Abstract
Psoriasis is an autoimmune, chronic, inflammatory skin disease characterized by epidermal hyperplasia, proliferation of blood vessels, and infiltration of leukocytes in dermis and epidermis. Several immunosuppressants such as methotrexate (MXT) and cyclosporine are used but they are associated with adverse effects due to down regulation of immune system. Numerous approaches have been explored to overcome the problems of conventional topical system such as high frequency of application, impermeability to skin barrier, and limited efficacy. Photodynamic therapy is another non-invasive technique currently used for skin diseases. The combination of two drugs is also commonly observed to achieve more effective therapy. In the present study, antipsoriatic activity of niosomal formulations for the treatment of psoriasis in combination with narrow and broad band UV radiation had been explored in experimental animal model.
Introduction
Psoriasis is an autoimmune disease of dermis and epidermis, characterized by increased proliferation of epidermis with dilation of dermal capillaries, infiltration of inflammatory cells in skin layers, deregulated localized skin growth which leads to scaly erythematous plaques along with itching, skin flaking, swelling, and pain (Nagle et al. Citation2011, Hashizume et al. Citation2007). Psoriasis is a chronic inflammatory skin disease, which may flare up at any cutaneous surface. It is most frequently seen at the extensor surfaces of the sacral areas, scalp, elbow, and knees (Ali et al. Citation2008). Approximately 1–3% of the population is affected by this disease and 1.5 million cases (annually) in the USA are diagnosed by physicians. There are mainly five types of psoriasis, such as plaque, flexural, guttate, nail, and psoriatic arthritis (Almutawa et al. Citation2013). Several available treatments can minimize skin lesions and associated symptoms but cannot provide complete cure of psoriasis. Biologic, phototherapy, topical, and systemic treatments can induce reduction of psoriasis for months to years but these type of treatments mainly depend on the severity of disease or extent of involvement, expense, adverse-effect profile, patient preference, and availability (Mason et al. Citation2013). For example, most patients with psoriasis have limited disease (less than 20% of their body surface area), so topical therapy is usually sufficient for such patients. MTX, a dihydrofolate reductase enzyme inhibitor is used in the treatment of certain types of neoplasia (at high doses), useful as an immunosuppressant and anti-inflammatory agent (at low doses) and thus have potential in controlling intractable psoriasis and adult rheumatoid arthritis (in a long-term therapy) (Lotti Citation2008). The use of MTX is limited due to many adverse effects such as anemia, leucopenia, hepatotoxicity, oligospermia, menstrual alteration, gastrointestinal disorder, hyperazotemia, depression, and other psychic disorders (Kocak et al. Citation2013). The oral uptake of MTX by the gastrointestinal tract is limited due to the saturation of the transporter, reduced folate carrier 1. The major problem with topical MTX is its limited capacity for passive diffusion across the stratum corneum (SC) due to its water soluble nature, high molecular weight (454.56 Da), and mostly occurs in the dissociated form at a physiological pH (Dogra et al. Citation2012). To overcome the barrier properties of the SC, various techniques such as iontophoresis, electroporation or the use of vehicles such as micro emulsions, liposomes, niosomes, emulsomes, transferosomes, and nanolipid carrier have been explored to enhance transdermal MTX delivery (Azeem et al. Citation2009, Garg et al. Citation2012). The encapsulation of MTX in unilamellar niosomes has been effective over other micro and nano encapsulation technologies because it offers high patient compliance, accommodates drug molecules with a wide range of solubility, vesicle formulations are variable as well as controllable, prolongs its action and also decreases the renal clearance of drug (Shirsand et al. Citation2012). Niosomal formulations can also increase the residence time of drugs in the SC and epidermis, while reducing the systemic absorption and can improve the horny layer properties by reducing transepidermal water loss and by increasing smoothness via replenishing lost skin lipids. Topical drug can be used only as short-term therapy so it is combined with photodynamic therapy (PUVA therapy) using trioxysalen drug (Silva et al. Citation2013). It causes DNA cross-linking when exposed to UV light which prevents cell replications and slows down the rate of epidermal cell turnover the skin thickening and finally may clear plaques easily. Individually PUVA therapy causes hyper pigmentation, erythema, and itching, so it is used along with MTX niosomes carrier (Dayal and Jain Citation2010). The combination of these two drugs is also commonly observed to achieve more effective therapy and reduces the side effects of MTX. Finally, the main aim of the present study was to formulate niosomal formulations for psoriasis treatment by using combination therapy.
