for 30 min at 37°C. After incubation, the enzymatic solution was carefully removed, and an equal volume of 0.25% trypsin-EDTA was added. The nerve segments were incubated for another 5 min at 37°C and then mechanically dissociated until they formed a homogeneous suspension. SC basal medium was then added to the suspension at a ratio of 4:1 in order to terminate the activity of the trypsin. The mixture was centrifuged at 800–1000 rpm for 5 min. The supernatant was discarded, and the cells were re-suspended in SC basal medium. The SCs were proliferated in the flask and for sub culturing they were washed using the PBS. Then the trypsin enzyme/EDTA was added to the flask (37°C) and the flask was incubated for 90 s. The culture media (FBS/DMEM) was added to the flask, and the cells were gently pipetted. Then the cell suspension was transferred to a tube (15 ml) [BD Falcon™] and centrifuged (400 g) [Germany 5702] for 5 min. The solution was removed and the precipitation was transferred to a new flask (75 cm) for re-culturing. Pieces of cell culture (1 cm × 1 cm) from the petri dish (Control) and the main sample were placed individually in one of the Petri dish wells by using a sterilized pincer. A total of 200,000 cells/well were seeded into a 24-well culture plate and then removed by a pipette and poured onto the control and the main samples. Afterwards, all samples were placed in binder incubator at 37°C for 48 h and studied using an Invert microscope (Wolf Laboratories, UK). Cell proliferation and viability in vitro was analyzed with the tetrazolium salt 3-(4, 5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide assay. Briefly, 5000 SCs were seeded on nanofibrous PHBV scaffolds. For analysis, 20 μl of MTT (sigma) substrate (2.5 mg/ml stock solution in phosphate-buffered saline [PBS]) was added to each well, and the plates were returned to standard tissue incubator conditions for an additional 4 h. Medium was then removed, the cells were solubilized in 100 μl of dimethyl sulfoxide, and colorimetric analysis was performed. For scanning electron microscopy study, the scaffolds and cultured SCs were washed by PBS, and then fixed by glutaraldehyde (2.5%) at 4°C for 2 h. The samples were dehydrated by alcohols, and then kept with tetroxide osmium vapors at 4°C for 2 h. The samples were kept in desiccator for 48 h, then coated with gold and investigated by a SEM (Cambridge Stereo-scan, S-360, Wetzlar, Germany).
Results
Structural characterization
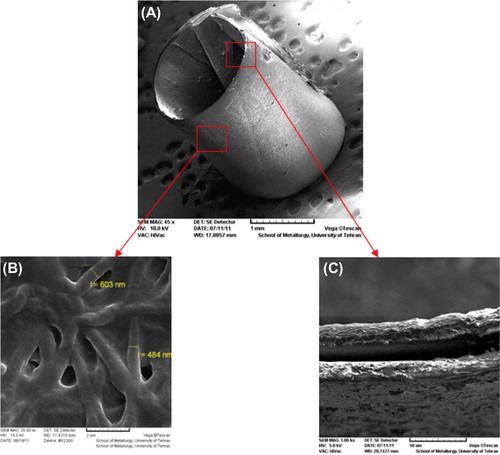
The nanofibrous scaffolds were prepared using the electrospinning method. shows the SEM images of the electrospun nanofibers and the chitosan-crosslinked nanofibers at different magnifications. The smooth and homologous nanofibers have clearly been shown in this figure. The average sizes for the un-crosslinked and the chitosan-crosslinked nanofibers were about 100 and 500 nm, respectively. Figures show SEM images of the un-crosslinked nanofibrous PHBV mat (A: 5000 ×, B: 20000 ×), and the chitosan-crosslinked nanofibrous PHBV mat (C: 5000 ×, D: 20000 ×). illustrates the designed tubular structure and diameter of tube wall.
Figure 2. SEM images of the un-crosslinked nanofibrous PHBV mat (A: 5000×, B: 20000×), and the chitosan-crosslinked nanofibrous PHBV mat (C: 5000×, D: 20000×).
