Abstract
Continuous delivery of proteins by engineered cells encapsulated in biocompatible polymeric microcapsules is of considerable therapeutic potential. However, this technology has not lived up to expectations due to inadequate cell–matrix interactions and subsequent cell death. In this study we hypothesize that the presence of fibronectin in an alginate matrix may enhance the viability and functionality of encapsulated human cord blood-derived mesenchymal stromal cells (MSCs) expressing the human Factor IX (FIX) gene. MSCs were encapsulated in alginate-PLL microcapsules containing 10, 100, or 500 μg/ml fibronectin to ameliorate cell survival. MSCs in microcapsules with 100 and 500 μg/ml fibronectin demonstrated improved cell viability and proliferation and higher FIX secretion compared to MSCs in non-supplemented microcapsules. In contrast, 10 μg/ml fibronectin did not significantly affect the viability and protein secretion from the encapsulated cells. Differentiation studies demonstrated osteogenic (but not chondrogenic or adipogenic) differentiation capability and efficient FIX secretion of the enclosed MSCs in the fibronectin-alginate suspension culture. Thus, the use of recombinant MSCs encapsulated in fibronectin-alginate microcapsules in basal or osteogenic cultures may be of practical use in the treatment of hemophilia B.
Introduction
Hemophilia B is an X-linked bleeding disorder caused by a lack of circulating functional Factor IX (FIX) (CitationSadler and Davie 1987, CitationBolton-Maggs and Pasi 2003). Current treatment for severe hemophilia B (patients with < 1% active FIX) involves the costly and life-long injection of plasma-derived or recombinant FIX. Gene therapy offers an attractive alternative treatment for hemophilia B. Current successful hemophilia B gene therapy strategies involve direct injection of viral vectors for FIX delivery, but there are associated complications with the host immune response (CitationMarshall 2001, CitationCheck 2003, CitationNathwani et al. 2011). Cell therapy technologies offer a variety of safe alternatives for hemophilia treatment. Encapsulation of recombinant cells within a semi-permeable membrane was first described 50 years ago (CitationChang 1964) and has since been used as a strategy for continuous delivery of therapeutic proteins for a variety of protein deficiency diseases, including hemophilia B, without altering the patient's genome (CitationLim and Sun 1980, CitationKoo and Chang 1993, CitationChang et al. 1994, CitationCieslinski and David Humes 1994, CitationHortelano et al. 1996, Citation1999, Citation2001, CitationOrive et al. 2003, Citation2004, Citation2009, CitationChang 2005, CitationWen et al. 2006, Citation2007, CitationThakur et al. 2010). However, a key limiting factor of this strategy for hemophilia B is the compromised viability of the implanted cells.
It is well known that adhesive peptides or proteins play central roles at the cell–surface interface by interacting with integrins to mediate survival signals (CitationShin et al. 2003). Although several studies have addressed the significance of certain proteins, such as laminin, fibrinogen, and fibronectin, in enhancing cell survival, different results have been reported depending on the cell line used and the cell-biomaterial combination (CitationWhitlock et al. 2000, CitationStupack and Cheresh 2002, CitationKaroubi et al. 2009). We have previously constructed fibrinogen-supplemented alginate that resulted in enhanced viability and functionality of enclosed FIX-engineered mesenchymal stromal cells (MSCs) (CitationSayyar et al. 2012). Here, we explore the effect of fibronectin as a cell-adhesive molecule on the viability and protein secretion of MSCs within alginate-based microcapsules.
Fibronectin, one of the most important extracellular matrix (ECM) proteins, is a multi-domain glycoprotein that possesses binding sites for a variety of other ECM components including collagen, heparin A and B, fibrin and chondroitin sulfate. It is present both in blood at 150–300 μg/ml and in the ECM and plays a critically important role in linking cells to the ECM via cell surface integrins and adaptor molecules (CitationSchoen and Mitchell 1996, CitationDolatshahi-Pirouz et al. 2011).
The objectives of this study were to assess whether the incorporation of fibronectin within alginate matrix can enhance the viability and FIX secretion level of encapsulated umbilical cord blood (CB)-derived MSCs and to determine effective doses of fibronectin. Furthermore, we evaluated the differentiation capabilities of the encapsulated cells into osteogenic, chondrogenic, and adipogenic lineages and examined the effect of fibronectin on cell viability and FIX secretion.
