Abstract
Recent research suggested that cardiac stem cells (CSCs) may have the clinical application for cardiac repair. However, their characteristics and the regulatory mechanisms of their growth and differentiation have not been fully investigated. Vascular endothelial growth factor (VEGF, VEGF-A) is a major regulator of physiological and pathological angiogenesis. But the homing role of VEGF for CSCs is unclear. In this report, CSCs were isolated, purified, and expanded in vitro from rat heart. VEGF, SU5416 (VEGF receptor blocker), and Wortmannin (PI3K/Akt signaling pathway inhibitor) were used for differentiation into vascular endothelial cells (VECs). Real-time qPCR was selected to confirm the role of PI3K/Akt signaling pathway in VECs differentiation from rat CSCs. The result of real-time qPCR demonstrated that PI3K/Akt signaling pathway plays an important role in rat CSCs differentiated into VECs. So, our research provides a theoretical basis and experimental evidence for therapeutic application of rat CSCs to treat cardiac repair.
Introduction
In recent years, cardiac disease has emerged as the leading cause of death worldwide, particularly in developed countries. The exact prevalence of heart failure is cardinal symptom and largely unknown but is growing because new treatments have increased the life expectancy of these patients. Whereas heart failure is often ischemic in origin, the origin of heart failure is more varied, including a spectrum of cardiomyopathies and arrhythmias. A novel emerging treatment for heart failure is cellular cardiomyoplasty, whereby stem cells are delivered to the dysfunctional myocardium.(Mishra et al. Citation2011, Yi et al. Citation2010) Due to its unique characteristics, stem cell transplantation is considered a potentially effective treatment for heart failure. Both embryonic stem cells and adult stem cells (ASCs) are being studied for clinical applications, and some types of autologous cells (skeletal myoblasts, bone marrow stem cells, mesenchymal stem cells, and endothelial progenitor cells) are being tested in clinical trials (Otsuru et al. Citation2012, Perez-Simon et al. Citation2011, Trounson et al. Citation2011, Bai et al. Citation2013).
Cardiac stem cells (CSCs) isolated from the myocardium have self-renewal capabilities in vitro and the ability to differentiate into all three major cardiac cell types: cardiomyocytes, vascular smooth muscle cells, and vascular endothelial cells (VECs) in vivo. VECs take part not only in vascularization during embryonic development, but also in postnatal vascularization and reparative processes post-trauma. Therefore, VECs hold extensive prospects for vascular tissue engineering and possible clinical application in heart failure. The expression of vascular endothelial growth factor (VEGF) increases in response to myocardial ischemia and promotes vascular repair. VEGF is widely recognized to be a potential therapeutic target for regulating angiogenesis (Freedman and Isner Citation2001, Zachary and Morgan Citation2011). However, whether VEGF is involved in CSCs differentiation remains unknown.
The Phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB or Akt) signal transduction cascade has been investigated extensively for its roles in angiogenesis. Initial studies implicated both PI3K and Akt in prevention of apoptosis. However, more recent evidence has also associated this pathway with regulation of angiogenesis and cell cycle. Here, we discuss PI3K/Akt-mediated signal transduction in differentiation of VECs from rat CSCs.
Material and methods
Isolation, culture, and purification of rat CSCs
Rat heart tissues were obtained from 20 to 30 neonatal rats. All animal experiments were performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee at Hebei Medical University. All operations were conducted aseptically. The heart tissues were washed 3 times with phosphate buffer saline (PBS) containing 100 IU/ml penicillin and 100 μg/ml streptomycin to remove pericardium and capillaries. The heart tissue pieces were digested with 200 U/ml collagenase Type II and collagenase Type I (both from Invitrogen) in Hank's Balanced Salt Solution for 60 min at 37°C, followed by a 10-min treatment with 0.125% trypsin. Enzymatic activity was then neutralized with the same volume of fetal bovine serum (GIBCO, USA). The cell suspension containing the majority of c-kit+ CSCs was obtained after being purified through flow cytometry, the antibodies (c-kit and Flk 1) were used in the cell purification.
