Abstract
Stearic acid-grafted chitosan (CS-SA) and glycyrrhetinic acid-conjugated stearic acid-grafted chitosan (GA-CS-SA) were synthesized and were further used for the preparation of micelles. The substitution degree (SD) of SA and GA on CS was measured. The physicochemical properties of CS-SA and GA-CS-SA micelles such as critical micelle concentration (CMC), aggregation number of hydrophobic micro-domain (AN), particle size, zeta potential, and morphology were also determined. The CMC of GA-CS-SA was about 17.49 μg/mL, which was relatively low. Its AN was 2.09. The GA-CS-SA micelles showed spherical shape with mean diameter of 121.1 nm and had positive charge, which suggested that GA-CS-SA could be a good carrier of cancer drug.
Keywords::
Introduction
Liver cancer is a common clinical disease (CitationDufour and Johnson 2010) and chemotherapy is usually used as its treatment. However, traditional chemical preparations are not efficient in hepatic tumor treatment. Molecules of these agents distribute evenly within human body through circulatory system. Consequently, the liver has a comparatively low concentration leading to low therapeutic effect, whereas other organs are likely to be impaired by the toxic effects of these anti-tumor drugs. Additionally, their in vivo instability may also reduce their anti-neoplastic effect.
Hepatic targeting drug delivery system (HTDDS) could deliver drug to the liver effectively (CitationSedlacek 2001), reducing systemic distribution, decreasing dosage and frequency of administration. Therefore, it improves the therapeutic index of drugs and reduces adverse reactions. The key procedure in HTDDS development is to develop targeting drug delivery carriers that provide high efficiency and enough physiological security, for example, lipids, proteins, and biodegradable polymers. One significantly advantageous kind of carriers is chitosan (CS) and its derivatives (CitationJayakumar et al. 2007). CS is a kind of natural, inexpensive, stable, and biocompatible amino polysaccharide. Low molecular weight CS has good water solubility and biological activity (CitationKumar et al. 2004).
Polymer micelles have self-assembled core-shell structures that are formed from amphiphilic polymers in aqueous solution. Polymeric micelles, which are a novel drug carrier, have many valuable characteristics such as stability, biodegradability, biocompatibility, solubilization, and passive targeting. The reactive groups on the hydrophilic shell can be connected with targeting ligands (CitationWang et al. 2012, CitationXu et al. 2012). Through interaction between ligands and receptors on external surface of cytomembrane, the polymer micelles can be targeted to specific organs, tissues, or cells. Glycyrrhetinic acid (GA) is an efficient and specific targeting ligand, which is a kind of pentacyclic triterpene in liquorice root. Due to GA receptor existing on liver (parenchyma) cell membrane surface, GA can be accumulated in the liver (CitationWang et al. 1995, CitationNegishi et al. 1991).
In this study, amphiphilic CS-SA polymer with different SDs were prepared. A liver-targeting drug carrier GA-CS-SA was obtained using GA as specific ligand. The CS-SA and GA-CS-SA micelles were further prepared, and the following properties are determined: critical micelle concentrations (CMCs), number of hydrophobic micro-domains, particle size, zeta potential, morphology, and morphology were investigated.
Materials
Reagents
Chitosan (CS, average molecular weight = 10 kDa, and deacetylation degree = 85.62%), Haidebei Marine Bioengineering Co., Ltd.; Stearic acid (SA, analytically pure), Huasheng Bio-technology Co., Ltd.; Glycyrrhetinic acid (GA, purity = 98%), Zelang Medical Technology Co., Ltd.; 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, purity > 99%), and 2,4,6-trinitrobenzene sulfonic acid(TNBS, 5%, w/v), Yuanye bio-technology Co., Ltd.; N-hydroxysuccinimide (NHS, purity = 98%), pyrene (purity = 98%), and 1-dodecylpyridinium chloride (DPC, purity > 98%), J&K Scientific Ltd. Other chemicals were all of analytical or chromatographic grade.