Materials and methods
Materials
MXT was kindly received as gift sample from Sigma pharmaceuticals and trioxysalen from Unitech pharmaceuticals, Ludhiana. Cholesterol, DMSO, and Span 85 were purchased from CDH laboratory reagents, New Delhi. Diethyl ether, dichloromethane, and sephadex were purchased from Sigma chemicals. Triton X-100 was purchased from Himedia India. All other solvents and chemicals used in the study were of analytical grade.
Preparation of drug-loaded niosomes
Reverse phase evaporation method
The required optimized amount of cholesterol and span 85 in different molar ratio (7:3) were dissolved in organic solvent. Then aqueous phase was added dropwise and sonicated for 2 min. A clear solution is formed which is further vortexed till organic solvent is evaporated (Ko and Bickel Citation2012). Finally, prepared niosomes were used for further characterization.
MTX-loaded niosomes—the organic solvent used was diethyl ether. The drug was dissolved in aqueous phase, that is, phosphate buffer (pH 7.4).
Trioxysalen-loaded vesicles—the organic solvent is dichloromethane. The drug was dissolved in organic solvent. The aqueous phase was phosphate buffer (pH 7.4).
Characterization of optimized niosomal formulations
The optimized formulations were characterized for various parameters like morphology, size and size distribution, particle charge, entrapment efficiency, in vitro drug release, skin permeation, stability studies, and finally in vivo studies.
Morphology of optimized formulations
Samples were analyzed by using Transmission electron microscopy to examine the shape and morphology of the niosomal formulations. The size and potential of niosomes were determined by photon correlation spectroscopy using zetasizer (Beckman coulter Delsa nano software, UK). The dispersions were put into the cuvetts of zetasizer and measured the size, poly dispersity index (PDI), and zeta potential.
Entrapment efficiency of niosomal formulations
The percent of MXT and trioxysalen in carrier system were determined by using mini column centrifuge of sephadex G-50 and then evaluation of entrapment efficiency by rupturing the carriers in suitable solvent (0.1% Triton X-100). Firstly, Sephadex G-50 (1 g) was swelled in 10 ml of 0.9% sodium chloride with occasional shaking (for 5 h at room temperature) and the formed gel was stored at 4°C. The hydrated gel was filled to the top of barrel of 1 ml disposable syringe. This syringe was plugged with Whatmann filter pad and then placed in centrifuge tubes. The tubes were centrifuged (at 3000 rpm for 3 min) to remove excess saline solution. A Niosomal formulation (2 ml) was added dropwise on the top of gel bed in the center. Elutes were removed and vesicles were disrupted by adding 1 ml of 0.1% Triton X-100 with the help of centrifugation and assayed for drug content by UV spectroscopy (Dubey et al. Citation2007).
Entrapment efficiency = Entrapped drug∕Total drug × 100.
In-vitro drug-release study of niosomal formulations
Niosomal formulations (passing through sephadex) were placed in dialysis membrane and were incubated in 100 ml phosphate buffer solution (PBS):methanol (9:1) medium in shaking incubator (Labtech, LSI-2005RL), which was maintained at 37°C at 100 rpm. At defined time intervals, samples were withdrawn periodically and the supernatant (3 ml) sampled was replaced with an equal volume of fresh release medium. The amount of drug was determined by measuring the drug concentration in the supernatant. The clear solution was analyzed by UV spectrophotometer using a detection wavelength of 250 nm for MXT, 258 nm for Trioxysalen, and for combination UV method was used to estimate both the drugs (Shirsand et al. Citation2012).
