Abstract
Encapsulation techniques have the potential to protect hepatocytes from cryoinjury. In this study, we comparatively evaluated the viability and metabolic function of primary rat hepatocytes encapsulated in calcium alginate microbeads, in chitosan tripolyphosphate beads, and in three-layered alginate–chitosan–alginate (ACA) microcapsules, before and after cryopreservation at −80°C and in liquid nitrogen (LN2) for 1 and 3 months. Findings demonstrated that LN2 was atop of −80°C in regard to preservation of viability (> 90%) and hepatic functions. LN2-cryopreserved hepatocytes encapsulated in ACA microcapsules retained metabolic function post-thawing, with > 90% of the albumin, total protein and urea syntheses activities, and > 80% of oxidative function.
Introduction
Acute liver failure is a destructive disease associated with high mortality. This disease leads to sudden loss of hepatic functions, and eventually causes progressive multiorgan failure (CitationBernal et al. 2010). The only available therapy with high survival rate is the liver transplantation; however, the main limitation is the shortage of organ donors. To overcome this, several types of treatment approaches, including artificial and bioartificial liver (BAL) systems as temporary support, and hepatocyte transplantation have been suggested (CitationDemetriou et al. 2004, CitationLaleman et al. 2006, CitationYu et al. 2012b). Artificial liver support devices based on the filtration of patient's blood in terms of hemodialysis and hemoperfusion have not been sufficient to manage an array of metabolic disorders seen in liver disease. BAL systems which incorporate hepatocytes are of paramount importance to artificial liver systems with regard to fulfilling metabolic functions. A number of designs including flat membrane, hollow fiber, and microcapsules have been proposed. These designs are based on the exclusion of immune system components, and allowance of nutrients and oxygen across the semipermeable membranes. Improvements in liver failure have been reported in early BAL studies on animal models (CitationDixit et al. 1993, CitationSosef et al. 2002).
Among BAL designs, hepatocyte encapsulation using biopolymers has drawn particular interest regarding the cell-friendly and easy fabrication procedure resulting in a favorable three-dimensional spherical geometry, with the possibility for scaling up. Thus, a number of natural and synthetic polymers including alginate, poly(ethylene glycol), chitosan, collagen, dextran, poly(vinyl alcohol), agarose (with or without gelatin), hyaluronic acid, and 2-hydroxyethyl methacrylate have been evaluated for cell encapsulation (reviewed by CitationMurua et al. 2008).
In principle, the ideal biopolymer to be used for encapsulation should display mechanical stability during use, should not collide with the viability of the cells, should be permeable to nutrients, wastes and gases, and most importantly should facilitate the metabolic functions of the hepatocytes (CitationKoc et al. 2007). Besides, the biocompatible polymer should be resistant to cryopreservation while providing a convenient environment to frozen hepatocytes.
Cryopreservation is currently considered as a practical method allowing on-demand usage of long-term stored primary hepatocytes. Cryopreserved hepatocytes can be used as a source for readily avaliable cell bank, even in emergency cases, such as for metabolic decompensation. Existence of fully characterized cryopreserved hepatocytes can enable the planning of transplantations beforehand (CitationStephenne et al. 2010). In this context, the high sensitivity of hepatocytes to freezing/thawing process is a major limitation. Several methods have been utilized to cryopreserve human and animal hepatocytes with variable performance, in terms of viable cell recovery, attachment, and metabolic function (CitationSun et al. 1990, CitationHubel et al. 1991, CitationChesne et al. 1993, CitationLee et al. 2000). For example, CitationGuyomard et al. (1996) have evaluated the survival rate and some hepatic functions of alginate microbead-entrapped rat hepatocytes before and after cryopreservation in vitro. CitationHang et al. (2010) have analyzed the in vitro properties of human hepatocytes encapsulated in alginate–poly-L-lysine (PLL)–alginate (APA) microcapsules.
Alginate, obtained from brown seaweeds, the monovalent salt form of alginic acid is a block copolymer composed of 1→4-linked β-D-mannuronic acid (M) and guluronic acid (G) residues in a linear polysaccharide chain which has been proposed as an immobilization matrix for cells (CitationSmidsrød and Skjåk-Braek 1990, CitationHuang et al. 2012). Alginate forms gels (or microbeads) in the presence of divalent cations (e.g., calcium and strontium ions), and destabilizes in the presence of phosphate, lactate or citrate. Alginate microcapsules can be formed by polycationic coating of microbeads and then removing the polymer core (CitationLim and Sun 1980, CitationThu et al. 1996). A number of polycationic coatings, such as PLL, poly-L-ornithine, chitosan, lactose-modified chitosan, and others have been evaluated. PLL has been a prominent polymer for coating alginate microbeads, but later its immunogenic properties were found out (CitationDarquy et al. 1994, CitationStrand et al. 2001). Chitosan [(1→4) linked 2-amino-2-deoxy-b-D-glucopyranose], being the result of the partial N-deacetylation of the natural biopolymer chitin, has structural similarity to glycosaminoglycans. This polycation has been proposed as a matrix for mammalian cell encapsulation (CitationZielinski and Aebischer 1994), and has been applied for alginate microbead coating (CitationBaruch and Machluf 2006, CitationPaul et al. 2010).