Surface marker expression analysis of rat CSCs
Cultures of rat CSCs were seeded on glass coverslips coated with poly-L-lysine. When cultures reached 60% confluence they were washed three times with PBS, then fixed with 4% paraformaldehyde for 15 min at room temperature, and again washed three times (5 min per wash) with PBS. Cells were permeabilized by incubating with 0.125% Triton X-100 for 10 min. Rat CSCs were then washed three times with PBS and incubated with 4% bovine serum albumin at room temperature for 30 min. The cells were then incubated with primary antibodies in a humidified chamber at 4°C overnight. After three washes with PBS for 5 min each, the cells were incubated with fluorescein isothiocyanate (FITC)-labeled secondary antibodies at room temperature for 1 h, and were rinsed three times with PBS for 5 min each. Finally, nuclei were labeled by incubation with 4,6 diamidino-2-phenylindole (Sigma, USA). The cells were examined by a phase contrast fluorescence microscope (Olympus, Japan).
VECs differentiation of rat CSCs
For differentiation into VECs, rat CSCs were incubated in VECs medium containing 20 ng/ml VEGF165 (Peprotech, USA), as Group A; Group B was set up by adding 5 μM SU5416 (VEGF receptor blocker) from Group A; Group C was set up by adding 100 nM Wortmannin (PI3K/Akt signaling pathway inhibitor) from Group A. While control cells were continued to be cultured in complete medium without any inducer. The medium was refreshed every 3 days; 1 week later, it was detected for the expression of the CD31 and CD144, the endothelial specific marker via immunofluorescence.
PI3K/Akt signaling pathway analysis in differentiation of VECs
After 8 days, RNA was extracted with Trizol reagent (Invitrogen, USA) to detect the expression of PI3K/Akt signaling pathway members and special genes of VECs using real-time qPCR in induced VECs. The members of PI3K/Akt signaling pathway include VEGFR2, PI3K, Akt, and endothelial nitric oxide synthase (eNOS). Special genes of VECs include CD31 and CD144. Information of gene-specific primer pairs is listed in .
Table I. Primer sequences used in real-time qPCR.
Statistical analysis
Statistical analyses of the data were performed with a one-way ANOVA followed by the Tukey–Kramer honestly significant difference test for the three sets of results. A P-value of less than 0.05 was considered significant. Statistical analyses were done with a JMP® Statistical Discovery Software (SAS Institute, Cary, NC).
Results
Morphological features and Phenotypic Characterization of rat CSCs
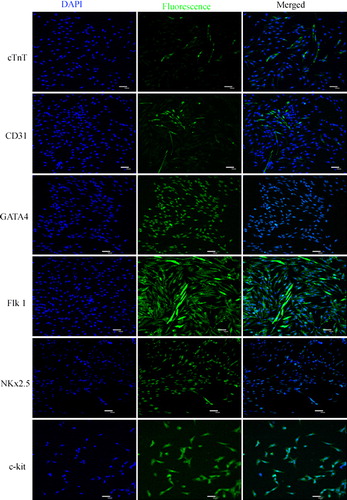
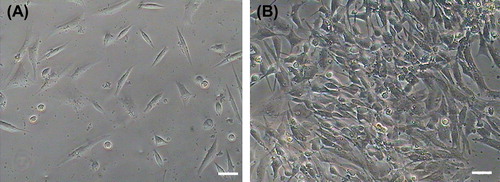
Primary cells isolated from heart tissue adhered to plates at 48 h and began to proliferate 72 h later. The cells proliferated quickly, displaying obviously karyokinesis and different morphologies including fusiform, triangular and irregular shape after purification (). Rat CSCs at various passages were positive for the c-kit, GATA 4, Nkx2.5, and Flk 1 cell surface markers but cTnT and CD31 were weakly expressed (). We did not observe a significant difference in the prevalence of c-kit positive cells at different passages (p > 0.05).
Figure 1. Morphology of rat cardiac stem cell and vascular endothelial cells. (A) Rat primary c-kit+ cardiac stem cells after purification through FCM. (B) Vascular endothelial differentiation of rat c-kit+ cardiac stem cells, cells converted from fusiform and triangular to roundness in shapes after day 6.