Instruments
Nuclear Magnetic Resonance Spectrometer (JNM-ECP600), JEOL; Zetasizer Nano instrument (Zetasizer Nano-ZS90), Malvern Instruments Ltd.; Transmission electronic microscope (JEM2010), JEOL; UV spectrophotometer (UV-3000), Shanghai Mapada Instruments Co., Ltd.; Fluorometer (F-4500), HITACHI, Ltd.; Ultrasonic cell crusher (BILON92-2D), Shanghai Bilon Experiment Equipment Co., Ltd.; Low-speed centrifuge (D-78532), Hettich; Freezing dryer (FD-1C-50), Beijing Boyikang Lab Instrument Co., Ltd.
Methods and results
Synthesis of CS-SA
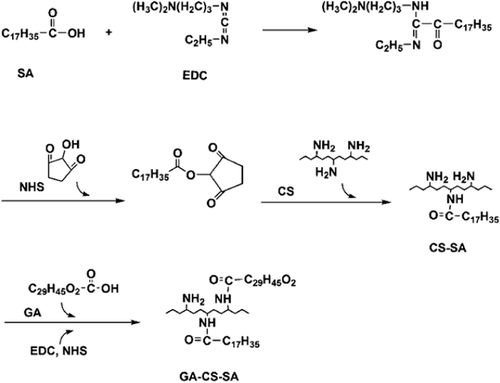
CS-SA was synthesized by amidation between the amino-groups of CS and the carboxyl groups of SA in the presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) (CitationTermsarasab et al. 2013). Briefly, 0.5 g of CS was dissolved in 20 mL of dimethylsulfoxide; and SA (the molar ratios of CS to SA were 1:0.05, 1:0.1, 1:0.15, 1:0.2, 1:0.25, 1:0.3, respectively.) was dissolved in 20 mL of dimethylsulfoxide, respectively. Both were stirred in water bath at 50°C for 15 min. Then, EDC and NHS were added into the SA solution, and then stirred in water bath at 50°C for 30 min to activate the SA. Then, the activated SA solution was added to the CS solution dropwise. Sequently, the mixture was kept in agitation at 37°C in dark condition for 8 h. The reaction mixture was poured into acetone (reaction mixture/acetone = 1/5,v/v), and then the liquid was centrifuged at 4000 rpm for 15 min to remove unreacted SA. Rinse the precipitate thrice with acetone. The precipitate was dispersed with and dialyzed against ultrapure water for 24 h using dialysis membrane (molecular weight cut-off = 3.5 kDa) to remove water soluble impurities. The dialyzed product was lyophilized to obtain CS-SA. The reaction process is shown in .
Synthesis of GA-CS-SA
GA-CS-SA was synthesized by amidation between the amino-groups of CS which had not reacted with SA and the carboxyl groups of GA in the presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). Briefly, CS-SA (the product of 1:0.3 group, 0.5 g), GA, EDC and NHS were dissolved in 40 mL of dimethylsulfoxide and then stirred at 50°C in dark condition for 12 h. The reaction mixture was poured into acetone (reaction mixture/acetone = 1/5,v/v), and the liquid was centrifuged at 4000 rpm for 15 min to remove unreacted GA. Rinse the precipitate thrice with acetone. The precipitate was dispersed with ultrapure water and dialyzed against ultrapure water for 24 h by using dialysis membrane (molecular weight cut-off = 3.5 kDa). The dialyzed product was lyophilized to obtain CS-SA. The reaction process was shown in .
Characterization of CS-SA and GA-CS-SA
1H NMR analysis of CS-SA and GA-CS-SA
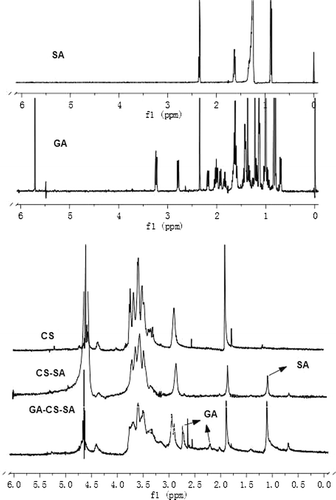
The chemical structures of CS-SA and GA-CS-SA were confirmed by 1H NMR analysis (). The 1H NMR spectra of CS-SA and GA-CS-SA were obtained by nuclear magnetic resonance (NMR) spectrometer (JNM-ECP600, JEOL, Japan) at 600 MHz. CS, CS-SA, and GA-CS-SA were dissolved in D2O, SA, and GA were dissolved in deuterated DMSO (DMSO-d6).
