Abstract
Ovarian cancer is one of the leading causes for death of women. Every year the percentage of mortality rate is increasing day by day. Various chemotherapeutic agents are used to increase the survival rate of patients with ovarian cancer, but the available conventional dosage forms/marketed preparations are associated with several limitations. The use of nanotechnology in drug delivery contributes to their small size (10–100 nm), which improves the circulation and enables superior accumulation of therapeutic drugs at the tumor sites. In future, the use of nanotechnology will enable passive targeting and further improvements can be made using targeting moieties.
Introduction
Cancer is defined as the uncontrolled growth of cells coupled with malignant behavior incursion and metastasis. Death from cancer worldwide is continuously rising with an estimate of 13 million deaths in 2030 (CitationVyas 2012). A woman in her life span maybe affected by five major types of cancers, which mainly affects her reproductive organs, these are cervical cancer, ovarian cancer, uterine cancer, vaginal cancer, and vulvar cancer. Of all these, Ovarian cancer is the most prominent type that begins in the ovaries. These are the most common cause of death by gynecological cancer in women (CitationZiller et al. 2004, CitationJemal et al. 2005). They prove to be the highest mortality rate compared to all the gynecologic cancers. In ovarian cancer, the tumor initially appears responsive to chemotherapy, but the recurrent resistance results in treatment failure (CitationOzols et al. 2004). However, the survival rate is only about 3 years and the majority of women treated recur within the first 2 years of subsequent diagnosis (CitationMc Guire and Markman 2003). It is also one of the most common cancers that occur during pregnancy (CitationAli et al. 2012) and displays enormous histopathological diversity.
Ovarian cancer is the fifth ubiquitous cancer among women with a lifetime risk of 1.4–1.8% in the US. According to the survey report of American cancer society approximately 15,000 women will die due to ovarian cancer. Ovarian cancer is the ninth most prevalent cancer in women excluding the non-melanoma type. In most of the developed countries, women encompass a lifetime risk of about 1.4% for ovarian cancer, which is considerably greater than the risk of cervical or endometrial cancers, but is well below the 7% menace of breast cancer. Practically 25% of all the ovarian neoplasm are malignant in nature, around 80% includes the primary growths of ovaries and the remainder being secondary usually carcinomata. The chance of developing ovarian cancer is most in older women. It is supplementary in white women than the African-American women and are particularly liable to become malignant. There are four different stages in ovarian cancer. Of all these stages, fourth stage is the most chronic stage and difficult to treat. The mean survival is primarily dependent on the disease stage of the patients. highlights the median survival rates of different stages of ovarian cancer. From the table, it is clear that the patients in Stage I of ovarian cancer can be treated successfully and their survival rate is maximum, that is, 90–95% whereas the patients, which are in the last/ fourth stage of ovarian cancer, have minimum survival rate of 15–25%.
Table I. Median survival rate for different stages of ovarian cancer.
Treatment
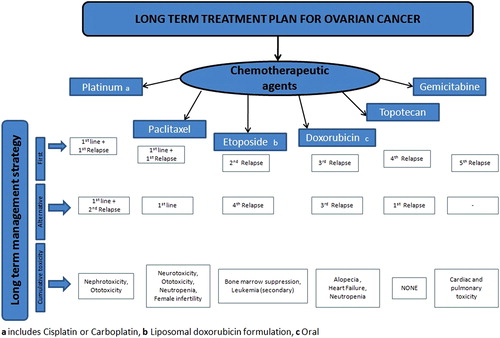
Once diagnosed, ovarian cancer can be treated by eliminating the cancerous tissue through surgery and further care must be taken to prevent the disease from recurring. Of different treatments, surgery alone is effective for only Stage I disease, whereas chemotherapy is necessary in all other stages (CitationArmstrong 2002). No single drug is able to treat ovarian cancer completely, it is essential to follow combination therapy, the various combination of anti-cancer drugs used in the long-term management of ovarian cancer are highlighted in . CitationYallapu et al. (2010) discussed the different combination therapy for treatment and management of ovarian cancer. One of the therapeutic approaches of treating ovarian cancer is surgical removal followed by systemic chemotherapy to destroy the malignant cells that have survived the surgery and to avoid metastasis and progression of tumor. Tumor markers are one of the indicators used to determine the clinical progress of ovarian cancers in women. Some of the examples are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), and Beta human chorionic gonadotropin (HCG) (CitationMani et al. 2007). The only suitable serum tumor marker that plays a promising role in monitoring ovarian cancer is CA 125 (CitationMeyer and Rustin 2000). The samples can be analyzed by using chromatographic techniques such as HPLC, HPTLC, and spectroscopic techniques such as UV Spectroscopy (CitationVyas et al. 2011). Regardless of the improved surgical technology and chemotherapy treatment, the long-term diagnosis of patients with advanced ovarian cancer has not markedly improved during the past 10 years (CitationOzols 1999). highlights the various marketed products of most of the drugs used in ovarian cancer. The national bodies currently working for the management of ovarian cancer in US are as follows:
a. National Cancer Institute—U.S. department of health and human services.