Skin permeation studies of niosomal formulations
The in vitro skin permeation of optimized formulation of both the drugs was studied by Franz diffusion cell. Full thickness of skin (freshly sacrificed rat) was excised and its subcutaneous fat was carefully removed using scalpel and scissor. Rat's skin was mounted between donor and receptor compartments of Franz diffusion cell and excessive skin at the sides was trimmed to minimize lateral diffusion. The donor compartments of the cell were filled with 2 ml of the formulation (after passing the formulation through dialysis bag). The receptor phase was filled with 10 ml of PBS:Methanol (9:1). The entire assembly was securely positioned on magnetic stirrer (Remi motor, RQT-122) at 100 rpm. Samples were withdrawn through sampling port of diffusion cell at predetermined interval over 24 h. The receptor was immediately replenished with equal volume of fresh solution. The apparent permeability coefficient (Papp) was calculated as follows:
where the term ∆Q/∆T was the steady-state of the linear portion of the plot of the amount of drug in the receptor chamber versus time. The available skin for diffusion (0.78 cm2), C0 the initial concentration of drug in the donor cell and 60 the conversion of units from minutes to seconds (Lunter and Daniels Citation2013).
Stability studies of niosomal formulations
Stability of a product is defined as capability of a particular formulation to remain with the physical, chemical, therapeutic, and toxicological specification. The optimized formulation was selected for stability study on the basis of its in vitro performance and stored in tightly closed container at ambient temperature (25 ± 1°C) and refrigerator (4 ± 1°C). The effect of storage on size and drug content were evaluated at different time interval (10, 20, and 30 days).
In vivo studies of niosomal formulations
The normal adult mouse tail contains regions of ortho and parakeratosis stratum corneum. Parakeratosis, an abnormal form of an epidermal keratinization pattern, is characterized by absence of a granular layer in the epidermis. In the rat tail skin, parakeratosis sets in by days 7–9 after birth and the scales start developing. During this period, small depressions are observed in the epidermis, which further develop and form the orthokeratotic area (i.e., normal pattern of keratinization), while the area between these notches shows an abnormal pattern of keratinization, known as the parakeratosis area. Any agent that causes the inhibition of parakeratosis and the restoration of orthokeratosis can be used for treating psoriasis. Hence, the mouse tail model is commonly employed for screening drugs for the treatment of psoriasis (Brady Citation2004).
Antipsoriatic activity
In the present study, four groups of five albino mice each were treated once daily with saline, MXT, trioxysalen, and combination of both drugs. One group of five animals acted as control (i.e., no treatment). The formulations were applied once daily with paint brush to the proximal half of the mouse tail for 4 weeks. After the application of formulation, UV treatment is given for half an hour to activate the drug, that is, trioxysalen. Twenty-four hours after the last application of formulations, the mice were killed by spinal dislocation, and the tail skin was cut by longitudinal dissection with a scalpel and stripped from the underlying cartilage. The skin samples obtained were appropriately processed and stained with hematoxylin and eosin. The processed skins were examined microscopically for the presence of granular layer in the scale regions and epidermal thickness. Induction of orthokeratosis in those parts of the adult mouse tail, which have normally a parakeratotic differentiation, was quantified measuring the length of the granular layer (A) and the length of the scale (B). Ten sequential scales were examined for each skin section. The proportion, that is, A/B*100 represents the percent orthokeratosis per scale. The drug activity was calculated by the following equation:
Results and discussion
Physicochemical characteristics of drug-loaded niosomes
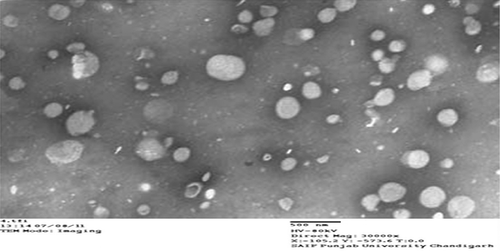
MTX- and trioxysalen-loaded niosomes, which have smooth and spherical surface, were formulated from a reverse-phase evaporation technique (). The vesicles were discrete and separate with no aggregation or agglomeration. The niosomes have regular spherical nanopore structures ranging from 400-500 nm. From TEM observation, it is clear that the structure of niosomes was uniform and independent of surfactant. The average size, PDI, and zeta potential of optimized niosomal formulations are reported in .