As shown in , it was clear that compared with CS, the 1H-NMR spectrum of the CS-SA (5:1) showed a new sharp signal at d = 1.0 ppm, which was attributed to the –CH2– of SA. Compared with CS-SA, the 1H-NMR spectrum of the GA-CS-SA (5:1) () showed some new signals at d = 3.5˜3.65 ppm, which belonged to GA. All above data indicated that SA and GA were successfully coupled to CS.
Substitute degree of amino groups of CS-SA and GA-CS-SA
The substitute degree of amino groups (SD) of CS-SA or GA-CS-SA (the number of SA groups per 100 amino groups of CS or the number of GA groups per 100 amino groups of CS-SA) were measured by 2,4,6-trinitrobenzene sulfonic acid (TNBS) method (CitationBernkop-Schnurch and Krajicek 1998). Briefly, CS solution with different concentrations were mixed with 2 mL of NaHCO3 (4%, w/v) and 2 mL of TNBS solution (0.1%, w/v). After incubated in water bath at 37°C for 2 h, 2 mL of HCl (2 mol/L) was added into the mixture. The UV absorbance of the final mixture was measured at 344 nm by UV spectroscopy. Then, the calibration curve was obtained. Two hundred microliters of CS-SA solution (1 mg/mL) and GA-CS-SA solution was prepared with the same processing. The SD percentage of CS-SA and GA-CS-SA were calculated from calibration curve.
TNBS is a chromogenic reagent that can react with the amino groups of the CS, CS-SA, and GA-CS-SA to develop a kind of yellow derivative. The derivatives had maximum UV absorbance at the wavelength of 344 nm, and the absorbance is proportional to the number of amino groups. The SD of CS-SA and GA-CS-SA were calculated from the standard curve, which was obtained from CS solutions with different concentration. As shown in the , the SD of SA and GA were 16.2% and 5.6%.
Table I. Physicochemical properties of GA-CS-SA and CS-SA.
CMC of CS-SA and GA-CS-SA
Pyrene, as a fluorescence probe, was used to determine CMC (CitationLee et al. 1998b). Briefly, 1 mL of pyrene solution (6 × 10− 7 mol/mL) with acetone as solvent was added to 10 test tubes, After the acetone was evaporated by vacuum drying at 50°C, 10 mL of CSO-LA (or GA-CS-SA) solution with different concentrations from 1.0 μg/mL to 1.0 × 103 μg/mL were added into the test tubes. The mixture was sonicated for 30 min in water bath at room temperature. The fluorescence emission spectrum of pyrene in the final solution was measured with fluorometer. The excitation wavelength was set at 334 nm, and the slit of excitation and emission at 10 nm and 2.5 nm, respectively. Then, the intensity ratios of the first fluorescence peak(I1, 374 nm) to the third fluorescence peak(I3, 384 nm) in the pyrene emission spectra were calculated. The CMC was obtained from trend changing of the intensity ratios.
CS-SA micelles and GA-CS-SA micelles were prepared by dissolving 20 mg CS-SA and GA-CS-SA in 20 ml ultrapure water, respectively. Then the solutions were probe-type ultra-sonicated in ice bath at 400 W for 30 min (pulse on 2.0 s, pulse off 3.0 s).
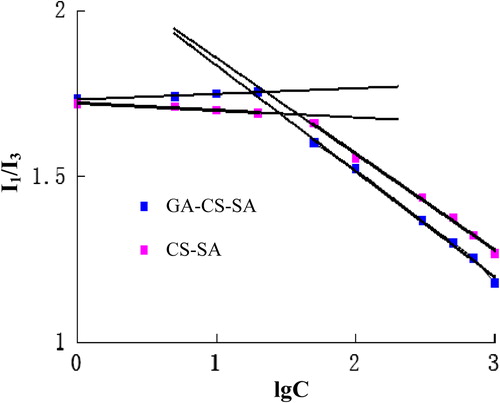
CMC is the lowest concentration of polymers to form micelles, which indicate the stability of micelles. Polymeric micelles with lower CMC are generally more stable in highly diluted conditions, which enable safe drug delivery to certain area.