Materials and methods
Cell culture
Umbilical cord blood was obtained after delivery with the mothers’ informed consent in accordance with the guidelines of the University of Alberta Health Research Ethics Board. Light density mononuclear cells (MNC) were separated by Percoll density gradient centrifugation (Amersham Biosciences, Uppsala, Sweden) and cultured in Iscove's modified Dulbecco's medium (IMDM; Invitrogen, Burlington, ON, Canada) supplemented with 10% fetal bovine serum and streptomycin (100 μg/ml) at 37°C in 5% CO2. After 24 h, non-adherent cells were removed and complete medium was replaced, as described previously (CitationSon et al. 2006). MSCs were used in experiments before reaching passage 6. Flow cytometric analysis showed positive expression for CD73, CD90, and CD105 but not for the hematopoietic markers CD34 and CD45 (data not shown), which is in agreement with the position statement of the International Society of Cellular Therapy on the minimal criteria defining MSCs (CitationDominici et al. 2006).
Engineering of MSC
The PLVX-Puro vector DNA (Clontech, Mountain View, CA, USA) was engineered with traditional restriction enzyme techniques to generate a FIX-expressing lentiviral DNA construct with CMV promoter. Viral particles were generated with the Lenti-X Expression System (Clontech) according to the manufacturer's protocol. Briefly, LentiX 293T cells were transfected with the 4th generation VSV-G packaging DNA and PLVX-FIXI expression plasmid using Xfect transfection reagent to generate viable virus particles. Two rounds of freshly produced viral supernatant were used to transduce CB MSCs (passage 4) for 24 h at a MOI of approximately 20. Transduced cells were selected with puromycin (3 μg/ml) (Clontech) after 15-day incubation at 37°C.
Cell encapsulation
MVG ultrapure alginate was purchased from FMC BioPolymer (Philadelphia, PA, USA). Human plasma fibronectin (Sigma Aldrich, Oakville, ON, Canada) was added to the alginate solution (1.56% alginate) to achieve concentrations of 10, 100, and 500 μg/ml of alginate solution. FIX-engineered CB MSCs were suspended in fibronectin-alginate or non-supplemented (control) alginate solutions at a concentration of 3 × 106 cells/ml. Microencapsulation was performed with an electrostatic encapsulator (Nisco Engineering Inc., Zurich, Switzerland) as previously described (CitationChang et al. 1994). Briefly, cell suspension was pumped through the electrostatic encapsulator (voltage: 7 kV) at a flow rate of 0.9 ml/min into a vial containing 1.1% CaCl2 yielding microcapsules 400 μm in diameter. Cell-loaded beads were then washed with saline solution and cross-linked with poly-L-lysine and with an outer layer of non-supplemented alginate.
Differentiation of human MSCs
For induction of osteogenic, chondrogenic, or adipogenic differentiation, encapsulated MSCs were cultured in StemPro Osteogenic, StemPro Chondrogenic, or StemPro Adipogenic differentiation media (GIBCO Invitrogen, Burlington, ON, Canada), respectively, and with appropriate supplements.
At week three post-osteogenic induction and at week two post-chondrogenic and adipogenic induction, encapsulated cells were washed with phosphate buffered saline (PBS), then fixed in 4% paraformaldehyde for 10 min. Encapsulated cells were stained with Alizarin Red, Alcian Blue, and Oil Red O dyes (Sigma Aldrich) for detection of calcium deposits, proteoglycans, and fat vacuoles as indication of osteogenic, chondrogenic, and adipogenic differentiation, respectively. Microcapsules were visualized with an inverted light microscope (Leica DM IL, Leica Microsystems, Richmond Hill, ON, Canada).