VECs differentiation of rat CSCs
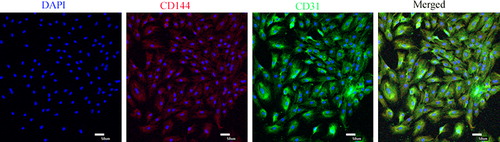
After the induction with osteoblast VECs, rat CSCs had apparent changes in appearance. From the 4th day after the induction, rat CSCs in Group A changed from fusiform to t roundness or irregularity, until the cells were very closely located and showed cobblestone shape (). But there were no obvious morphological changes in Group B, Group C, and control. Immunofluorescence results showed that CD31 and CD144, markers of VECs, were expressed in the differentiated cells ().
PI3K/Akt signaling pathway analysis in VECs differentiation
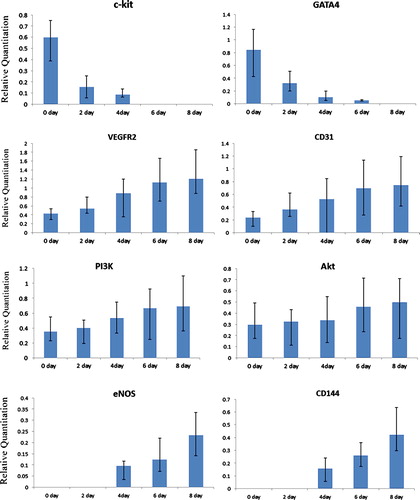
Real-time qPCR was used to detect the gene expression of members of PI3K/Akt signaling pathway, including VEGFR2, PI3K, Akt, and eNOS. The result demonstrated that after incubation with VEGF, the specific genes, including VEGFR2, PI3K, Akt, eNOS, CD31, and CD144, markers of PI3K/Akt signaling pathway and VECs, were expressed and gene expression level showed a time-lapse increase in Group A. However, the markers of rat CSCs, including c-Kit, GATA 4, and Nkx2.5, the gene expression level showed a time-lapse decrease (). There are no significant differences in Group B, Group C, and control group.
Figure 4. Real time qPCR analyses of PI3K/Akt cell signaling pathway members and vascular endothelial cell markers in endothelial cell differentiation. The result indicated that after endothelial cell induction the specific genes, including PI3K/Akt cell signaling pathway members (PI3K and Akt), and endothelial cell markers (CD31, VEGFR-2 and CD144) were detected, and gene expression level showed a time-lapse increase, cardiac stem cells markers (c-kit and GATA 4) showed a time-lapse gradually decrease.
Discussion
VEGF is an endothelial cell-specific mitogen which induces angiogenesis and vascular permeability in vivo. Previous evidence suggested that VEGF is an important angiogenesis factor during heart form and embryonic development (Millauer et al. Citation1993, Ferrara Citation2001). The discovery of the existence of multiple VEGF isoforms raised several questions regarding their relative significance (Plate and Warnke Citation1997). Instead, the VEGF165 is potentially the most important isoform and was found, similar to native VEGF, to be a soluble heparin-binding endothelial cell mitogen (Leung et al. Citation1989). Subsequent studies confirmed that native VEGF from numerous sources corresponds to VEGF165 (Houck et al. Citation1992). Therefore, VEGF165 was selected to induce rat CSCs and activate PI3K/Akt signaling pathway for differentiation into VECs in our research. Although originally identified as a factor that induces vascular permeability, VEGF exhibits multiple biological activities in VECs, including the enhancement of VECs survival. VEGF effects on cell survival have been shown to be mediated by VEGFR2/PI3K/Akt signaling pathway (Gerber et al. Citation1998, Fujio and Walsh Citation1999). And VEGF enhances endothelial cell migration and capillary-like structure formation in vitro and these activities of VEGF are PI3K-Akt-dependent.