b. Ovarian cancer national alliance.
c. U.S. Department of Health and Human Services Center for Disease Control and Prevention.
Table II. Marketed drug products used in ovarian cancer.
Chemotherapeutic agents used in treatment of ovarian cancer
Chemotherapy is the ultimate solution that helps to recover the survival of patients with ovarian cancer. The chemotherapeutic drugs mainly used are cisplatin (CP), paclitaxel (PTX), doxorubicin (DOX), decitabine, gemcitabine (GB), and their combinations. Research is continuously going on to identify the novel therapeutic agents and improve the efficacy of existing therapeutic modalities. Also, a number of randomized trials using combination of hexamethyl melamine, cyclophosphamide (CPP), methotrexate (MTX), and fluorouracil have achieved higher survival rates compared to single therapeutic agent (CitationCarmo-Pereira et al. 1981). enlists the various chemotherapeutic drugs used in treatment of ovarian cancer. The list distinguishes the drugs into three parts viz. FDA approved drugs, drugs not FDA approved but listed in compendium and drugs neither FDA approved nor listed in compendium. Most of the chemotherapeutic agents/drugs used in ovarian cancer possess a significant therapeutic outcome, but are associated with following drawbacks:
a. They exhibit several adverse/side effects.
b. They are not effective in treating the recurrence of ovarian cancer.
c. They eventually become resistant to therapy (CitationDunton 2002).
Table III. Chemotherapeutically active agents/drugs used in ovarian cancer.
Therefore, drug resistance remains the major obstacle in ovarian cancer treatment. These above-mentioned drawbacks or limitations can be surmounted by utilizing the concept of nanotechnology. In nanotechnology-based formulations, the drug is encapsulated, conjugated, or entrapped/loaded in micro/nanocarriers. These formulations have considerable benefit of promoting the controlled delivery of chemotherapeutic drugs in a targeted way acting directly on the cancer site for prolonged duration with minimal or no normal organ toxicity. Therefore, the present review focuses on the development of novel formulations for treatment of ovarian cancer. This novel therapeutics has proved to enhance the drug efficacy, reduce the toxicity in healthy tissues and improve patient compliance. Many advances have reported the use of these nanoparticles for cancer therapies (CitationHaley and Frenkel 2008).
Nanotechnology-based therapy
Nanoparticles are the nanosized particles, which carry and transport pharmaceutical agents to achieve improved or enhanced pharmacological effects. Nanotechnology has a significant impact on therapeutic efficiency of drugs (CitationZhang et al. 2008). The use of nanotechnology with combination chemotherapy provides synergistic and or additive effect in drug delivery. Advantages of nanoparticle-based drug delivery therapy include the following:
It enhances therapeutic effectiveness, reduces side effects of the drug payloads by improving their pharmacokinetics properties, provides long circulation half lives, enhanced permeation and retention effect, drug safety, and patient compliance (CitationGreco and Vicent 2009). These nano carriers serve as promising candidate and provide unique platform capable of replacing the conventional available chemotherapeutic treatment, where in the intravenous injection of most of cytotoxic agents poses a serious threat to healthy tissues and results in dose-limiting side effects. Drug resistance, which is the most critical issue associated with the delivery of almost all the antineoplastic agents can be minimized or reduced by applying the concept of nanotechnology together with combination therapy (CitationHu et al. 2010).