Table I. Surface characteristics of optimized drug-loaded niosomal formulations.
Entrapment efficiency study of optimized formulations
The entrapment efficiency of MXT-loaded niosomes, trioxysalen-loaded niosomes, and niosomes containing both the drugs were estimated and repeated three times. All the formulations showed good reproducible results and the entrapment efficiency of optimized formulation is shown in . Solubility of drugs and cholesterol concentrations play an important role in the entrapment efficiency of drugs in the niosomal formulation. MTX entrapment efficiency is higher due to its hydrophilic nature as compared with trioxysalen's (lipophillic nature). The entrapment efficiency tends to increase with increase in cholesterol from low level to intermediate level due to interchlelation of cholesterol into bilayer of vesicles that also increases the width of the bilayer. Further, the bilayer structure gets disrupted due to increase in space at high level of cholesterol concentration.
Table II. Entrapment efficiency of optimized niosomal formulation.
In vitro-release study of optimized formulations
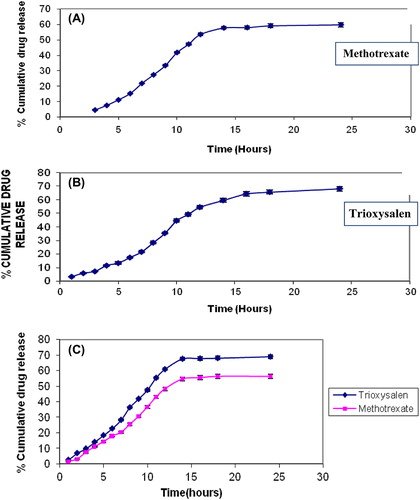
The drug-release studies were carried out on all formulations. The percentage of drug release after 24 h was 59.8 ± 1.25 (A) for MXT and 68.21 ± 1.31 for trioxysalen (), and that for combined dosage containing both the drugs was 69 ± 1.21 for trioxysalen and 56.31 ± 1.19 for MXT (). The release of MXT is less as compared with that of trioxysalen due to hydrosolubility of the drug and its high molecular weight (454.56 g/mol), and the presence of drug mostly in dissociated form at physiological pH further limits its capacity for passive diffusion (Dubey et al. Citation2007).
Permeation studies of optimized formulations
The amount of drug permeated in 24 h from niosomes for MXT is 44.2 ± 4.09 (flux 6.1 μg/cm/h), for Trioxysalen 71.54 ± 3.12 (flux 9.5 μg/cm/h), and for combined dosage form 47.51 ± 3.17 (flux 9.2 μg/cm/h) for MXT and 74 ± 3.18 (flux 11.5 μg/cm/h) for trioxysalen due to fusion of niosome vesicles to the surface of the skin results in high permeation due to direct transfer of the drug into the skin. and show the permeation release parameters of optimized niosomal formulations.
Figure 3. Permeation studies of Trioxysalen (A), MXT (B), and combination of both drugs (C) in methanolic PBS (7.4).
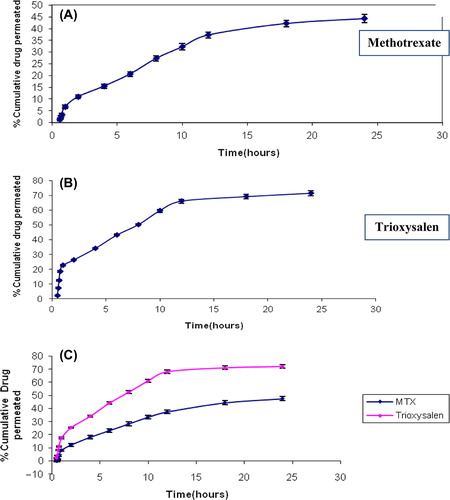
Table III. Permeation parameters of niosomal formulations.