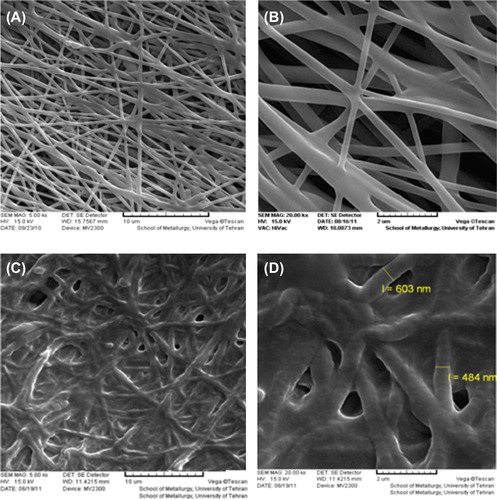
Figure 3. SEM images of the chitosan-crosslinked nanofibrous conduit. (A) The tubular conduit (45×), (B) The nanofibrous structure of designed conduit (20000×), (C) The diameter of tube wall (1000×).
Mechanical and physical properties of the nanofibrous PHBV scaffolds have been presented in . The contact angles of 105° and 57° were measured for the electrospun and the chitosan-crosslinked nanofibers, respectively. The 48° difference in the contact angle shows a better hydrophilicity of the crosslinked nanofibers compared with the un-crosslinked samples. The porosity of nanofibrous PHBV and the chitosan-crosslinked scaffolds were calculated 91.62% and 79.58% and their pore size also measured as 0.45 ± 0.25 μm and 0.28 ± 0.33 μm, respectively. The specific surface area for the electrospun nanofibrous scaffolds was about 138 m2/g and for the crosslinked nanofibrous ones was about 105 m2/g. It is discernible that the electrospun nanofibrous mats have higher porosity and higher level of specific surface area, as well.
Table I. The mechanical and physical properties of elecrospun PHBV mats.
Cellular culture
shows the results of the MTT assay for the control (TCPS: tissue culture polystyrene), the un-modified nanofibrous PHBV mat and the chitosan-crosslinked nanofibrous mat. These results indicated that there is a high degree of cell-viability for the nanofibrous samples. On the other hand, more numbers of SCs were adhered to both the nanofibrous surfaces and could proliferate. shows the attached cells on the un-modified and modified nanofibrous samples and the control ones. Cellular images show a good cell growth in the vicinity of nanofibrous mat crosslinked with chitosan. shows SEM images of cultured Schwann cells on the nanofibrous samples. Figures show high viability of the attached SC on both the nanofibrous surfaces especially for the chitosan-crosslinked nanofibrous mat.
Figure 4. Cell culture on the nanofibrous mats, and the control. (A) The control (TCPS), (B) The un-modified nanofibrous PHBV mat, (C) The chitosan-crosslinked nanofibrous mat.
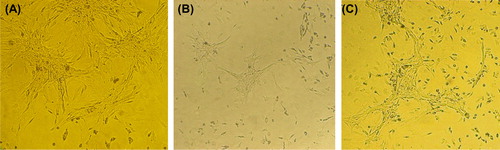
Figure 5. SEM images of cultured Schwann cells on the nanofibrous mats. (A) The un-modified nanofibrous PHBV mat, (B) The chitosan-crosslinked nanofibrous mat (Mag: 2000×).
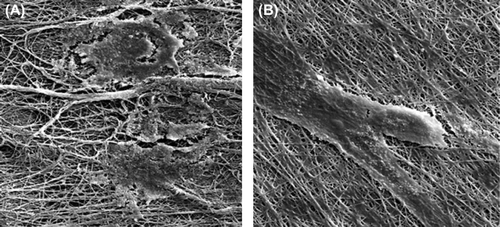
Table II. Results of MTT assay for the un-crosslinked, and the chitosan-crosslinked nanofibrous PHBV mats.