Some of the nanoparticles used in ovarian cancer are discussed with examples as under:
Liposomes
Liposomes were investigated since 1970 as the drug carriers for humanizing the delivery of therapeutic agents/drugs in the body. They contain several concentric lipid bilayers, which enclose an internal aqueous volume. The unique advantage of liposomes is that they can enhance or increase the solubility of insoluble drugs from 100- to 10,000-folds and in the small intestine, they are digested in the existence of bile and enzymes, where the solubilized compound is liberated and further solubilized in bile and digested lipids. Some of the examples of chemotherapeutic agents/drugs loaded in liposomes are explained below:
DOX, a cytotoxic anthracycline available in the form of DOX HCl liposome injection (Doxil or Adiramycin®) has proved to be an efficient therapeutic agent for treatment of cancer from several years. One of the formulations of DOX, that is, Pegylated liposomal DOX has been approved for the management of refractory ovarian cancer (CitationThigpen et al. 2005). Furthermore CitationSun et al. (2010) developed the liposomal formulation (NaDC-Lip) of Altretamine, commonly known as Hexamethylmelamine (HMM), an alkylating anti-tumor agent), using sodium deoxycholate (NaDC) for oral delivery. Results of in-vivo studies revealed that the pharmacokinetics study of HMM NaDC-Lip was found better. It was approximately 9.8 and 1.2 folds higher as compared to HMM solution and HMM liposomes respectively. This indicates that HMM NaDC-Lip can be used as a potential carrier for oral drug administration.
Niosomes
Niosomes are non-ionic surfactant vesicles formed by self assembly of hydrated surfactant monomers. They have a bilayer structure and can entrap both hydrophilic and lipophilic drugs in aqueous layer and vesicular membrane. Niosomes as drug delivery carriers can overcome the drawback of severe side effects and lesser therapeutic effects associated with antineoplastic agents or with the chemotherapy of cancer. Ucheqbu et al. (1996) prepared niosomal formulation of DOX, which led to improved pharmacokinetic and tumoricidal activity. He demonstrated the superiority of niosome-based formulation over free drug. The activity of DOX C16G2 (a hexadecyl diglycerol ether)-based niosomes were evaluated against naive and established MAC (mouse adenocarcinoma) tumor models. The C16G2 niosomes were equiactive with DOX solution. A comparative study of the activity of DOX C16G2 niosomes and Span 60 niosomes was conducted against human ovarian cancer cell line, results showed a slight reduction in IC50 against the resistant cell line with Span 60 niosomes in comparison with the drug in solution. Thus, niosomes as drug delivery carriers can be utilized against multidrug resistance (Uchegbu et al. 1996).
Moreover, the negatively charged niosomes of PTX showed slow drug release with reduced toxic side effects and efficient oral delivery (CitationBayindir and Yuksel 2010). This formulation led to increase in solubility and bioavailability and stability of PTX.
Dendrimers
Dendrimers are nanoscale delivery carriers used for identification and treatment of cancer. They are utilized to achieve targeted therapy using folate receptors. The unique advantage of dendrimers is that they can be synthesized and designed according to the specific required applications. Due to their feasible topology, functionality/dimensions, and size (very close to most of the important biological polymers such as DNA and proteins) they are considered as ideal drug-delivery systems. Ovarian cancer cells consistently and uniformly overexpress folate receptors as much as two orders of magnitude higher relative to normal, healthy ovarian epithelial cells. Folate-conjugated dendrimers of CP, carboplatin, and PTX led to effective targeted delivery of these chemotherapeutic agents with lower dosage, improved solubility/bioavailability and lower toxicity (CitationBai et al. 2006). Dendrimers encapsulated with CP provided greater control over release profile. Results revealed the initial burst release of CP followed by sustained release over several hours.