In this study, we encapsulated primary rat hepatocytes in calcium alginate (Alg) microbeads, in chitosan tripolyphosphate (Chi) beads, and in three-layered alginate–chitosan– alginate (ACA) microspheres, then compared the viability and metabolic function of hepatocytes in abovementioned encapsulation forms and in free monolayer culture. Later, the encapsulated and free hepatocytes were subjected to two cryopreservation conditions (either −80°C or vapor phase of liquid nitrogen, LN2) for 1 and 3 months duration. The viability and metabolic function of freeze-thawed hepatocytes were comparatively evaluated. Findings demonstrated that ACA microcapsules were suitable for primary hepatocytes to retain considerable level of viability with metabolic function following cryopreservation.
Materials and methods
Chemicals
Sodium alginate (low viscosity), chitosan (85% deacetylated, medium molecular weight), sodium citrate, penicillin/streptomycin, insulin, and all other chemicals were obtained from Sigma/Aldrich Chemical Company (St. Louis, MO, USA). Total protein and albumin kits were purchased from Clonital (Lombardia, Italy).
Isolation of primary rat hepatocytes
Hepatocytes were isolated from young male Wistar rats (weighing 125–150 g) using the in situ modification of the portal vein collagenase (Serva, Type IV, 200 U/ml) perfusion method (CitationSeglen 1976) and purified using differential centrifugation in Dulbecco's Modified Eagle's Medium (DMEM) at 4°C (CitationElcin et al. 1998). Donor animals were handled in accordance with the standards of international regulations. Cell isolation was performed under avertin anesthesia, and all efforts were made to minimize suffering. Initial viability of the hepatocytes was tested using the trypan blue exclusion test (> 93% viability). Rat hepatocyte yield averaged > 2.7 × 108 cells/rat.
Encapsulation of primary rat hepatocytes
Hepatocyte concentrations of 1.0 × 106, 2.0 × 106, and 3.0 × 106 cells/ml gel were evaluated in all encapsulation methods, and 1.0 × 106 cells/ml gel was found to be optimal in maintaining a smoother encapsulation surface with mechanical stability in culture. Cell encapsulation yield was found to be usually > 90% for all types of methods, and was calculated from the initial number of cells and from the non-encapsulated number of cells which had remained in the washing solutions.
Primary rat hepatocytes were encapsulated as follows:
(i) Alg microbeads were formed by transferring 2% (w:v) sodium alginate containing hepatocytes into 0.4 M CaCl2 solution from a syringe pump connected to an electromagnetically driven vibrating 200-μm-nozzle inside a closed encapsulation device (Nisco Engineering, Zurich, Switzerland) under sterile conditions at 4°C. The Alg microbeads with a diameter of ˜ 300–350 μm were collected after 10 minutes, and transferred to culture after washing.
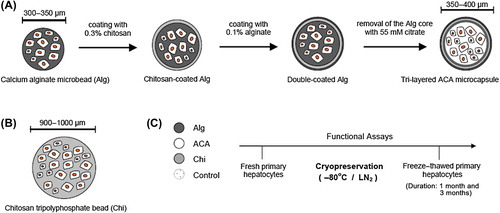
(ii) To form ACA microcapsules, hepatocyte- encapsulated alginate microbeads were first coated by immersing in 0.3% (w:v) chitosan solution for 30 minutes, then were kept in 0.1% (w:v) sodium alginate solution for 10 minutes. At the last step, double-coated alginate microbeads were transferred into a solution of sodium citrate (55 mM) to remove the alginate core. The resulting microcapsules (˜ 350–400 μm-diameter) were intact, demonstrating the interaction of alginate and chitosan, thus the formation of the three-layered membrane system containing the cells. ACA microcapsules were transferred to culture after washing.
(iii) Hepatocytes were encapsulated in chitosan by extruding 2% (w:v) chitosan containing hepatocytes into 0.6% (w:v) sodiumtripolyphosphate (TPP; anionic oligomer solution) containing 0.85% (w:v) NaCl (pH 7.4) using the same encapsulation device at 4°C, except a nozzle size of 350 μm was used since the suspension had higher viscosity. TPP polyanion interacts with the protonated amine groups of chitosan through electrostatic forces, forming the chitosan beads with a diameter of around 900– 1000 μm. After the washing steps, Chi beads were transferred to culture.
Morphological evaluation
The shape and the uniformity of the microbeads/ microcapsules were evaluated and photographed using a Nikon TS 100 model (Tokyo, Japan) inverted microscope at Hoffman modulation. The distribution of the hepatocytes (as single cells, in clumps, or both) following encapsulation was also examined. The average diameters of the Alg microbeads, ACA microcapsules, and Chi beads were determined from digital images (60 measurements on 6 images, each from three independent experiments).
Surface morphology of ACA microcapsules and alginate microbeads was examined using scanning electron microscopy (SEM). The effect of hepatocyte encapsulation density on the smoothness of bead surface was also evaluated. The samples were fixed in 2.5% (w/v) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) at 4°C for a week, and then dehydrated by immersing in a series of ethanol–water solutions (50–100%; v/v). The surfaces were examined using a JEOL JSM 5600 model SEM (Tokyo, Japan) after sputter-coating with gold.