Pyrene fluorescent probe was used to determine the CMC. Pyrene is very hydrophobic, and sensitive to changes of polarity within microenvironment. When pyrene concentration is greater than the CMC, the fluorescence intensity of pyrene almost had no change. Once the micelles are formed, pyrene molecules will be integrated into the hydrophobic cores, leading to the increase of fluorescence intensity. The fluorescence intensity of the third peak (I3) increases more significantly than that of the first one (I1). Consequently, after micelles had formed, the ratio of I1 to I3 (I1/I3) was decreased.
As shown in , the CMC was obtained from the intersection of two straight lines, which represent different changing trends. shows that the CMC of CS-SA and GA-CS-SA were 23.20 μg/mL and 17.94 μg/mL, respectively.
Aggregation number of hydrophobic micro-domain(AN) per CSO-SA or GA-CS-SA molecule
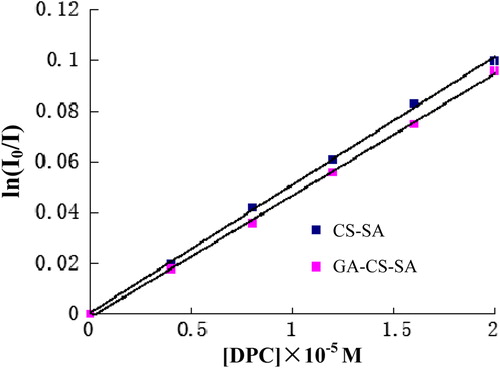
Steady-state fluorescence-quenching method (with pyrene as fluorescence probe and DPC as quencher) was used to estimate the AN per CSO-SA or GA-CS-SA molecule (CitationLee et al. 1998a). Briefly, 0, 0.2, 0.4, 0.6, 0.8, and 1.0 mL of DPC solution (1.0 × 10− 4 M) was added into different test tubes. After the ethanol was evaporated by vacuum drying at 80°C, 5 mL of CS-SA (or GA-CS-SA) (C = 1.0 mg/ml) solution with pyrene (6 × 10− 7 mol/mL) was added. The fluorescence emission spectrum of pyrene in the final solution was measured using fluorometer. The excitation and emission wavelengths were set at 334 and 393, respectively. The slits were set at 5 nm.
The AN per CS-SA and GA-CS-SA molecules were detected by steady-state fluorescence-quenching method and calculated using the following formulas:
where NNH2 is the total number of amino groups per CS-SA or GA-CS-SA molecule; NA is the number of SA or GA groups per hydrophobic micro-domain; [A] is the concentration of SA or GA; I0 and I are the fluorescence emission intensity in the absence and presence of quencher, respectively; [DPC] is the concentration of DPC; [M] is the concentration of hydrophobic stearate micro-domains in micelles.
The plot of ln (I0/I) against DPC concentration in the presence of 1.0 mg/mL CSO-SA and GA-CS-SA is shown in . indicate that the AN of CS-SA and GA-CS-SA are 1.96 and 2.09, respectively.
Particle size and zeta potential determination of CS-SA and GA-CS-SA micelles
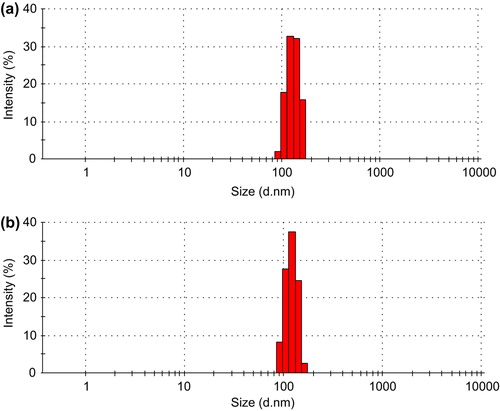
CS-SA and GA-CS-SA micelles with the concentration of 1 mg/mL (the micelle solution was filtered using 0.22 μm ultrafiltration membrane) were prepared to measure the sizes and zeta potential using dynamic light scattering. Each sample was measured thrice. The GA-CS-SA micelles have a unimodal size distribution and a small size (mean diameter of 121 nm), which was lower than that of CS-SA (). As shown in , the Z-average diameters of CS-SA micelles and GA-CS-SA micelles were 131.7 nm and 121.1 nm and the zeta potential were 19.7 mV and 17.2 mV.