Polymeric nanoparticles
Solid lipid nanoparticles (SLNs) are a class of colloidal carrier system that possesses advantage of easy preparation and cheaply available ingredients for their production (CitationJenning et al. 2002, CitationLee et al. 2003). These SLNs have higher dispersibility in water and can provide controlled particle size with an extended drug release (CitationMuller et al. 1995, CitationSiekmann and Westesen 1994, CitationCarsten and Oliver 2002). Some of the examples of polymeric and lipid-based nanoparticles are discussed as under: PTX, a prominent antitumor agent has been proved for the effective treatment of ovarian cancer. The commercially available formulation of PTX contains solvent cremophor and dehydrated ethanol. But, the frequent use of cremophor in PTX formulation is relatively high and is associated with serious toxicities and hypersensitivity reactions. Furthermore, CitationGelderblom et al. (2001), CitationKloover et al. (2004), and CitationLu et al. (2007) prepared polymeric drug delivery system for PTX and evaluated whether these nanoparticles can inhibit growth of ovarian carcinoma xenografts in Fisher344 (F344) rats by intraperitoneal administration. Ultrasonic emulsification technique was used to prepare the nanoparticles. Results showed that PTX nanoparticles and the formulation PTX (Cremophor) showed equivalent cytotoxic activity in vitro. The nanoparticles loaded with PTX led to reduction of tumor weight and ascite volume. The results showed 20-fold higher concentration in pelvic lymph nodes in presence of PLA. Thus, the intraperitoneal administration of PTX nanoparticles is effective for lymph targeting (CitationColeman et al. 2011). Also, the albumin-bound nanoparticles of PTX are effective for the management of persistent platinum-resistant ovarian and peritoneal cancer (CitationFeng and Chien 2003).
Lipid nanoparticles
The use of nanoparticles (NP) for controlled delivery of anticancer chemotherapeutic agents allows the enhancement of their therapeutic efficiency (CitationGref et al. 1995). These colloidal drug carriers provide protection against in-vivo degradation; the patient's comfort is also increased by avoiding the repetitive bolus injection or the use of perfusion pumps and led to better drug pharmacokinetics (CitationHoarau et al. 2004). It has been established that passive targeting of solid neoplasm by systemic drug carriers administration can be achieved if particles present long-circulating properties and adequate particle size for optimal extravasion at tumoral sites, that is, in the range of 50–200 nm (CitationMalzert-Freon et al. 2006).
CitationMalzert-Freon et al. (2006) formulated nanocapsules of tripentone by phase inversion method (CitationZhang et al. 2010). Tripentone is widely used in treatment of cancer especially in resistant tumoral ovarian cells. The drawbacks of tripentone are its poor solubility in biological fluids and cytotoxicity; to overcome the drawbacks, its nanocapsules were prepared by using the mixture of lipid, surfactant, and water. Results of TEM study showed that particles with a homogeneous size of about 50 nm were observed. The encapsulation efficiency of nanocapsules was above 90%. A little deformation of the NP, from spherical to hexagonal, is visible in some case, but it may be due to the evaporation process consecutive to the preparation and the analysis of the samples under vacuum. Also, sustained release of the encapsulated tripentone NP at a slower release rate was observed and about 60% tripentone was released in 10 days. The very low IC50 values confirmed the strong cytotoxic activity of the tripentone. Moreover, IC50 values were unchanged between free tripentone and tripentone-loaded NP, which proved that the activity of tripentone was recovered in vitro after encapsulation. Recently, CitationZhang et al. (2010) evaluated antitumor efficacy of docetaxel-loaded SLNs in a murine ovarian cancer model (CitationLi et al. 2011). In this study, SLN bio-distribution from RES more toward the circulatory system was observed. Moreover, the in-vivo anti-ovarian cancer activity was better in SLNs as compared with free drug. However, the PTX-loaded pegylated SLNs were taken up by the RES after parenteral (intravenous) administration in rats, with results of 8-fold and 3-fold higher levels in organs such as liver and spleen respectively, and 8 h after administration compared with Taxol® (CitationLiggins and Burt 2002).