Cell culture
Hepatocytes (either as monolayer or in encapsulated forms) were cultured in DMEM supplemented with 10% fetal calf serum (FCS; Hyclone), 10 μg/ml insulin, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C, 5% CO2, and 95% humidity. While the control hepatocytes were plated in T-25 flasks, encapsulated hepatocytes were placed in 12-well plates (20 microbead or microcapsule/well) for culture. Culture medium was replaced daily, and the medium samples were collected to perform some of the metabolic functional analyses.
Cell viability
Viability of hepatocytes was determined using the 3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay on days 1, 3, and 9 of culture, and 24 h after thawing. The assay is based on the ability of viable cells converting soluble yellow MTT into the insoluble purple formazan salt by the mitochondrial dehydrogenase enzyme. MTT was added to each well and the plates were incubated for 5 h at 37°C. The dark purple crystals were dissolved in 0.1 N HCl in anhydrous isopropyl alcohol (IPA), and the optical density was read at 570 nm using a Biotrak II microplate reader (Amersham Biosciences, Buckinghamshire, UK). MTT assay was applied similarly to the encapsulated hepatocytes, except the microbeads or microcapsules (20/well) were ruptured to extract formazan, then filtered through 1.2 μm-membrane filter, and transferred to isopropanol.
Cryopreservation and thawing of hepatocytes
Cells were cryopreserved under two conditions: at −80°C and LN2 for 1 month and 3 months. For freezing, monolayer hepatocyte cultures were detached from the plates using 0.05% trypsin/0.53-mM EDTA, washed twice and pelleted using centrifugation for 5 min at 1000 rpm. Then, the pellet was suspended in the cryogenic medium (CM) consisting of DMEM supplemented with 10% FCS, 20% dimethylsulfoxide (DMSO), and 10 μg/ml insulin, inside freezing vials (Nunc, Rockilde, Denmark) at 2.0 × 106 cells/ml concentration. For encapsulated cells, hepatocytes containing microbeads/microcapsules were washed twice for 3 min with the CM, resuspended in fresh CM and placed inside cryogenic vials. For progressive slow freezing, the vials were placed in Mr. Frosty freezing container (Cryo 1°C; Thermo Scientific Nalgene, Rochester, NY) filled with IPA and placed at −80°C. Twenty four hours later, half of the vials were transferred to LN2 storage vessel. For thawing, the vials (either at −80°C or in LN2) were placed in a water bath at 37°C until ice dissolved. Free and encapsulated hepatocytes were gently pipetted, washed three times with the DMSO-free culture medium and transferred to culture plates. Viability and the metabolic activity of the cryopreserved/thawed hepatocytes were determined.
Metabolic function assays
Functional assays were performed on collected samples from culture media. Standard curves were generated for each assay according to the protocols.
The amount of total protein secretion from the hepatocytes was determined using a commercial kit (cat no. KC198; Clonital) based on biuret method. Briefly, 10 μl of sample was added into 1000 μl biuret reagent and incubated for 15 min at 25°C; then the absorbances were measured at 546 nm. Total protein secretion rates were calculated from protein levels in a 24-h culture supernatant divided by the number of viable cells determined using the MTT assay.
Urea concentrations of the samples were determined using the quantitative Urease/Berthelot colorimetric method (CitationChaney and Marbach 1962). Ten microliter of sample was dissolved in 1250 μl of urease solution in phosphate buffer. Solution was incubated for 5 min at 37°C. Following this step, 1250 μl hypochlorite solution was added and incubated for an additional 5 min at 37°C, and the absorbances were measured at 600 nm. Urea release rates were calculated by dividing urea concentrations measured in supernatant derived from 24-h culture by the number of viable cells determined by the MTT assay.
The amount of albumin secretion from the hepatocytes was determined using a commercial kit (cat no. KC197; Clonital) that utilizes the bromocresol green (BCG) method in the absence of FCS. Ten μl of sample was added into 1500 μl BCG reagent. Then, the solution was incubated for 10 min at 25°C, and the absorbances were measured at 625 nm. Albumin secretion rates were calculated by dividing albumin concentrations measured in the supernatant obtained from 24-h culture by the number of viable cells determined by the MTT assay.