Assessment of viability and proliferation of encapsulated cells
Viability and proliferation of encapsulated MSC were assessed using the Live/Dead viability assay kit (Sigma Aldrich, Oakville, ON, Canada) according to the manufacturer's instructions adapted to encapsulated cells. The kit contained the acetoxymethyl ester of calcein (Calcein-AM) and the nuclei staining dye propidium iodide, which stain viable and dead cells, respectively. Briefly, a cell suspension in 200 μl PBS was prepared at a density of 1 × 105/ml, and 100 μl of assay solution was added. The mixture was incubated at 37°C for 15 min. Using a fluorescence microscope with 490 nm excitation, viable cells were monitored based on green fluorescence (515 nm emission) while dead cells were monitored based on red fluorescence (617 nm).
F-actin cytoskeleton staining of encapsulated cells
Encapsulated cells (100 μl of capsules) were fixed in 4% paraformaldehyde. As indicated in manufacturer's protocol, cells were then washed in pre-warmed PBS and permeabilized by incubation in 0.1% Triton X-100 in PBS for 10 min, followed by two washes in PBS. Cells were stained with Alexa Fluor 633 phalloidin (15 μl methanolic stock solution in 200 μl PBS, for 30 min in the dark) (Molecular Probes Invitrogen, Burlington, ON, Canada) containing 1% bovine serum albumin to reduce non-specific background. A sample was analyzed by inverted confocal microscopy (Zeiss 510, Carl Zeiss Inc., Toronto, ON, Canada).
Transmission electron microscopy
Transmission electron microscopy (TEM) studies were done in the electron microscopy facility at McMaster Children's Hospital (Hamilton, ON, Canada). Encapsulated cells were fixed with 2% glutaraldehyde (2% v/v) in 0.1 M sodium cacodylate buffer (pH 7.4). The samples were rinsed 2X in buffer solution, then post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 h and dehydrated through a graded ethanol (EtOH) series (50% to 100%). The final dehydration of the TEM samples was done in 100% propylene oxide (PO). Infiltration with Spurr's resin was done through a graded series of PO:Spurr's 2:1, 1:1, 1:2, 0:1 with rotation of the samples in between solution changes. The samples were transferred to embedding moulds which were then filled with fresh 100% Spurr's resin and polymerized overnight in a 60°C oven. Thin sections were cut on a Leica UCT ultramicrotome (Leica Microsystems, Richmond Hill, ON, Canada) and picked up onto copper grids. Sections were post-stained with uranyl acetate and lead citrate and viewed in a JEOL JEM 1200 EX TEMSCAN transmission electron microscope (JEOL, Peabody, MA, USA) at an accelerating voltage of 80 kV.
FIX ELISA assay
Human FIX antigen in culture media was quantified by an ELISA assay (Affinity Biologicals Inc., Ancaster, ON, Canada) as previously described (CitationWen et al. 2006).
Statistical data analysis
Analysis of variance (ANOVA) was done to determine significant differences between groups. Student's t-test or Tukey HSD was conducted as post hoc tests to compare pairs of data. Differences were considered significant when P < 0.05. Data are expressed as means ± SD.
Results
Assessment of cell viability and proliferation
MSCs encapsulated in alginate containing either 10, 100, or 500 μg fibronectin/ml alginate were cultured in vitro for 1 month, during which the viability of the cells was monitored with a LIVE/DEAD assay, and compared to cells encapsulated without fibronectin. Initially, no significant difference was observed (). However, after 2 weeks of culture, the viability of cells enclosed within 100 μg/ml fibronectin and 500 μg/ml fibronectin was significantly higher than those in control microcapsules. Interestingly, 10 μg/ml fibronectin did not significantly affect cell viability. This difference in viability persisted until day 28, when the viability of control and 10 μg/ml fibronectin microcapsules dropped to 54 ± 2 and 61 ± 2%, respectively. In contrast, viability remained significantly high for cells within 100 μg/ml fibronectin (67 ± 2%), and 500 μg/ml fibronectin (76 ± 7%) relative to control (P < 0.05) ().
Figure 1. Viability of CB MSCs. The effect of incorporation of 10, 100, and 500 μg/ml fibronectin on (A) % viability of the encapsulated cells and (B) viable cells (% control). Data are means ± SD, n = 4, Student's t-test. *Significant difference of 100 μg/ml fibronectin and 500 μg/ml fibronectin from control, **Significant difference of 100 μg/ml fibronectin and 500 μg/ml fibronectin from 10 μg/ml fibronectin, P < 0.05.