Polymeric micelles
The therapeutic efficacy of poor chemotherapeutic anti-cancer drugs can be improved by formation of polymeric micelles. They also help to minimize the side effects associated with the drug. The primary function of polymer micelles is to protect and improve the solubility and stability of hydrophobic (lipophilic) drugs. These micelles can increase the poor solubility of chemotherapeutic anti-cancer drugs by many folds (25,000 or more) (CitationLiu et al. 2004, CitationKataoka et al. 2001). A significant characteristic of polymer micelles is the small size (10–100 nm) of particles, which improves the drug distribution and enables superior accumulation at the tumor sites. Polymeric micelles are formed by a hydrophobic core layered with hydrophilic chains through a spontaneous self- assembly of block or graft copolymers (CitationHwang et al. 2005, CitationKim and Park 2002). CitationKim and Park (2002) developed a surface cross-linking PLGA-β-PEG copolymer in order to improve the stability of polymer micelles by using vinyl pyrrolidone (CitationLee et al. 2009). Hyaluronic acid, a carbohydrate-derived co-polymer can be used as target-specific micelle carriers for delivery of DOX in conjugation with PLGA polymer (CitationKim et al. 2001). This formulation allowed loading of 4.8–7.2 w% DOX (i.e., DOX-HA-g-PLGA) which exhibited 5.2-fold greater cytotoxicity in the cancer cells over free DOX (IC50 value of DOX-HAg- PLGA = 0.67 mg.mL− 1 and free DOX = 3.48 mg.mL− 1). Similarly, a mixed micelle nanoformulation of DOX-loaded TPGS/PLGA-b-PEG-b-FOL (TPGS = atocopheryl succinate esterified to polyethylene glycol 1000 and FOL = folate) has shown higher cellular uptake of DOX, which resulted a higher degree of apoptosis in drug- resistant cancer cells (CitationLoftsson et al. 2007).
Use of cyclodextrins
Another aspect of increasing the poor solubility of chemotherapeutic drugs in water and biological fluids is formation of inclusion complexes. Cyclodextrins are water-soluble macrocyclic oligosaccharides, which are covalentently linked with glucopyranose rings. Cyclodextrins (α, β, and γ) and their derivatives are multifunctional excipients used in pharmaceuticals to enhance/improve the solubility and bioavailability of poorly water-soluble or insoluble compounds (CitationPiel et al. 1999, CitationVyas and Saraf 2008). These carbohydrate derivatives have the tendency to form inclusion complex in such a way that camouflages the undesirable features/physicochemical properties of the drug. These versatile pharmaceutical carriers are classified into hydrophilic, hydrophobic, and ionic derivatives. Thus, the versatility of CDs and their derivatives creates the capability for alleviating the undesirable physicochemical features of the drug (CitationPurgholami and Wangoo 2008). One example in this context is of albendazole, which showed enhanced proliferative activity or cytotoxicity in ovarian cells (1A9, OVCAR-3 and SKOV-3) after its solubility enhancement by complexation with hydroxy propyl beta cyclodextrin (CitationChauhan et al. 2009). This proved that the potency of anticancer drugs could be enhanced by using cyclodextrins as complexing agent.
Nanostructured lipid carriers
Nanoparticles, mainly the SLNs possess certain advantages over other colloidal or particulate carrier-based delivery systems. These include biocompatibility, sterility, scale up, and protection of incorporated active ingredients against chemical degradation (CitationMuller et al. 2000). Still, these SLNs have some potential limitations such as limited drug-loading capacity and drug expulsion during storage. These limitations hinder their use in development of delivery system, thus nanostructured lipid carriers (NLCs) came into existence, as they are prepared by mixing solid lipids with various chemically different liquid lipids/oils. One of the prominent examples is the NLCs of PTX which is used for successful and effective treatment of ovarian cancer, overcoming the limitations associated with delivery of PTX. The cholesterol NLCs loaded with PTX were prepared by solvent emulsification–diffusion method using poloxamer 188 and oleic acid. These NLCs provided targeted delivery without any side effect associated with cremophor EL-containing formulations (CitationEmami et al. 2012). Thus, NLCs as carriers can provide targeted and intracellular drug delivery of various antineoplastic agents. enlists examples of nanocarriers used in treatment of ovarian cancer.
Table IV. Nanocarrier-based delivery system for ovarian cancer.