Stability study of optimized formulations
The storage stability studies performed on optimized formulation containing both the drugs indicated that formulation stored at 4 ± 1°C was more stable than 25 ± 1°C on the basis of size and drug content. shows that the particle sizes of optimized formulations were increased and drug contents of both the drugs were decreased at 25 ± 1°C as compared to 4 ± 1°C because niosomal formulation has shown leaky and Fusogenic characteristic at 25 ± 1°C. Studies revealed that formulations were stable at 4 ± 1°C and were stored at 4 ± 1°C for its better therapeutic efficacy.
Table IV. Stability of effect of storage temperature on size and drug content.
Antipsoriatic activity (Rat tail model)
In psoriasis, lymphocytes may change the growth of epidermis leading to keratinocytes proliferation and abnormal differentiation of apoptotic cells. The rat tail model was used for studying the efficacy of the formulation in vivo. In rat's skin, parakeratosis is seen 7–9 days after birth and after that scales start developing. The normal pattern of keratinization is called orthokeratosis and retention of nuclei and abnormal maturation of epidermal keratinocytes is called parakeratosis (Ruchusatsawat et al. Citation2011). In this process granular layer is also decreased. indicates histological appearance of untreated mouse tail skin after staining with hematoxylin and eosin, showing alternate scale (S) and interscale (IS) epidermal regions. The granular layer was only apparent in the orthokeratotic interscale regions and not in the parakeratotic scale regions. The treatment was done for 4 weeks. indicates the treatment with MXT and the most common keratosis is orthokeratosis; in only one point nuclei are caught in the keratin (parakeratosis). indicates treatment for 4 weeks with trioxysalen and it shows hyperplasia of epidermis with multiple layers of cells specially the basal cells. The top most layer of keratin shows parakeratosis. indicates treatment for 4 weeks with MXT and trioxysalen. The epidermis is totally orthokeratotic and has continuous granular cell layer. The most common reasons for parakeratosis are increase in mitosis and decrease in transit time of differentiating keratinocytes at the time of cornification. The transverse section of rat tail skin was treated with niosomes formulation for 4 weeks and the percentage of orthokeratosis calculated in drug activity is shown in and . The control group showed orthokeratosis, that is 11% only in the notches or no scaly regions, while the scaly regions showed parakeratotic differentiation. MXT and trioxysalen showed 25% and 39% orthokeratosis, respectively, while combined group showed an almost 52% orthokeratosis (i.e., presence of granular layer per scale).This shows the effectiveness of combination therapy to induce normal differentiation pattern in the epidermis of the skin.
Figure 4. Skin histology: (A) Control group, (B) Skin treated with MXT, (C) Skin treated with Trioxysalen, and (D) Skin treated with MXT and trioxysalen.
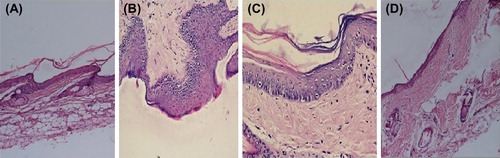
Figure 5. Photographs of Skin treated with (A) MXT (400 ×), (B) Trioxysalen (400 ×), and (C) MXT and trioxysalen (400 ×).
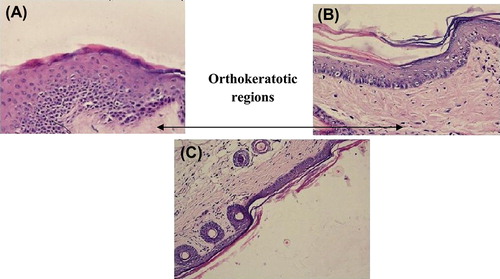
Table V. Percentage orthokeratosis and drug activity.