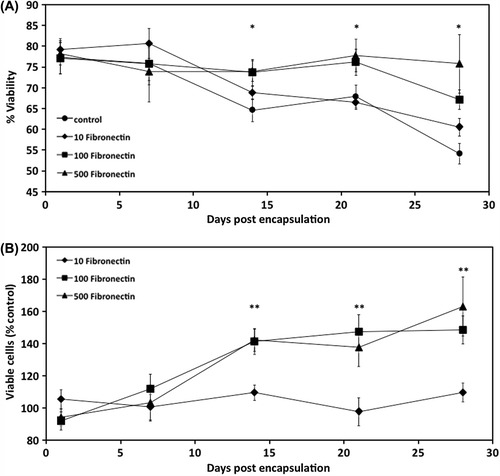
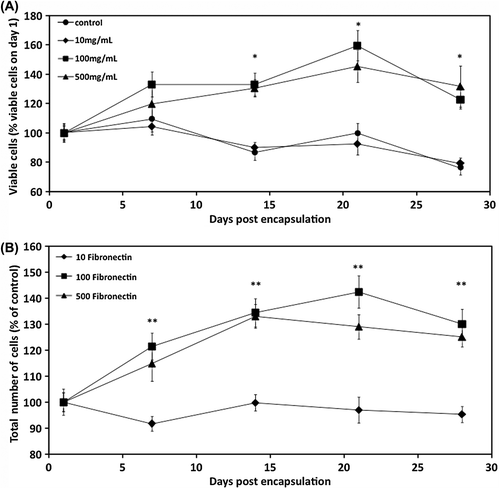
To better analyze cell viability, the total number of viable cells in each fibronectin-supplemented group was normalized to the total number of viable cells in the control group. After 2 weeks of culture, 100 and 500 μg/ml fibronectin, but not 10 μg/ml fibronectin, significantly improved the number of viable cells compared to the control group ().
Additionally, incorporation of 100 and 500 μg/ml fibronectin into the alginate microcapsules enhanced proliferation of the encapsulated MSCs during the 28-day culture. After 2 weeks, the number of viable cells (% number of viable cells on day 1) in 100 and 500 μg/ml fibronectin was comparable and was significantly higher than the control group (). By day 28, the number of cells was 30% higher than the control. In contrast, 10 μg/ml fibronectin did not affect the total number of cells significantly ().
Figure 2. Proliferation of CB MSCs. (A) The effect of fibronectin-alginate microcapsules on viability of encapsulated cells. (B) Comparison of total number of cells in fibronectin-alginate microcapsules. Cell proliferation was calculated using the LIVE/DEAD viability assay. Data are means ± SD, n = 4, Student's t-test. *Significant difference of 100 μg/ml fibronectin and 500 μg/ml fibronectin from control, **Significant difference of 100 μg/ml fibronectin and 500 μg/ml fibronectin from 10 μg/ml fibronectin, P < 0.05.
FIX secretion from encapsulated cells
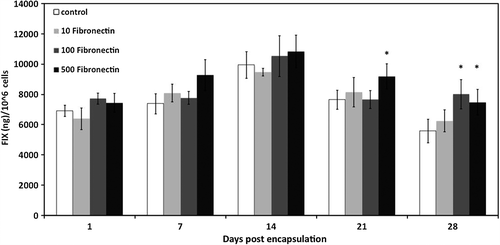
Consistent with improved viability and proliferation, incorporation of 100 and 500 μg/ml fibronectin within the alginate matrix resulted in enhanced FIX secretion from the encapsulated cells. As measured by ELISA, FIX secretion from 100 μg/ml fibronectin and 500 μg/ml fibronectin was above 3000 ng/ml (capsules)/24 h on day 14 and remained above 2000 ng/ml (capsules)/24 h after 28 days of in vitro culture. On the other hand, FIX secretion from 10 μg/ml fibronectin and control groups was approximately 2000 ng/ml (capsules)/24 h on day 14 and decreased to around 1000 ng/ml (capsules)/24 h on day 28 ().