Gene therapy
Now-a-days a new approach based on nanoteched gene therapy of drug is utilized for successful treatment of ovarian cancer. This technique reduces the tumors of ovarian cancer 80–85% and stops further growth of tumor in chemotherapeutic-resistant ovarian cancer tissues. In this technique the gene (RNA) is loaded into nanoparticles and developed into delivery system. Small inhibitory RNA (siRNA), a snippet of genetic material interferes with the gene expression of ephA2 and stops the cancer cells from growing (CitationHouston 2013). These siRNA cannot be injected directly into the body as the enzymes present in the blood and inside cells would destroy them before reaching the target cancer cells. So, a protective shield of lipid carrier-based nanoparticles called nanoliposome is used. The liposome-clad siRNA can easily reach the targeted cancer cells, where the liposome is absorbed.
These micro/nano carriers can be loaded in suitable dosage form such as tablets and capsules for oral delivery, creams and ointments for topical delivery, and in injectable or parenteral dosage form. Of all the available dosage forms, oral delivery is the most preferred. Various simple methods are available for encapsulating these carriers and stable microcapsules or tablets ranging from 1 to 100 μ in diameter can be formed (CitationChang 1964, Citation1976). In future, laboratory work can be explored focusing on the use of biocompatible artificial cells for regeneration of metastatic cancers. This can also be done through stem cells or gene therapy (CitationChang 2007, Citation2013).
Conclusion
Anticancer drugs used in treatment of ovarian cancer are more diverse in terms of physicochemical properties and molecular structure. The major challenges/obstacles in the effective ovarian cancer chemotherapies are inadequate drug concentrations reaching the tumor site, their rapid elimination, systemic toxicity, and adverse effects. Nanotechnology has the potential to overcome the current chemotherapeutic barriers in ovarian cancer treatment and to solve the problems associated with traditional chemotherapy and multidrug resistance. In the coming years, the development of a new generation of other nanocarriers of anticancer drugs based on nanotechnology with combination therapy will provide more effective treatment of ovarian cancer. The use of nanotechnology will lead to decrease in mortality rate and increase the survival rates of patients with ovarian cancer. Also, the use of nanotechnology in drug delivery system will enable passive targeting to the tumors due to their nano size and further improvements in tumor localization can be made using targeting moieties. In future, several factors affecting the designing and development of new nanoscale drug delivery system includes ratiometric drug-loading capacity of the carrier, temporal drug release, targeted delivery, and manufacturability of the therapeutic nanoparticles.
Acknowledgment
The authors are thankful to Director, University Institute of Pharmacy, Pt Ravi Shankar Shukla University Raipur, Chhattisgarh for providing necessary infrastructural facilities.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
The authors are thankful to UGC-MRP F. No. 42-706/2013 (SR) and CCOST/MRP/2012 Endt. No. 1926 for providing financial assistance relating to this work.
References
- Ali E, Rahimi E, Akahavan S. 2012. Cancer during pregnancy. A review of 10 years of experience. Pak J Biolog Sci. 15:341–346.
- Armstrong DK. 2002. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncol. 7:20–28.
- Bai S, Thomas C, Rawat A, Ahsan F. 2006. Recent progress in dendrimer-based nanocarriers. Crit Rev Ther Drug Carrier Syst. 23:437–495.
- Bayindir ZS, Yuksel N. 2010. Characterization of niosomes prepared with various non ionic surfactants for paclitaxel oral delivery. J Pharm Sci. 99:2049–2060.
- Carmo-Pereira J, Costa FO, Henriques E, Ricardo JA. 1981. Advanced ovarian carcinoma: a prospective and randomized clinical trial of cyclophosphamide versus combination cytotoxic chemotherapy. Cancer. 48:1947–1951.
- Carsten OK, Oliver RH. 2002. Enzymatic degradation of dynasan 114 SLN – effect of surfactants and particle size. J Nanopart Res. 4: 121–129.
- Chang TMS. 1964. Semi permeable Microcapsules. Science. 146: 524–525.
- Chang TMS. 2007. Monograph on “Artificial Cells: Biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation, cell/stem cell therapy.” Singapore and London: World Scientific Publisher/Imperial College Press, p. 435.
- Chang TMS. 2013. Introduction chapter on “Artificial Cells that started Nanomedicine” in book on Selected Topics in Nanomedicine. Singapore and London: World Science Publisher/Imperial College Press, p. 3659.