Discussion
The attempt to replace the lack of certain damaged or entirely destroyed anatomic structures is a challenge and a problem of continuous present interest in many medical fields. In this respect, the research concerning nerve regeneration cannot overlook the vast field of biocompatible materials, as some of them may be suitable to be used as nerve conduits. The aim is to produce a structure that promotes nerve regeneration to a level at least comparable to that found in nerve grafts, and to eliminate the need of sacrificing healthy nerves in order to repair a damaged one. For peripheral nerve repair, much effort has been devoted to developing artificial nerve grafts to replace traditional autograft techniques, which exhibit some drawbacks. Although artificial nerve grafts constructed from nonresorbable materials (e.g., conduits made from silicone or polyethylene) have yielded some degree of functional recovery, long-term complications often mean that a second surgical procedure is necessary to remove the conduits. These may actually become detrimental by virtue of toxicity or tendency to constrict the nerve (Fields et al. Citation1989). A nerve graft made of bioresorbable (gelatin (Gamez et al. Citation2004), hyaluron (Jansen et al. Citation2004), lactosorb (Francel et al. Citation2003), fibronectin (Ahmed et al. Citation2003), and poly (DL-lactide- co-caprolactone) (Meek et al. Citation1997)) is thus a promising alternative for promoting successful nerve regeneration. From that perspective the PHBV tube is a candidate that might replace the nerve graft, at least for the repair of long nerve defects. The tube is very easy to be handled; it can be easily placed and sutured on the nerve, thus eliminating the need of a very accurate nerve suture technique (Yucel et al. Citation2010, Kalbermatten et al. Citation2008). The electro-spinning technique is widely recognized as a straightforward way to fabricate nanoscale fibrous structures. Since this technique can produce nano- or submicron fibrous scaffolds which mimic the structure of natural ECM, it has elicited extensive research interest (Xiao et al. Citation2007). Another material is chitosan which has been evaluated extensively to bridge sciatic nerve gaps after injury (Meng et al. Citation2008, Belkas et al. Citation2004, Zhang et al. Citation2005, Citation2006, Xie et al. Citation2005, Rosales-Cortes et al. Citation2003, Patel et al. Citation2006). A recent study in dogs evaluated the immunological response to a chitosan prosthesis which was used for sciatic nerve repair after resection of a 26 mm nerve segment. They found that the material did not alter the cellular or humoral immune response of the organism during sciatic nerve repair. They also concluded that nerve repair, evaluated both morphologically and functionally, was similar to the nonoperated intact control group (Xie et al. Citation2005). More recently, our group has demonstrated that bridging resected sciatic nerves with chitosan nerve guides can increase functional recovery (Rosales-Cortes et al. Citation2003). These studies indicate that chitosan does have the potential to provide an ideal environment to promote nerve repair. Chitosan material has also been fabricated as a tubular scaffold with a variety of materials including alginate (Abernethy et al. Citation1992), hydroxyapatite (Politis et al. Citation1982), and poly-glycolic acid (Guertin et al. Citation2005). In this study, the nanofibrous conduit cross-linked with chitosan showed suitable physical, mechanical and structural, and cellular properties as the nerve graft.
Conclusion
In this study, a 3D, biodegradable, porous, polymeric nerve guidance conduit was described for use in the restoration of the function of injured nerve tissues. The nanofibrous PHBV nerve conduit was fabricated by electrospinning, and cross-linked with chitosan. The crosslinked nanofibrous conduit showed suitable physical, mechanical, and structural properties as nerve graft, also SCs well adhered on the nanofibrous surfaces especially on the chitosan-crosslinked nanofibrous surface. Furthermore, it is possible that this study will allow improvements to meet clinical trial requirements in the future.
Declaration of interest
The authors have no conflicts of interest to report in this work. The authors alone are responsible for the content and writing of the paper.
References
- Abernethy DA, Rud A, Thomas PK. 1992. Neurotropic influence of the distal stump of transected peripheral nerve on axonal regeneration: absence of topographic specificity in adult nerve. J Anat. 180:395–400.
- Ahmed Z, Underwood S, Brown RA. 2003. Nerve guide material made from fibronectin: assessment of In vitro properties. Tissue Eng. 9:219–231.
- Ai J, Heidari SK, Ghorbani F, Ejazi F, Biazar E, et al. 2011. Fabrication of Coated-Collagen Electrospun PHBV Nanofiber Film by Plasma Method and Its Cellular Study. J. Nanomater. 2011:1–8.
- Archibald SJ, Shefner J, Krarup C, Madison RD. 1995. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci. 15:4109–4123.
- Barcelos AS, Rodrigues AC, Silva MD, Padovani CR. 2003. Inside-out vein graft and inside-out artery graft in rat sciatic nerve repair. Microsurgery. 23:66–71.
- Belkas JS, Shoichet MS, Midha R. 2004. Peripheral nerve regeneration through guidance tubes. Neurol Res. 26:151–160.
- Biazar E, Montazeri N, Khorasan MT, Pourshamsian K, Daliri M, Mostafa Rezaei T, et al. 2010a. Types of neural guides and using nanotechnology for peripheral nerve reconstruction. Int J Nanomed. 5:839–852.
- Biazar E, Zhang Z, Heidari S. 2010b. Cellular orientation on micro- patterned biocompatible PHBV film. J. Paramed Sci. 1:74–7.