Figure 3. FIX secretion by CB MSC. Comparison of FIX secretion from MSCs in non-supplemented (control), and fibronectin-supplemented microcapsules (10 μg/ml, 100 μg/ml, 500 μg/ml fibronectin). FIX secretion was measured using ELISA assay and reported as (A) the amount of FIX (ng) secreted from 1 ml of microcapsules in 24 h. (B) The amount of FIX secretion from fibronectin-alginate microcapsules/the amount of FIX secretion from control microcapsules. Data are means ± SD, n = 4. P < 0.05, Student's t-test. *Significant difference of 100 μg/ml fibronectin and 500 μg/ml fibronectin from control, **Significant difference of 100 μg/ml fibronectin and 500 μg/ml fibronectin from 10 μg/ml fibronectin, P < 0.05.
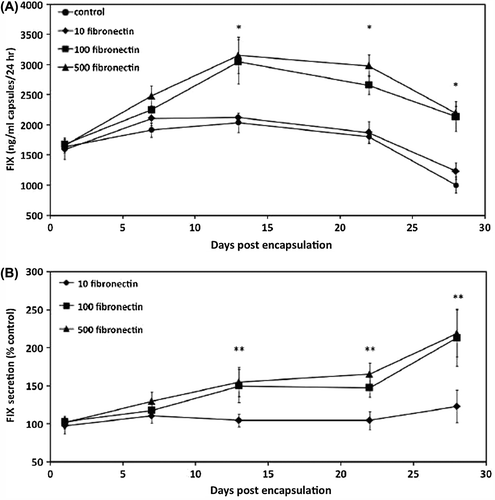
To better compare the FIX secretion profile from different microcapsules, FIX secretions were normalized with respect to the FIX secretion value from the control microcapsules (). After 2 weeks of culture, FIX secretion from 100 and 500 μg/ml fibronectin, but not 10 μg/ml fibronectin, was significantly higher than FIX secretion from the control. On day 28, FIX secretion from 100 and 500 μg/ml fibronectin reached about 210% of FIX secretion from the control and 10 μg/ml fibronectin.
An aim of this study was to detect whether or not the enhanced FIX secretion profile in 100 and 500 μg/ml fibronectin was caused by an increase in the number of viable cells in these two microcapsule systems. To address this, we introduced a parameter called the FIX secretion index (defined as the amount (ng) of FIX secreted from 106 viable cells in a 24-h period) and compared its value among 10, 100, and 500 μg/ml fibronectin, and control microcapsules during 1-month in vitro culture. As shown in , FIX secretion index on day 28 in 100 and 500 μg/ml fibronectin was significantly higher than that of the control. This confirms the positive effects of these two microcapsule systems on the FIX protein secretion capability of the enclosed cells in addition to their enhancing effect on viability and proliferation.
Cell–matrix analysis
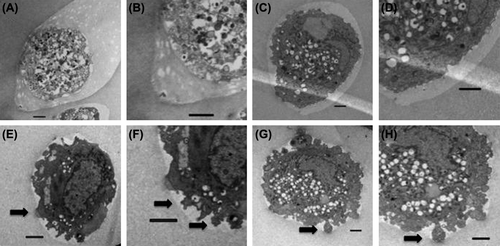
Cell-cytoskeleton studies by confocal microscopy revealed that cells in all encapsulated groups showed a rounded morphology with no distinct alignment of F-actin filaments (data not shown). In order to better assess the cell–matrix interactions, encapsulated MSCs were examined by transmission electron microscopy (TEM; ). Cells encapsulated in both 100 and 500 μg/ml fibronectin demonstrated the presence of filopia-like membrane extensions into the surrounding matrix (), which were observed to a lesser extent in 10 μg/ml fibronectin () and were not observed in the control group ().
Figure 5. Transmission electron microscopy images of CB MSCs encapsulated in control microcapsules (A, B); 10 μg/ml fibronectin microcapsules (C, D); 100 μg/ml fibronectin microcapsules (E, F); 500 μg/ml fibronectin microcapsules (G, H). Cells were fixed 10 days after encapsulation and processed for electron microscopy. Bars indicate 2 microns.
Effect of differentiation induction on cell viability and FIX secretion
It is well known that MSCs have the capacity to differentiate into osteogenic, chondrogenic, and adipogenic lineages. Understanding the effect of differentiation induction on cell viability and functionality would allow modulation of cell differentiation status before implantation for optimum functionality. Therefore in this study, we assessed the effect of the differentiation induction on cell viability, proliferation and FIX secretion.