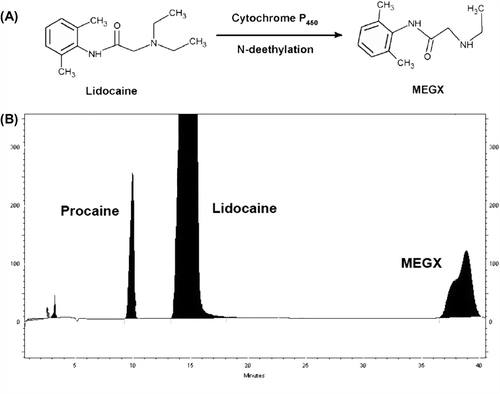
Lidocaine is converted to monoethylglycinexylidide (MEGX) by N-deethylation reaction catalysed by the cytochrome P-450 enzyme (CYP450). This is used as a test for the metabolic assessment of liver cell bioreactors (CitationGerlach et al. 2010). The rate of conversion of lidocaine (ω-diethylamino-2,6-dimethylacetanilide) to MEGX was measured using the HPLC method described by CitationChen et al. (1992) with slight modification. The HPLC system (Finnigan Surveyor, Thermo Fischer Scientific, Dreieich, Germany) consisted of a Surveyor LC pump, a Surveyor Auto sampler, a Shim-Pack CLC-C8 (M) column (250 4.6 mm), and a PDA detector. The mobile phase for the chromatographic separation buffer was composed of 10% acetonitrile and 90% H3PO4/KH2PO4 (pH 3.0). This mobile phase was pumped through the HPLC system at 1.5 ml/min. The HPLC detector was operated between a wavelength of 200 and 360 nm. Later, 1 ml sample and then 10 ml of ethyl acetate were added to the tube. The mixture was mechanically shaken for 10 min and centrifuged at 2000xg for 10 min. Seven milliliters of the organic phase was transferred to a tube containing H2SO4 and procaine hydrochloride (internal standard). The mixture was shaken and centrifuged as described above; then most of the organic phase was removed by aspiration. The acidic aqueous phase was neutralized by the NaOH titration. Remaining ethyl acetate and aqueous layer were removed. The residue was reconstituted in 100 μl double-distilled water. Five to 50 μl of the sample was injected onto the HPLC column. The concentration of lidocaine was calculated from the ratios of the peak heights of the internal standard and from the standard calibration curve generated for lidocaine concentrations between 0.001 and 5 mM.
Statistical analysis
Each value represents mean ± standard deviation (SD) of samples from three independent experiments. Statistical significance was determined by unpaired Student's t-test. A value of p < 0.05 was used for statistical significance.
Results and discussion
Encapsulation, cell density, and viability
In this study, primary rat hepatocytes were encapsulated under sterile conditions in three different forms ():
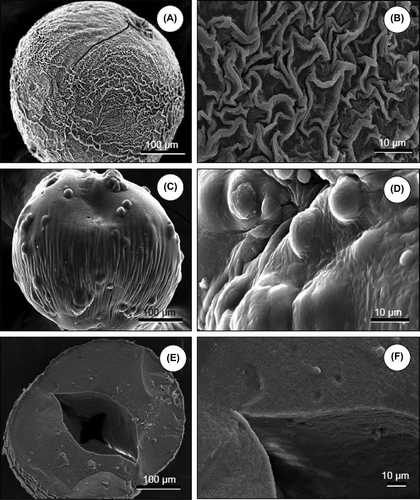
(i) Hepatocytes were encapsulated in 2% (w:v) Alg microbeads having a diameter of around 300–350 μm (Figures 2A,C and 3A,B). Alg microbeads were stable under culture conditions and after cryopreservation, without any significant change in size. Cell leakage was negligible in Alg microbeads.
(ii) Secondly, the hepatocytes-encapsulated Alg microbeads were coated with a thin intermediate layer of 0.3% (w/v) chitosan, and then with 0.1 (w/v) alginate. Then, the inner alginate matrix was removed using 55 mM citrate solution which resulted in three-layered transparent ACA microcapsules with a diameter of ˜ 350–400 μm (). The small increase of ˜ 50 μm in the diameter can mainly be attributed to the loosening of the core alginate network altering diffusional properties. However, the electrostatic interactions between the oppositely charged polymer chains were sufficient to form a stable polyelectrolyte multilayer complex that lasted for the duration of experiments. It was previously shown that time (30 min) needed for the interaction of chitosan with alginate did not have a significant effect on cell viability (CitationHaque et al. 2005). While HepG2 cells were used in that particular study, we also experienced similar results with rat primary hepatocytes. In this study, primary rat hepatocytes encapsulated in ACA microcapsules retained > 90% of their viability, quite comparable to the cases of Alg microbeads and Chi beads. We preferred to use chitosan for the polycationic coating of alginate, instead of the extensively used PLL, since PLL was shown to be immunogenic (CitationStrand et al. 2001). Besides, the stability of ACA microcapsules is shown to be higher than that of the alginate–PLL–APA microcapsules to enzymatic digestion, and the binding of chitosan to alginate is stronger than the binding of PLL to alginate (CitationLin et al. 2008).
(iii) As the third form, hepatocytes were encapsulated in Chi beads of around 900–1000 μm-in diameter (). Related to the viscosity of the cell– chitosan suspension, 350 μm-nozzle was used to create chitosan beads of around 900–1000 μm- diameter (instead of 200 μm-nozzle, as was the case for Alg and ACA fabrication). It was not possible to produce stable and uniform Chi beads in smaller dimensions. Different than Alg and ACA, chitosan beads became loosened with time, and started to rupture during culture, especially after the freeze– thawing step. The ionotropic gelation of chitosan with TPP results in a basically non-toxic cell encapsulation environment; however, the poor mechanical strength is usually the limiting factor for many intended applications. Encapsulated hepatocytes gradually became darker, attaining a necrotic form, which could be explained as a consequence of poor bidirectional mass transfer of oxygen and nutrients inside the Alg microbeads and Chi beads (not shown). This was observed to a lesser extent at a later stage of post-cryopreservation, for hepatocytes inside ACA microcapsules.