- Biazar E, Heidari SK, Pouya M, Rad H, et al. 2013a. Nanofibrous nerve conduits for repair of 30-mm-long sciatic nerve defects Neural Regen Res. 8:2266–2274.
- Biazar E, Heidari SK, Pouya M. 2013b. Behavioral evaluation of regenerated rat sciatic nerve by a nanofibrous PHBV conduit filled with schwann cell as artificial nerve graft. Cell commun adhes. 20:93–103.
- Biazar E, Heidari SK. 2013.A nanofi brous PHBV tube with Schwann cell as artificial nerve graft contributing to Rat sciatic nerve regeneration across a 30-mm defect bridge. Cell commun adhes. 20:41–49.
- Brunelli GA, Battiston B, Vigasio A, Brunelli G, Marocolo D. 1993. Bridging nerve defects with combined skeletal muscle and vein conduits. Microsurgery. 14:247–251.
- Burnett MG, Zager EL. 2004. Pathophysiology of peripheral nerve injury: brief review. Neurosurg Focus. 11:161–167.
- Ducker TB, Hayes GJ. 1970. Peripheral nerve grafts: experimental studies in the dog and chimpanzee to define homograft limitations. J Neurosurg. 32:236–243.
- Evans GR. 2000. Tissue engineering strategies for nervous system repair. Prog Brain Res. 128:349–363.
- Fansa H, Schneider W, Wolf G, Keilhoff G. 2002. Host responses after acellular muscle basal lamina allografting used as a matrix for tissue engineered nerve grafts. Transplantation. 74:381–387.
- Fields RD, Le Beau JM, Longo FM, Ellisman MH. 1989. Nerve regeneration through artificial tubular implants. Prog Neurobiol. 33:87–134.
- Firouzi M, Moshayedi P, Saberi H, Mobasheri H, Abolhassani F, Jahanzad I, Raza M. 2006. Transplantation of Schwann cells to subarachnoid space induces repair in contused rat spinal cord. Neurosci Lett. 402:66–70.
- Francel PC, Smith KS, Stevens FA, Kim SC, Gossett J, Gossett C, et al. 2003. Regeneration of rat sciatic nerve across a lactosorb bioresorbable conduit with interposed short-segment nerve grafts. J. Neurosurg. 99:549–554.
- Gamez E, Goto Y, Nagata K, Iwaki T, Sasaki T, Matsuda T. 2004. Photofabricated Gelatin-based Nerve Conduits: Nerve Tissue Regeneration Potentials. Cell. Transplant. 13:549–564.
- Ghaemmaghami F, Behnamfar F, Saberi H. 2009. Immediate grafting of transected obturator nerve during radical hysterectomy. Int J Surg. 7:168–169.
- Godard CW, de Ruiter MD, Spinner RJ, Yaszemski MJ, Windebank AJ. 2009. Nerve tubes for peripheral nerve repair. Neurosurg Clin N Am. 1:91–105.
- Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA. 2005. Microanatomy of axon/glial signaling during wallerian degeneration. J Neurosci. 25:3478–3487.
- Heath CA, Rutkowski GE. 1998. The development of bioartificial nerve grafts for peripheral nerve regeneration. Trends Biotechnol. 16:163–168.
- Heidari SK, Biazar E, Rezaei-Tavirani M, Rahmati-Roodsari M, et al. 2014. The healing effect of unrestricted somatic stem cells loaded in collagen-modified nanofibrous PHBV scaffold on full-thickness skin defects. Artif Cell Nanomed Biotech. 42:210–216.
- Hromadka M, Collins JB, Reed C, Han L, Kolappa KK, Cairns BA, et al. 2008. Nanofiber applications for burn care. J Burn Care Res. 29:695–703.
- Hughes RAC. 2002. Peripheral neuropathy: regular review. BMJ. 324:466–469.
- Jansen K, van der Werff JF, van Wachem PB, Nicolai JP, de Leij LF, van Luyn MJ. 2004. A Hyaluronan-based nerve guide: in vitro cytotoxicity, subcutaneous tissue reactions, and degradation in the rat. Biomaterials. 25:483–489.
- Kalbermatten DF, Erba P, Mahay D, Wiberg M, Pierer G, Terenghi G. 2008. Schwann cell strip for peripheral nerve repaire. J Hand Sur. 33:587–594.
- Keeley R, Atagi T, Sabelman E, Padilla J, Kadlcik S, Keeley A, et al. 1993. Peripheral nerve regeneration across 14-mm gaps: a comparison of autograft and entubulation repair methods in the rat. J Reconstr Microsurg9:349–358.