Since 100 and 500 μg/ml fibronectin microcapsules were not significantly different from each other in terms of cell viability, proliferation and FIX secretion, microcapsules with 100 μg/ml fibronectin were used as a model for differentiation analysis.
Upon culture in osteogenic, chondrogenic, and adipogenic media, the total number of viable cells grown in differentiation media was normalized with respect to the total number of viable cells in the basal medium (). The viability of MSCs cultured in osteogenic medium was not significantly different from that of cells grown in basal medium but was significantly higher than the viability of the cells grown in chondrogenic or adipogenic media, suggesting the inability of the 100 μg/ml fibronectin alginate microcapsules to effectively support chondrogenic and adipogenic differentiation of MSCs.
Figure 6. Viability and FIX secretion of CB MSCs in differentiation media. (A) Number of viable MSC in 100 μg/ml fibronectin microcapsules cultured in differentiation media normalized to the number of viable cells grown in basal media. (B) Comparison of FIX secretion from the MSCs in 100 μg/ml fibronectin microcapsules grown in differentiation media. Data are means ± SD, n ≥ 3. Viable cells were measured calculated using the LIVE/DEAD viability assay. FIX secretion was measured using ELISA. P < 0.05, Student's t-test. *Significant difference from 100%.
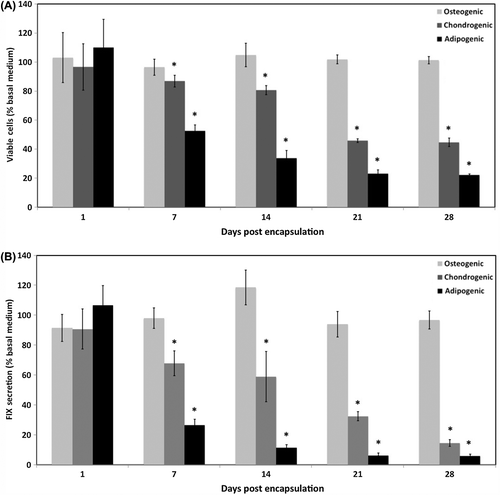
The effect of differentiation induction on FIX secretion from encapsulated cells was also investigated. The low FIX secretion from the encapsulated cells grown in chondrogenic and adipogenic media is consistent with the lower number of viable cells in these conditions compared with the number of viable cells in basal or osteogenic media. Similarly, and consistent with cell viability data, FIX secretion from the cells grown in osteogenic medium was not significantly different from the FIX secretion from the cells cultured in basal medium (), a finding which is also supported by comparison of the FIX secretion index.
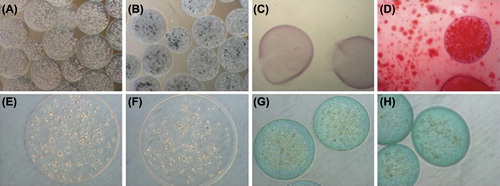
MSCs grown in 100 μg/ml fibronectin-alginate suspension cultures were also evaluated for their ability to differentiate into the three mesodermal lineages namely osteocytes, chondrocytes, and adipocytes (). Calcium deposits were evident in the microcapsules grown in osteogenic culture even without staining (), but their presence of was further confirmed by Alizarin Red, 3 weeks post-encapsulation (). In contrast, encapsulated cells grown in chondrogenic or adipogenic media did not differentiate into chondrocytes or adipocytes (as detected using Alcian Blue and Oil Red-O, respectively, 2 weeks post-encapsulation) ().
Figure 7. Differentiation potential of microencapsulated FIX-engineered CB MSCs. (A–D) Osteogenic differentiation analysis, (A) unstained in basal medium, (B) unstained in osteogenic medium, stained with Alizarin Red in basal (C) and osteogenic medium (D); (E, F) Adipogenic differentiation analysis, stained with Oil Red-O in basal (E) and adipogenic medium (F); (G, H) Chondrogenic differentiation analysis, stained with Alcian Blue in basal (G) and chondrogenic medium (H).