Conclusions
Developed formulations improve the local concentration of encapsulated drugs and reduce the systemic side effects due to their limited systemic access. The maximum percentage of orthokeratosis 52% and drug activity 58% was found to be with combine niosomal delivery of MXT and trioxysalen using photodynamic therapy. Thus, combination therapy causes good healing of psoriasis. This approach will offer selective and targeted therapy as well as satisfactory safety profile, enabling treatment for prolonged periods of time. The utilization of combined delivery of MXT and trioxysalen within niosomal formulations provides a novel and effective alternative for the treatment of psoriasis. Progress in the academic field of drug formulations may be modified to become commercial products in the future.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ali MF, Salah M, Rafea M, Saleh N. 2008. Liposomal methotrexate hydrogel for treatment of localized psoriasis: preparation, characterization and laser targeting. Med Sci Monit. 14:PI66–74.
- Almutawa F, Alnomair N, Wang Y, Hamzavi I, Lim HW. 2013. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 14:87–109.
- Azeem A, Khan ZI, Aqil M, Ahmad FJ, Khar RK, Talegaonkar S. 2009. Microemulsions as a surrogate carrier for dermal drug delivery. Drug Dev Ind Pharm. 35:525–547.
- Brady SP. 2004. Parakeratosis. J Am Acad Dermatol. 50:77–84.
- Dayal S, Jain VK. 2010. Comparative evaluation of NBUVB phototherapy and PUVA photochemotherapy in chronic plaque psoriasis. Indian J Dermatol Venereol Leprol. 76:533–537.
- Dogra S, Krishna V, Kanwar AJ. 2012. Efficacy and safety of systemic methotrexate in two fixed doses of 10 mg or 25 mg orally once weekly in adult patients with severe plaque-type psoriasis: a prospective, randomized, double-blind, dose-ranging study. Clin Exp Dermatol. 37:729–734.
- Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. 2007. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 123:148–154.
- Garg T, Singh O, Arora S, Murthy R. 2012. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Hashizume H, Ito T, Yagi H, Takigawa M, Kageyama H, Furukawa F, et al. 2007.Efficacy and safety of preprandial versus postprandial administration of low-dose cyclosporin microemulsion (Neoral) in patients with psoriasis vulgaris. J Dermatol. 34:430–434.
- Ko YT, Bickel U. 2012. Liposome-encapsulated polyethylenimine/oligonucleotide polyplexes prepared by reverse-phase evaporation technique. AAPS PharmSciTech. 13:373–378.
- Kocak AY, Kocak O, Aslan F, Tektas M. 2013. Methotrexate toxicity presenting as cutaneous ulcerations on psoriatic plaques. Cutan Ocul Toxicol. 32:333–335.
- Lotti TM. 2008.Dermatologic therapy issue on “New and emerging treatments in dermatology”, Dermatol Ther. 21:85.
- Lunter D, Daniels R. 2013. In vitro skin permeation and penetration of nonivamide from novel film-forming emulsions. Skin Pharmacol Physiol. 26:139–146.
- Mason AR, Mason J, Cork M, Dooley G, Hancock H. 2013. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 3: CD005028.
- Nagle A, Goyal AK, Kesarla R, Murthy RR. 2011. Efficacy study of vesicular gel containing methotrexate and menthol combination on parakeratotic rat skin model. J Liposome Res. 21:134–140.
- Sebok B, Bonnekoh B, Kerenyi M, Gollnick H. 2000. Tazarotene induces epidermal cell differentiation in the mouse tail test used as an animal model for psoriasis. Skin Pharmacol Appl Skin Physiol. 13:285–291.
- Shirsand S, Para M, Nagendrakumar D, Kanani K, Keerthy D. 2012. Formulation and evaluation of Ketoconazole niosomal gel drug delivery system. Int J Pharm Investig. 2:201–207.
- Silva FS, Oliveira H, Moreiras A, Fernandes JC, Bronze-da-Rocha E, Figueiredo A, et al. 2013. Cytotoxic and genotoxic effects of acitretin, alone or in combination with psoralen-ultraviolet A or narrow-band ultraviolet B-therapy in psoriatic patients, Mutat Res. 753:42–47.
- Ruchusatsawat K, Wongpiyabovorn J, Protjaroen P, Chaipipat M, Shuangshoti S, Thorner PS, Mutirangura A. 2011. Parakeratosis in skin is associated with loss of inhibitor of differentiation 4 via promoter methylation. Hum Pathol. 42:1878–1887.