PI3K pathway exerts its effects through Akt, its downstream target molecule, and thereby regulates various cell functions including cell proliferation, cell transformation, apoptosis, tumor growth, and angiogenesis (Ma et al. Citation2009). Previous studies demonstrated that VEGF-induced increase in NO release from endothelial cells is attenuated by PI3K inhibitors (Papapetropoulos et al. Citation1997). Subsequently, it was demonstrated that VEGF stimulates Akt-mediated eNOS phosphorylation at Ser1177, leading to an increase in eNOS activity (Fulton et al. Citation1999, Dimmeler et al. Citation1999). It was also reported that production of NO in response to fluid shear stress in cultured endothelial cells was controlled by Akt-dependent phosphorylation of eNOS, (Dimmeler et al. Citation1999) and another study demonstrated that shear stress induces eNOS phosphorylation predominantly through a protein kinase A-dependent, Akt-independent mechanism (Boo Citation2006). In addition to its role in angiogenesis, Akt has also been involved as a general regulator of organ growth. Studies in Drosophila have demonstrated that components of the insulin/IGF signaling pathway including Akt are involved in the regulation of organ growth and body size in response to nutritional input (Verdu et al. Citation1999, Weinkove and Leevers Citation2000). In brief, PI3K/Akt signaling pathway also regulates cardiovascular homeostasis and vessel integrity at least in part by controlling NO synthesis. And this pathway is activated by a variety of stimuli in endothelial cells and regulates multiple critical steps in angiogenesis, including endothelial cell survival, migration, and capillary structure formation. Therefore, real-time qPCR was used to confirm the function of PIAK/Akt signaling pathway in VECs differentiation from CSCs.
In this study, CSCs were isolated and purified from rat heart tissue, and their morphology and surface marker expression were investigated. We also demonstrated that VGEF can regulate rat CSCs to differentiate into VECs via stimulating PI3K/Akt signaling pathway. The present study has important bearing on the potential application of CSCs as an ASC source for tissue engineering.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bai C, Hou L, Zhang M, Wang L, Guan W, Ma Y. 2013. Identification and biological characterization of chicken embryonic cardiac progenitor cells. Cell Prolif. 46:232–242. Epub 2013/03/21.
- Boo YC. 2006. Shear stress stimulates phosphorylation of protein kinase A substrate proteins including endothelial nitric oxide synthase in endothelial cells. Exp Mol Med. 38:453. Epub 2006/ 09/20.
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 399:601–605. Epub 1999/06/22.
- Ferrara N. 2001. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 280:C1358–1366. Epub 2001/05/15.
- Freedman SB, Isner JM. 2001. Therapeutic angiogenesis for ischemic cardiovascular disease. J Mol Cell Cardiol. 33:379–393. Epub 2001/02/22.
- Fujio Y, Walsh K. 1999. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 274:16349–16354. Epub 1999/05/29.
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. 1999. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 399:597–601. Epub 1999/06/22.
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. 1998. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343. Epub 1998/11/07.
- Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. 1992. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 267:26031–26037. Epub 1992/12/25.
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 246:1306–1309. Epub 1989/12/08.
- Ma J, Sawai H, Ochi N, Matsuo Y, Xu D, Yasuda A, et al. 2009. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem. 331:161–171. Epub 2009/05/14.
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. 1993. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 72:835–846. Epub 1993/03/26.
- Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, et al. 2011. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 123:364–373. Epub 2011/01/19.
- Otsuru S, Gordon PL, Shimono K, Jethva R, Marino R, Phillips CL, et al. 2012. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood. 120:1933–1941. Epub 2012/07/26.
- Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. 1997. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 100:3131–3139. Epub 1998/01/31.
- Perez-Simon JA, Lopez-Villar O, Andreu EJ, Rifon J, Muntion S, Campelo MD, et al. 2011. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft- versus-host disease: results of a phase I/II clinical trial. Haematologica. 96:1072–1076. Epub 2011/03/12.
- Plate KH, Warnke PC. 1997. Vascular endothelial growth factor. J Neurooncol. 35:365–372. Epub 1998/01/24.
- Trounson A, Thakar RG, Lomax G, Gibbons D. 2011. Clinical trials for stem cell therapies. BMC Med. 9:52. Epub 2011/05/17.
- Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ. 1999. Cell- autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat Cell Biol. 1:500–506. Epub 1999/12/10.
- Weinkove D, Leevers SJ. 2000. The genetic control of organ growth: insights from Drosophila. Curr Opin Genet Dev. 10:75–80. Epub 2000/02/19.
- Yi BA, Wernet O, Chien KR. 2010. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 120:20–28. Epub 2010/01/07.
- Zachary I, Morgan RD. 2011. Therapeutic angiogenesis for cardiovascular disease: biological context, challenges, prospects. Heart. 97:181–189. Epub 2010/10/05.