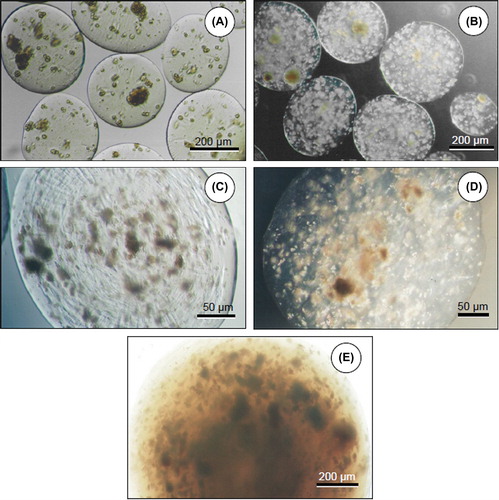
Findings demonstrated that hepatocytes could be encapsulated in cell densities of 1.0 × 106, 2.0 × 106, and 3.0 × 106 cells/ml gel; however, surface deformities related to high cell density could be observed when 3.0 × 106 cells/ml gel was used (). Some of the encapsulated hepatocytes exposed to the surface of the Alg microbeads are evident in scanning electron micrograph given in . Preliminary studies also showed lower mechanical stability during procedures when 2.0 × 106 and 3.0 × 106 cells/ml gel concentrations were used. Hence, 1.0 × 106 cells/ml gel concentration was selected for all experiments in order to acquire a more homogenous distribution of hepatocytes. The cross-section of an Alg microbead SEM image indicates the retained round morphology and the homogenous distribution of the hepatocytes ().
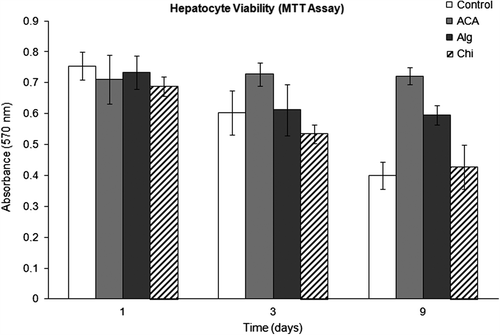
Initial viability of freshly isolated rat hepatocytes was found to be > 93% as confirmed using trypan blue exclusion test. The cell viability in monolayer culture and in encapsulated forms is presented in . While the hepatocyte viability in all groups was quite the same on day 1 of culture, a gradual decrease in monolayer culture and in cells encapsulated in Chi beads (and to a lesser extent in Alg microbeads) was observed on days 3 and 9. On the other hand, the cell viability in ACA microcapsules was practically unchanged for the culture period of 9 days, demonstrating the compatibility of the microcapsule cultivation conditions to hepatocytes. Conforming observations corroborating the positive influence of microencapsulation on maintaining the cell viability have also been reported in other studies (CitationAoki et al. 2005, CitationYu et al. 2012a).
Metabolic function of hepatocytes
Albumin synthesis
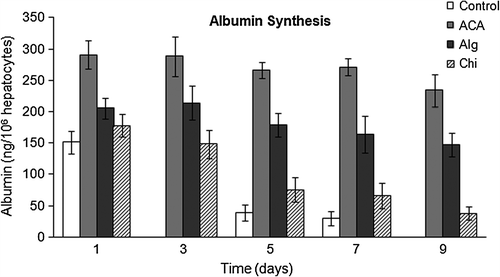
As a marker of hepatic function, the albumin synthesis activity of hepatocytes was evaluated on samples collected from the culture media at predetermined time points. Albumin secretion of primary rat hepatocytes in monolayer culture and in encapsulated forms is presented in . A reduction in the albumin synthetic activity was determined by culture time; furthermore no albumin secretion from monolayer cultures was observed on day 9 of culture. In addition, the marked reduction in the albumin synthetic activity was proportionally higher than the loss of hepatocyte viability presented in . This signifies that albumin synthetic function of the viable cells is not equal under different culture/encapsulation conditions. The only exception is the hepatocytes encapsulated in ACA microcapsules, which seem to have a stable albumin secretion level of ˜180–210 ng/106 cells for at least 9 days.
Total protein synthesis
presents the total protein synthesis of rat hepatocytes in monolayer and in three-dimensional encapsulated culture. The differences in total protein secretion levels on day 1 of culture were not significant in any group. However this difference was significant (p < 0.05) on day 3 for hepatocytes encapsulated in Chi beads, as well as on days 5 and 7 for both monolayer and Chi beads. The differences in the total protein synthetic activity became significant (p < 0.05) on day 9 for hepatocytes in ACA microcapsules and in Alg microbeads, in favor of the ACA group. Hepatocytes encapsulated in ACA microcapsules synthesized total protein at 300–350 mg/106 cells level for at least 9 days of culture.