Discussion
In this study we investigated the functionality of FIX-engineered CB MSCs in biomimetic fibronectin-alginate microcapsules. We further evaluated the effect of different concentrations of supplemented fibronectin on the behavior of the encapsulated cells. Our data indicates that incorporation of 100 and 500 μg/ml fibronectin, but not 10 μg/ml, within the alginate microcapsules reduced cell death and significantly enhanced cell viability and proliferation compared to the non-supplemented control group. CitationKaroubi et al. (2009) have previously reported enhanced viability and greater metabolic activity of human marrow stromal cells encapsulated in agarose microcapsules incorporated with fibrinogen and fibronectin molecules due to re-introduction of cell–matrix interactions. We have also previously reported enhanced viability of MSCs encapsulated in RGD-alginate or fibrinogen-alginate microcapsules (CitationSayyar et al. 2012, Citation2013). Improved proliferation was also detected for cells encapsulated within protein-supplemented alginate (fibrinogen-alginate) but not for the cells within RGD-coupled alginate. Although further studies are required to analyze the mechanisms of cell–matrix interactions, we hypothesize that as a cell adhesion molecule, fibronectin has the advantage of providing the cells with matrix cues for enhanced interaction and subsequent survival and proliferation. MSCs are able to attach to fibronectin through several integrins, which may activate the survival pathways phosphoinositide 3-kinase (PI3-kinase) and mitogen-activated protein kinase (MAPK). Integrins can activate the MAPK pathway by either of two cascades, one of which involves focal adhesion kinase (FAK) and the other the adaptor protein Shc (CitationWhitlock et al. 2000). It has been previously demonstrated that integrins αvβ3 and α5β1, the binding receptors of fibronectin, are among the few integrins that recruit and activate Shc and play a critical role in the survival of adherent MSCs (CitationStupack and Cheresh 2002, CitationZvibel et al. 2002). CitationKaroubi et al. (2009) reported that increased viability of encapsulated MSCs in fibrinogen and fibronectin-supplemented agarose is likely via the Shc signaling pathway, an essential intermediate of the MAPK cascade. Therefore, it is conceivable that the enhancement of MSC proliferation and viability observed in this study was modulated by MSCs interacting directly with fibronectin.
It is widely accepted that cell shape reveals critical information about the adherent cells (CitationMcBeath et al. 2004, CitationKilian et al. 2010, CitationDolatshahi-Pirouz et al. 2011). A non-flattened and round shape is typical for non-proliferating cells which are likely to enter the apoptosis pathways while a well-spread cell shape is an indicator of a healthy cell (CitationDolatshahi-Pirouz et al. 2011). Based on our data, we postulate that the presence of 100 and 500 μg/ml fibronectin molecules within the alginate microcapsules results in the formation of filopia-like membrane extensions into the matrix demonstrating cell attachment. These membrane extensions were significantly less evident for the cells encapsulated in 10 μg/ml fibronectin and not detectable for cells in control group. Fibronectin molecules potentially re-introduce the necessary cell–matrix interactions required for cell survival and proliferation. However, our cell-cytoskeleton studies using confocal microscopy demonstrated that cells in all encapsulated groups were rounded with no distinct alignment of F-actin filaments. It is possible that other concentrations of alginate or chemical cross-linking the fibronectin molecules to the alginate matrix may result in stronger cell–matrix attachment that can be detectable by confocal analysis.
Encapsulation of FIX-expressing MSCs in 100 and 500 μg/ml fibronectin microcapsules resulted in enhanced FIX secretion, which was not detected in cells cultured in 10 μg/ml fibronectin or control microcapsules. Although the FIX secretion dropped after 2 weeks of culture, perhaps due to the decreased viability of cells at the core of microcapsules, FIX secretion remained significantly higher from 100 and 500 μg/ml fibronectin microcapsules compared to 10 μg/ml fibronectin and control microcapsules. It is noteworthy that the enhanced FIX secretion from the 100 and 500 μg/ml fibronectin microcapsules was not due only to the increased number of viable cells in these two types of microcapsules. Indeed, on day 28, FIX secretion index in 100 and 500 μg/ml fibronectin, but not in 10 μg/ml, was significantly higher than that of the control (). Therefore, 100 and 500 μg/ml fibronectin microcapsules also enhanced protein secretion.