- Lundborg G, Rosen B, Dahlin L, Danielsen N, Holmberg J. 1997. Tubular versus conventional repair of median and ulnar nerves in the human forearm: early results from a prospective, randomized, clinical study. J Hand Surg22:99–106.
- Majdi A, Biazar E, Heidari S. 2011. Fabrication and comparison of electro-spun PHBV nanofiber and normal film and its cellular study. Orient. J. chem. 27:523–8.
- Montazeri M, Rashidi N, Biazar E, Rad H, et al. 2011. Compatibility of Cardiac Muscle Cells on Coated-Gelatin Electro-Spun Polyhydroxybutyrate/valerate Nano Fibrous Film. Biosci Biotech Res ASIA. 8:515–521.
- Meek MF, den Dunnen WFA, Bartels HL, Robinson PH. 1997. Long term evaluation peripheral nerve regeneration and functional nerve recovery after reconstruction with a thin-walled biodegradable poly (DL-lactide-caprolactone) nerve guide. Cell Mater. 7:53–57.
- Meng W, Xing ZC, Jung KH. 2008. Synthesis of gelatin-containing PHBV nanofiber mats for biomedical application. J Mater Sci: Mater Med. 19:2799–2807.
- Patel M, Vandevord PJ, Matthew HW, Wu B, Desilva S, Wooley PH. 2006. Video-Gait analysis of functional recovery of nerve repaired with chitosan nerve guides. Tissue Eng. 12:3189–3199.
- Politis MJ, Ederle K, Spencer PS. 1982. Tropism in nerve regeneration In Vivo. Attraction of regenerating axons by diffusible factors derived from cells in distal nerve stumps of transected peripheral nerves. Brain Res. 253:1–12.
- Rezaei-Tavirani M, Biazar E, AI J, Heidari S, et al. 2011. Fabrication of Collagen-Coated Poly (beta-hydroxy butyrate-co-beta- hydroxyvalerate) Nanofiber by Chemical and Physical Methods. Orient. J. chem. 27(2):385–95.
- Rosales-Cortes M, Peregrina-Sandoval J, Banuelos-Pineda J, Sarabia-Estrada R, Gomez-Rodiles CC, Albarran-Rodriguez E, Zaitseva GP, Pita-Lopez ML. 2003. Immunological study of a chitosan prosthesis in the sciatic nerve regeneration of the axotomized dog. J Biomater Appl. 18:15–23.
- Tong XJ, Hirai K, Shimada H, Mizutani Y, Izumi T, Toda N, et al. 1994. Sciatic nerve regeneration navigated by laminin-fibronectin double coated biodegradable collagen grafts in rats. Brain Res. 663:155–162.
- Venugopal J, Low S, Choon AT, Ramakrishna S. 2008. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater. 84:34–48.
- Wei Y, Zheng Z, Wang A, Zhou J, Ao Q, Gong Y, Zhang X. 2009. An improved method for isolating Schwann cells from postnatal rat sciatic nerves. Cell Tissue Res. 337:361–375.
- Williams SF, Martin DP, Horowitz DM, Peoples OP. 1999. PHA applications: addressing the price performance. Int J Biol Macromol. 25:111–116.
- Xiao XQ, Zhao Y, Chen GQ. 2007. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials. 28: 3608–3616.
- Xie F, Li QF, Zhao LS. 2005. Study on using a new biodegradable conduit to repairing rat's peripheral nerve defect. Zhonghua Zheng Xing Wai Ke Za Zhi. 21:295–298.
- Yin Q, Kemp GJ, Frostick SP. 1998. Neurotrophins neurones and peripheral nerve regeneration. J Hand Surg Br. 23:433–437.
- Yucel D, Kose GT, Hasirci V. 2010. Polyester based nerve guidance conduit design. Biomaterials. 31:1596–1603.
- Zhang PX, Jiang BG, Zhao FQ, Fu ZG, Zhang DY, Du C, Zhang HB. 2005. Chitin biological tube bridging the peripheral nerve with a small gap. Zhonghua Wai Ke Za Zhi. 43:1344–1347.
- Zhang WG, Lu DC, Fu CY, Qu W. 2006. Favorable effect of chitosan sustained-release FK506 incorporated conduits on axonal regeneration in rat sciatic nerve. Zhonghua Yi Xue Za Zhi. 86: 1065–1068.