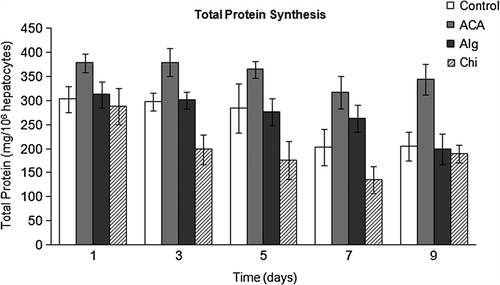
The level of protein secretion by the encapsulated hepatocytes has been accounted for diffusion and permeability properties of the capsule membrane, that is, properties including charge distribution, porosity, hydrophilicity, as well as the properties and the size of the substance being transferred across the membrane (CitationChang 1972, CitationUludag et al. 2000). These characteristics would vary depending on the interaction of the polycation (whether being chitosan, PLL, etc.) with alginate (CitationAngelova and Hunkeler 2002).
Urea synthesis
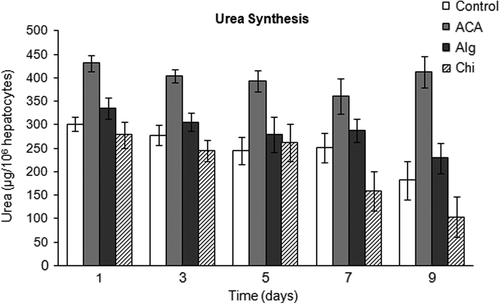
Urea production was assessed as a detoxification function of hepatocytes cultured as monolayers and in encapsulated forms. Findings are presented in . Hepatocytes encapsulated in Alg microbeads synthesized urea in a level ≥ 250 μg/106 cells for 9 days of culture. Yet, ACA microcapsules provided the best microenvironment for primary rat hepatocytes, in terms of urea synthesis with ˜300–350 μg/106 cells for the duration of in vitro experiments. Urea production by the cells in ACA was significantly higher than that of all other groups (p < 0.05), between days 3 and 9 of culture.
Maintenance of the encapsulation form and hepatic functions after cryopreservation
Alg microbeads (> 95%) and ACA microcapsules (> 85–90%) essentially maintained their mechanical stability to a large extent for the duration of experiments. Upon thawing, ACA microcapsules demonstrated a blebby appearance, yet gained their smooth surface morphology after a day of culture. Another observation on ACA after thawing was that, the primary hepatocytes had formed small aggregates inside microcapsules providing cell–cell contact that possibly leads to improved metabolic function over that of Alg and Chi groups. As indicated in a study by CitationBhatia et al. (1999), cell–cell interactions (either homotypic or heterotypic) in hepatocytes can be crucial for maintaining viability and function in extended cultures. CitationYu et al. (2012a) have also observed the aggregation of hepatocyte in the core of heparin–chitosan–alginate microcapsules; however, they were able to show albumin synthesis and glucose removal of the encapsulated hepatocytes.
Concerning Chi beads, the freeze/thaw process did affect their integrity to a large extent, with an initial swelling (˜ 30–35% increase in bead diameter) followed by disintegration, usually on day 3 post-thawing.
Findings from the metabolic activity experiments of freeze–thawed primary rat hepatocytes after 3 months of cryopreservation are summed up in . Synthetic activity of hepatocytes was evaluated on days 1 and 9 post-thawing, with the exclusion of certain time points in the Chi group related to disruption of the chitosan beads. In terms of retaining cell viability, LN2 was found to be a better option for cryopreservation than −80°C (compare 78.1–93.6% with 69.2–85.4%) (). Secondly, the metabolic activity of encapsulated primary rat hepatocytes (all encapsulation types) was higher than that of monolayer cultures, after normalizing the number of viable cells. In accordance with the findings of the pre-cryopreservation experiments, hepatocytes in ACA microcapsules provided the best encapsulation environment, followed by the Alg microbeads, and then the Chi beads. Hepatocytes in ACA microcapsules had a synthetic activity of > 320 mg total protein, > 230 ng albumin, and > 460 μg urea per million cells, on day 9 post-cryopreservation (LN2) ().
Table I. Total protein, albumin, and urea syntheses of freeze–thawed hepatocytes after 3 months of cryopreservation.