The morphological examinations of CS-SA and GA-CS-SA micelles
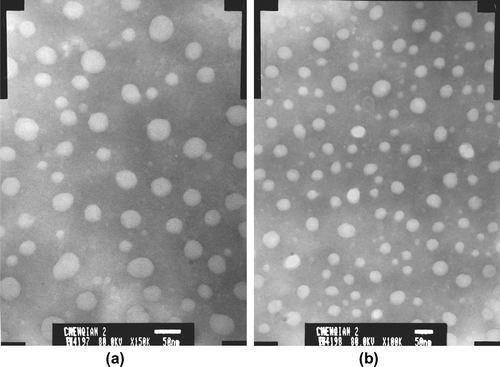
The appearance and shape of the both micelles (the micelle solution was filtered using 0.22 μm ultrafiltration membrane) were observed by transmission electron microscopy (TEM). As shown in , the two micelles are regular spherical particles, and their surfaces are smooth and not conglutinate.
Discussion
CS-SA was synthesized by amide reaction in the presence of EDC and NHS, for NHS can greatly improve the reaction efficiency of EDC (CitationErhan et al. 2002, CitationGómez et al. 2006). First, the carboxyls of SA reacted with EDC on the formation of an unstable intermediate. Secondly, the intermediate further reacted with EDC to form an active ester. Finally, the active ester reacting with the free amino of chitosan, which connected SA to the chitosan and generated CS-SA. While GA-CS-SA was synthetized by a similar reaction process with CS-SA, GA was connected to the remaining free amino which had not reacted with SA.
The increasing ratio of SA to CS gave rise to an growing substitution degree, whereas the growth gradually decreased. The reason may be the increase of steric hindrance. In addition, when the ratio was greater than 0.3:1, the solubility of products reduced significantly. This situation is likely to influence the study of CS-SA and the further reaction with GA. Consequently, the product of 0.3:1 group was used to connect with GA.
Because the water-solubility of CS-SA and GA-CS-SA were lower than that of CS, the micelles should be prepared under ultrasonic. The CMC of CS-SA was increased as SD accumulates. The CMC of GA-CS-SA was 17.94 ± 0.48 μg/ml, which was much lower than that of low molecular weight surfactants (CitationRiess 2003). The result suggested that the GA-CS-SA micelles are able to keep a stable structure in diluted condition.
Most stearic chains are gathered in the cores, but there are still some stearic chains assembled to form hydrophobic micro-domains on the shell, which resemble the secondary nucleus structure of the protein. These hydrophobic micro-domains can boost micelles to interact with cell surface, promoting endocytosis of micelles (CitationZuo et al. 2008).The AN rises with the increase of hydrophobic group number. The AN of GA-CS-SA was 2.09, which was larger than CS-SA. The result suggested that the uptake of CS-SA micelles into cells is easier than that of GA-CS-SA micelles.
With an increasing substitution degree, the Z-average diameter and zeta potential declined, which might be caused by growing hydrophobic group number and reducing free amino number, respectively. The zeta potential of GA-CS-SA micelles was lower than that of CS-SA micelles. The surface of micelle has a negative potential, which was able to absorb positively charged drugs. In addition, the interaction between positively charged GA-CS-SA micelles and negatively charged cell surface probably enhanced endocytosis (CitationMao et al. 2005). Particle size plays an important role in aggregation of nanoparticles in tumor tissue, and the size of nanometer particle used in tumor therapy should be 70–200 nm (CitationLiu et al. 1992). The smooth and round surface was in favor of improving its stability.