- Chang TMS. 1976. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J Bioeng. 1:25–32.
- Chauhan SC, Kumar D, Jaggi M. 2009. Mucins in ovarian cancer diagnosis and therapy. J Ovarian Res. 2:21–26.
- Coleman RL, Brady WE, McMeekin DS, Rose PG, Soper JT, Lentz SS, et al. 2011. A phase II evaluation of nanoparticles, albumin-bound paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 122:111–5.
- Dunton CJ. 2002. Management of treatment-related toxicity in advanced ovarian cancer. Oncol. 7:11–19.
- Emami J, Rezazadeh M, Varshosaz J, Tabbakhian M, Aslani A. 2012. Formulation of LDL Targeted Nanostructured Lipid Carriers Loaded with Paclitaxel: A Detailed Study of Preparation, Freeze Drying Condition, and In Vitro Cytotoxicity. J Nanomater. 2012:1–10.
- Feng SS, Chien S. 2003. Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem Eng Sci. 58: 4087–4114.
- Gelderblom H, Verweij J, Nooter K. 2001. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 37:1590–1598.
- Greco F, Vicent MJ. 2009. Combination therapy: opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines. Adv Drug Deliv Rev. 61:1203–1213.
- Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. 1995. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev. 16:215–233.
- Haley B, Frenkel E. 2008. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 26:57–64.
- Hoarau D, Delmas P, David S, Roux E, Leroux JC. 2004. Novel long circulating lipid nanocapsules. Pharm Res. 21:1783–1789.
- Houston, TX. 2013. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clin Cancer Res. 19:1806–1815.
- Hu, CM, Aryal S, Zhang, L. 2010Nanoparticle-assisted combination therapies for effective cancer treatment. Therapeutic Delivery. 1, 2:323–334.
- Hwang MJ, Suh JM, Bae YH, Kim SW, Jeong B. 2005. Caprolactonic poloxamer analog: PEG-PCL-PEG. Biomacromolecules. 6:885–890.
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ. 2005. Cancer statistics. CA-Cancer J Clin. 55:10–30.
- Jenning V, Lippacher A, Gohla SH. 2002. Medium scale production of solid lipid nanoparticles SLN by high-pressure homogenization. J Microencapsul. 19:1–10.
- Kataoka K, Harada A, Nagasaki Y. 2001. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 47:113–7.
- Kim HK, Park TG. 2002. Surface Stabilization of Diblock PEG-PLGA Micelles by Polymerization of N-Vinyl-2-pyrrolidone. Macromol Rapid Commun. 23:26–31.
- Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, Seo MH. 2001. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 72:191–202.
- Kloover JS, Bakker MA, Gelderbolm H. 2004. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Canc. 90:304–305.
- Lee H, Ahn CH, Park TG. 2009. Poly [lactic-co-glycolic acid]-Grafted Hyaluronic acid copolymer micelle nanoparticles for target-specific delivery of doxorubicin. Macromol Biosci. 9:336–42.
- Lee KE, Cho SH, Lee HB, Jeong SY, Yuk SH. 2003. Microencapsulation of lipid nanoparticles containing lipophilic drug. J Microencapsul. 20:489–496.
- Li R, Eun JS, Lee MK. 2011. Pharmacokinetics and biodistribution of paclitaxel loaded in pegylated solid lipid nanoparticles after intravenous administration. Arch Pharm Res. 34:331–7.
- Liggins RT, Burt HM. 2002. Polyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulations. Adv Drug Deliv Rev. 54:191–202.
- Liu J, Xiao Y, Allen C. 2004. Polymer-drug compatibility: a guide to the development of delivery systems for the anticancer agent, ellipticine. J Pharm Sci. 93:132–143.
- Loftsson T, Vogensen SB, Brewster ME, Konradsdottir F. 2007. Effects of cyclodextrins on drug delivery through biological membranes. J Pharm Sci. 96:2532–2546.
- Lu H, Li B, Kang Y, Jiang W, Huang Q, Chen Q, et al. 2007. Paclitaxel nanoparticle inhibits growth of ovarian cancer xenografts and enhances lymphatic targeting. Cancer Chemother Pharmacol. 59:175–181.