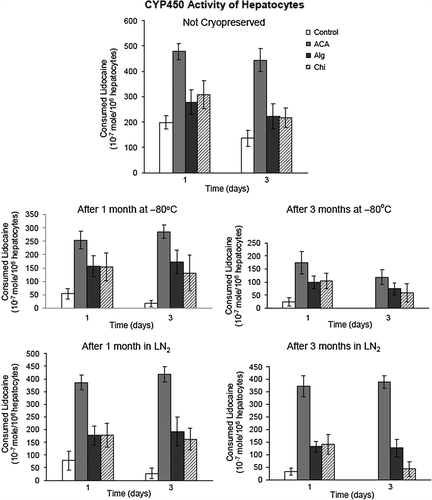
Lidocaine (xylocaine) catabolism was harnessed to evaluate oxidative function (phase I activity) of rat primary hepatocytes in monolayer and in encapsulated cultures. This method is based on the conversion of lidocaine to pharmacologically-active metabolite MEGX by the N- deethylation reaction catalyzed by the active CYP450 enzyme of rat hepatocytes (). demonstrates the procaine (internal standard), lidocaine, and MEGX peaks in a representative HPLC chromatogram. Lidocaine concentrations of samples were calculated from the ratios of the peak heights of procaine and from the standard calibration curves generated for lidocaine. Findings are presented in . The CYP450 activity of free hepatocytes (cells from the monolayer cultures) was quite low, even on day 1 of isolation (198.5 × 10−7 mole/106 cells). As a matter of fact, this limited activity gradually decreased and was completely lost after 3 months of cryopreservation. Results exhibited that cryopreserved hepatocytes maintained their CYP450 activity in all encapsulation systems after thawing. It is apparent from that this activity was further conserved when the cells were cryopreserved in the vapor phase of LN2, instead of −80°C. The most active lidocaine metabolism was detected for the ACA group with a consumed lidocaine concentration of ˜388.7 × 10−7 mole/106 cells (˜ 81% of activity of fresh hepatocytes) after 3 months of storage in LN2, whereas this was 126.2 × 10−7 mole/106 cells, and 42.5 × 10−7 mole/106 cells, for hepatocytes in Alg microbeads and in Chi beads on day 3, respectively (p < 0.05). It is clear that the oxidative function is substantially retained inside ACA microcapsules during cryopreservation, when the lidocaine consumption of fresh hepatocytes of 444.5 to 478.4 × 10−7 mole/106 cells is considered. The prolonged lidocaine metabolizing activity of primary hepatocytes suggests the potential of cryopreserved ACA microcapsule system as a ready source (bank) for use in BAL support systems.
Overall, the tri-layered ACA microcapsules demonstrated good mechanical stability, provided higher cell viability yield, and eventually a suitable microenvironment for hepatocytes maintaining biological functions, possibly due to the rapid transport of gases, nutrients and products to and from the entire encapsulation volume, and the formation of small hepatocyte aggregates particularly after cryopreservation. The durability of Alg microbeads brought out a higher cell encapsulation yield with function, compared to Chi beads with poor mechanical stability. As previously shown, blended forms of chitosan provide a suitable attachment substrate for primary hepatocytes (CitationElcin et al. 1999). Keeping this in mind, it is difficult to come through a definitive conclusion whether Alg or Chi beads provide a better matrix microenvironment for hepatocytes, since Chi beads used in this study had a diameter much larger (900–1000 μm) than that of Alg microbeads (300–350 μm), a much longer retention time for molecules and a distance to travel through.
In conclusion, we made a comparative evaluation of the three biopolymeric encapsulation systems in respect to providing primary hepatocytes viability and metabolic function, in terms of albumin, total protein and urea synthesis, as well as CYP450 activity, before and after cryopreservation. Findings indicated that ACA microcapsules provided the most suitable environment for primary rat hepatocytes and showed potential for use as a tool for primary hepatocyte banking.
Declaration of interest
The authors declare no competing interests. The authors are alone responsible for the content and writing of the paper.
YME acknowledges the support of TÜBA (The Turkish Academy of Sciences).
References
- Angelova N, Hunkeler D. 2002. Permeability and stability of chitosan-based capsules: effect of preparation. Int J Pharm. 242:229–232.
- Aoki T, Jin Z, Nishino N, Kato H, Shimizu Y, Niiya T, et al. 2005. Intrasplenic transplantation of encapsulated hepatocytes decreases mortality and improves liver functions in fulminant hepatic failure from 90% partial hepatectomy in rats. Transplantation. 79: 783–790.
- Baruch L, Machluf M. 2006. Alginate-chitosan complex coacervation for cell encapsulation: effect on mechanical properties and on long-term viability. Biopolymers. 82:570–579.
- Bernal W, Auzinger G, Dhawan A, Wendon J. 2010. Seminar. Acute liver failure. Lancet. 376:190–201.
- Bhatia SN, Balis UJ, Yarmush ML, Toner M. 1999. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 13:1883–1900.
- Chaney AL, Marbach EP. 1962. Modified reagents for determination of urea and ammonia. Clin Chem. 8:130–132.
- Chang TMS. 1972. Artificial Cells. Monograph. Illinois: CC Thomas Publisher.
- Chen Y, Potter JM, Ravenscroft PJ. 1992. A quick, sensitive high- performance liquid chromatography assay for monoethylglycinexylidide and lignocaine in serum/plasma using solid phase extraction. Ther Drug Monit. 14:317–321.
- Chesne C, Guyomard C, Fautrel A, Poullain M, Fremond B, De Jong H, Guillouzo A. 1993. Viability and function in primary culture of adult hepatocytes from various animal species and human beings after cryopreservation. Hepatology. 18:406–414.
- Darquy S, Pueyo ME, Capron E, Reach G. 1994. Complement activation by alginate-polylysine microcapsules used for islet transplantation. Artif Organs. 18:898–903.
- Demetriou AA, Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. 2004. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 239:660–667.
- Dixit V, Darvisi R, Arthur M, Lewin K, Gitnick G. 1993. Cryopreserved microencapsulated hepatocytes-transplantation studies in Gunn rats. Transplantation. 55:616–622.
- Elcin YM, Dixit V, Gitnick G. 1998. Hepatocyte attachment on biodegradable modified chitosan membranes: in vitro evaluation for the development of liver organoids. Artif Organs. 22:837–846.