Conclusions
We synthesized CS-SA and GA-CS-SA which were further used to prepare micelles. The GA-CS-SA showed good property to form micelles in aqueous medium by self-assembling. The CMC and particle size of GA-CS-SA micelles are lower than that of CS-SA, and the number of hydrophobic micro-domains is higher than that of CS-SA micelles. The zeta potential of GA-CS-SA micelles is lower than that of CS-SA micelles, and it was still negative potential. The CMC of GA-CS-SA is relatively low, which indicated that GA-CS-SA micelles could keep the core-shell structure stable under diluted conditions. The mean diameter of GA-CS-SA micelles was about 121 nm and with a narrow size distribution. The surface of GA-CS-SA micelles was smooth and no conglutination, and the zeta potential was positive. Therefore, GA-CS-SA micelles are more likely to be integrated into tumor cells than are CS-SA micelles. All the results suggest that GA-CS-SA is a potential excellent liver-targeting drug carrier. We will study the drug-loading capacity and liver targeting of GA-CS-SA micelles in our further research.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Notes
This work was supported by the key scientific and technological projects of Shandong Province in 2008.
References
- Bernkop-Schnurch A, Krajicek ME. 1998. Mucoadhesive polymers as platforms for peroral peptide delivery and absorption: synthesis and evaluation of different Chitosan-EDTA conjugates. J Control Release. 50:215–223.
- Dufour JF, Johnson P. 2010. Liver cancer: from molecular pathogenesis to new therapies: summary of the EASL single topic conference. J Hepatol. 52:296–304.
- Erhan E, Keskinler B, Akay G, Algur OF. 2002. Removal of phenol from water by membrane-immobilized enzymes: Part I. Dead-end filtration. J Membrane Sci. 206:361–373.
- Gómez JL, Bódalo A, Gómez E, Bastida J, Hidalgo AM, Gómez M. 2006. Immobilization of peroxidases on glass beads: an improved alternative for phenol removal. Enzyme Microb Technol. 39: 1016–1022.
- Jayakumar R, Nwe N, Tokura S, Tamura H. 2007. Sulfated chitin and chitosan as novel biomaterials. Int J Biol Macromol. 40:175–181.
- Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. 2004. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 104:6017–6084.
- Lee KY, Jo WH, Kwon IC, Kim YH, Jeong SY. 1998a. Structural determination and interior polarity of self-aggregates prepared from deoxycholic acid-modified chitosan in water. Macromolecules. 31:378–383.
- Lee KY, Kwon IC, Kim YH, Jo WH, Jeong SY. 1998b. Preparation of chitosan self-aggregates as a gene delivery system. J Control Release. 51:213–220.
- Liu DX, Mori A, Huang L. 1992. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta. 1104:95–101.
- Mao S, Germershaus O, Fischer D, Linn T, Schnepf R, Kissel T. 2005. Uptake and transport of PEG-graft–trimethyl-chitosan copolymer-insulin nanocomplexes by epithelial cells. Pharm Res. 22:2058–2068.
- Negishi M, Irie A, Nagata N, Ichikawa A. 1991. Specific binding of glycyrrhetinic acid to the rat liver membrane. Biochim Biophys Acta. 1066:77–82.
- Riess G. 2003. Micellization of block copolymers. Prog Polym Sci. 28:1107–1170.
- Sedlacek HH. 2001. Pharmacological aspects of targeting cancer gene therapy to endothelial cells. Crit Rev Oncol Hemat. 37:169–215.
- Termsarasab U, Cho HJ, Kim DH, Chong S, Chung SJ, Shim CK, et al. 2013. Chitosan oligosaccharide-arachidic acid-based nanoparticles for anti-cancer drug delivery. Int J Pharm. 441:373–380.
- Wang XH, Tian Q, Wang W, Zhang CN, Wang P, Yuan Z. 2012. In vitro evaluation of polymeric micelles based on hydrophobically-modified sulfated chitosan as a carrier of doxorubicin. J Mater Sci Mater Med. 23:1663–1674.
- Wang Z, Nishioka M, Kurosaki Y, Nakayama T, Kimura T. 1995. Gastrointestinal absorption characteristics of glycyrrhizin from glycyrrhiza extract. Biol Pharm Bull. 18:1238–1241.
- Xu W, Cui Y, Ling P, Li LB. 2012. Preparation and evaluation of folate - modified cationic Pluronic micelles for poorly soluble anticancer drug. Drug Deliv. 19:208–219.
- Zuo A, Sun P, Liang D, Liu W, Zhao R, Guo G, et al. 2008. Improved transfection efficiency of CS/DNA complex by CO-transfected chitosanase gene. Int J Pharm. 352:302–308.