- Malzert-Freon A, Vrignaud S, Saulnier P, Lisowski V, Benot JP, Rault S. 2006.Formulation of sustained release nanoparticles loaded with a tripentone, a new anticancer agent. Int J Pharma. 320:157–164.
- Mani R, Jamil K, Vamsy MC. 2007. Specificity of serum tumor markers CA 125, CEA, AFP and Beta HCG for ovarian malignancies. Trends Med Res. 2:128–134.
- McGuire WP 3rd, Markman M. 2003. Primary ovarian cancer chemotherapy: current standards of care. Br J Canc. 89:3–8.
- Meyer T, Rustin GJ. 2000. Role of tumor markers in epithelial ovarian cancer. Br J Cancer. 82:1535–1538.
- Muller RH, Mader K, Gohla S. 2000. Solid lipid nanoparticles SLN for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 50:161–177.
- Muller HR, Mehnert W, Lucks JJ, Schwartz C, Zur Muhlen A, Weyhers H, et al. 1995. Solid lipid nanoparticles SLN as an alternative colloidal carrier system for controlled drug delivery. Eur J Pharm Biopharm. 41:62–68.
- Ozols R. 1999. Treatment of gynecologic cancer: the US experience. Tumori, 85, S5–S11.
- Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, et al. 2004. Focus on epithelial ovarian cancer. Cancer Cell. 5:19–24.
- Piel G, Evrard B, Van Hees T, Llabres G, Delattre L. 1999. Development of a parenteral and of an oral formulation of albendazole with cyclodextrins. STP Pharma Sci. 9:257–260.
- Purgholami MH, Wangoo KT. 2008. Albendazole-cyclodextrin complex: enhanced cytotoxicity in ovarian cancer cells. Anticancer Res. 28:2775–2780.
- Siekmann B, Westesen K. 1994. Melt-homogenized solid lipid nanoparticles stabilized by the nonionic surfactant tyloxapol - I. Preparation and particle size determination. Pharmacol Lett. 3:194–197.
- Sun J, Deng Y, Wang S, Cao J, Gao X, Dong X. 2010. Liposomes incorporating sodium deoxycholate for hexamethylmelamine HMM oral delivery: development, characterization, and in vivo evaluation. Drug Deliv. 17:164–70.
- Thigpen JT, Aghajanian CA, Alberts DS, Campos SM, Gordon AN, Markman M, et al. 2005. Role of pegylated liposomal doxorubicin in ovarian cancer. Gynecol Oncol. 96:10–8.
- Uchegbu IF, Double JA, Kelland LR, Turton JA, Florence AT. 1996The activity of doxorubicin niosomes against an ovarian cancer cell line and three in vivo mouse tumour models. J Drug Targeting. 3: 399–409.
- Vyas A, Saraf S. 2008. Cyclodextrin based novel drug delivery systems. J Incl Phenom Macrocycl Chem. 62:23–42.
- Vyas A, Shukla SS, Pandey R. 2011. Development and validation of spectrophotometric method for estimation of cephalexin in bulk and tablet dosage forms. Oriental J Chemistry. 27:359–362.
- Vyas A. 2012. Preparation, characterization and pharmacodynamic activity of supramolecular and colloidal systems of rosuvastatin– cyclodextrin complexes. J Incl Phen Macrocyclic Chem. 66: 251–259.
- Yallapu M, Jaggi MM, Chauhan SC. 2010. Scope of nanotechnology in ovarian cancer therapeutics Scope of nanotechnology in ovarian cancer therapeutics. J Ovarian Res. 3:19–29.
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. 2008Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 83, 5:761–769.
- Zhang P, Chen L, Zhang Z, Lin L, Li Y. 2010. Pharmacokinetics in rats and efficacy in murine ovarian cancer model for solid lipid nanoparticles loading docetaxel. J Nanosci Nanotechnol. 10:7541–4.
- Ziller C, Lincet H, Muller CD, Staedel C, Behr JP, Poulain L. 2004. The cyclin-dependent kinase inhibitor p21cip1/waf1 enhances the cytotoxicity of ganciclovir in HSV-tk transfected ovarian carcinoma cells. Cancer Lett. 212:43–52.