- Elcin YM, Dixit V, Lewin K, Gitnick G. 1999. Xenotransplantation of fetal porcine hepatocytes in rats using a tissue engineering approach, Artif Organs. 23:146–152.
- Gerlach J, Brayfield C, Puhl G, Borneman R, Müller C, Schmelzer E, Zeilinger K. 2010. Lidocaine/monoethylglycinexylidide test, galactose elimination test, and sorbitol elimination test for metabolic assessment of liver cell bioreactors. Artif Organs. 34: 462–472.
- Guyomard C, Rialland L, Fremond B, Chesne C, Guillouzo A. 1996. Influence of alginate gel entrapement and cryopreservation on survival and xenobiotic metabolism capacity of rat hepatocytes. Toxicol Appl Pharmacol. 141:349–356.
- Hang H, Shi X, Gu GX, Wu Y, Gu J, Ding Y. 2010. In vitro analysis of cryopreserved alginate-poly-L-lysine-alginate microencapsulated human hepatocytes. Liver Int. 30:611–622.
- Haque T, Chen H, Ouyang W, Martoni C, Lawuyi B, Urbanska AM, Prakash S. 2005. In vitro study of alginate-chitosan microcapsules: an alternative to liver cell transplants for the treatment of liver failure. Biotechnol Lett. 27:317–322.
- Huang X, Zhang X, Wang X, Wang C, Tang B. 2012. Microenvironment of alginate-based microcapsules for cell culture and tissue engineering. J Biosci Bioeng. 114:1–8.
- Hubel A, Toner M, Cravalho EG, Yarmush ML, Tompkins RG. 1991. Intracellular ice formation during freezing of hepatocytes cultured in a double collagen gel. Biotechnol Prog. 7:554–559.
- Koc A, Durkut S, Elcin AE, Tan E, Elcin YM. 2007. Evaluation of modified CMC and CMC-PVA as miscible polymer blend membranes for hepatocytes. Macromol Biosci. 7:681–689.
- Laleman W, Wilmer A, Evenepoel P, Verslype C, Fevery J, Nevens F. 2006. Review article: non-biological liver support in liver failure. Aliment Pharmacol Ther. 23:351–363.
- Lee KW, Park JB, Yoon JJ, Lee JH, Kim SY, Jung HJ, et al. 2000. The viability and functon of cryopreserved hepatocyte spheroids with different cryopreservation solutions. Transplant Proc. 36: 2462–2463.
- Lim F, Sun AM. 1980. Microencapsulated islets as bioartificial endocrine pancreas. Science. 210:908–910.
- Lin J, Yu W, Liu X, Xie H, Wang W, Ma X. 2008. In vitro and in vivo characterization of alginate-chitosan-alginate artificial microcapsules for therapeutic oral delivery of live bacterial cells. J Biosci Bioeng. 105:660–665.
- Murua A, Portero A, Orive G, Hernandez RM, de Castro M, Pedraz JL. 2008. Cell microencapsulation technology: towards clinical application. J Control Release. 132:76–83.
- Paul A, Shum-Tim D, Prakash S. 2010. Investigation on PEG integrated alginate–chitosan microcapsules for myocardial therapy using marrow stem cells genetically modified by recombinant baculovirus. Cardiovasc Eng Technol. 1:154–164.
- Seglen PO. 1976. Preparation of isolated rat liver cells: the enzymatic preparation of isolated intact parenchymal cells from rat liver. Methods Cell Biol. 13:29–83.
- Smidsrød O, Skjåk-Braek G. 1990. Alginate as immobilization matrix for cells. Trends Biotechnol. 8:71–78.
- Sosef MN, Abrahamse LS, van de Kerkhove MP, Hartman R, Chamuleau RA, van Gulik TM. 2002. Assesment of the AMC-bioartificial liver in the anhepatic pig. Transplantation. 73:204–209.
- Stephenne X, Najimi M, Sokal ME. 2010. Hepatocyte cryopreservation: is it time to change the strategy. World J Gastroenterol. 16:1–14.
- Strand BL, Ryan L, In't Veld P, Kulseng B, Rokstad AM, Skjåk-Bræk G, Espevik T. 2001. Poly-L-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 10:263–275.
- Sun EL, Aspar DG, Ulrich RG, Melchior GW. 1990. Cryopreservation of cynomolgus monkey (Macaca fascicularis) hepatocytes for subsequent culture and protein synthesis studies. In Vitro Cell Dev Biol. 26:147–150.
- Thu B, Bruheim P, Espevik T, Smidsrød O, Soon-Shiong P, Skjåk-Braek G. 1996. Alginate polycation microcapsules. I. Interaction between alginate and polycation. Biomaterials. 17: 1031–1040.
- Uludag H, De Vos P, Tresco PA. 2000. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 42:29–64.
- Yu S, Han B, Peng C. 2012a. A preliminary study of alginate, heparin-chitosan-alginate and heparin microencapsulated hepatocytes system. Hepatogastroenterology. 59:1234–1240.
- Yu Y, Fisher JE, Lillegard JB, Rodysill B, Amiot B, Nyberg SL. 2012b. Cell therapies for liver diseases. Liver Transpl. 18:9–21.
- Zielinski BA, Aebischer P. 1994. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials. 15:1049–1056.