Understanding the effects of differentiation induction on encapsulated cell behavior would allow modulation of cell differentiation status before implantation for optimum functionality. Cells encapsulated in 100 μg/ml fibronectin and cultured in osteogenic or basal media exhibited enhanced viability, proliferation, and FIX secretion compared to encapsulated cells grown in chondrogenic or adipogenic media. Also, the FIX secretion index revealed that encapsulated cells grown in basal and osteogenic medium had the same FIX index values. Therefore, 100 μg/ml fibronectin not only supported osteogenic differentiation of encapsulated MSCs, but also maintained their FIX secretion. The presence of calcium deposits confirmed the support of osteogenic differentiation in suspension culture. In contrast, differentiation into chondrocytes or adipocytes was not fully achieved, in agreement with our recent findings with fibrinogen-alginate microcapsules (CitationSayyar et al. 2012). Consistently, it was previously reported that cell aggregates are needed to induce chondrogenic differentiation in MSCs in 3D cultures (CitationGoren et al. 2010). The low cell viability in adipogenic media was predicted based on results from our previous monolayer differentiation studies (CitationSayyar et al. 2012) and was expected due to the inefficiency of CB MSCs to differentiate into the adipogenic lineage (CitationRebelatto et al. 2008).
Conclusions
Various fibronectin concentrations (10, 100, and 500 μg/ml) were incorporated in an alginate matrix to evaluate their effect on encapsulated MSCs in terms of their effect on cell viability, proliferation, and FIX secretion. The highest fibronectin concentrations, 100 and 500 μg/ml, had a positive effect on cell viability, proliferation as well as FIX secretion. In contrast, the low 10 μg/ml fibronectin dose did not significantly affect cell viability or protein secretion.
Differentiation studies confirmed that 100 μg/ml fibronectin alginate microcapsules support osteogenic (but not chondrogenic or adipogenic) differentiation of cultured encapsulated MSCs, indicating their potential for cell-based gene therapies for hemophilia B. Future work ought to address the characterization of mechanisms of cell–matrix interactions of MSCs in a fibronectin matrix.
Acknowledgements
Thanks to Marcia Reid and Marnie Timleck for processing the TEM and SEM samples.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was funded in part by a grant from Canadian Blood Services to G. H. and A. J.-W. and from NSERC IDEM CREATE to G. H. and B. S.
References
- Bolton-Maggs PH, Pasi KJ. 2003. Haemophilias A and B. Lancet. 361:1801–1809.
- Chang PL, Hortelano G, Awrey DE, Tse M. 1994. Growth of recombinant fibroblasts in alginate microcapsules. Biotechnol Bioeng. 43:925–933.
- Chang TM. 1964. Semipermeable microcapsules. Science. 146: 524–525.
- Chang TMS. 2005. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 4:221–235.
- Check E. 2003. Harmful potential of viral vectors fuels doubts over gene therapy. Nature. 423:573–574.
- Cieslinski DA, David Humes H. 1994. Tissue engineering of a bioartificial kidney. Biotechnol Bioeng. 43:678–681.
- Dolatshahi-Pirouz A, Jensen TH, Kolind K, Bünger C, Kassem M, Foss M, Besenbacher F. 2011. Cell shape and spreading of stromal (mesenchymal) stem cells cultured on fibronectin coated gold and hydroxyapatite surfaces. Colloids Surf B Biointerfaces. 84:18–25.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8:315–317.
- Goren A, Dahan N, Goren E, Baruch L, Machluf M. 2010. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. FASEB J. 24:22–31.
- Hortelano G, Al-Hendy A, Ofosu FA, Chang PL. 1996. Delivery of human factor IX in mice by encapsulated recombinant myoblasts: a novel approach towards allogeneic gene therapy of hemophilia B. Blood. 87:5095–5103.
- Hortelano G, Xu N, Vandenberg A, Solera J, Chang PL, Ofosu FA. 1999. Persistent delivery of factor IX in mice: gene therapy for hemophilia using implantable microcapsules. Hum Gene Ther. 10:1281–1288.
- Hortelano G, Wang L, Xu N, Ofosu FA. 2001. Sustained and therapeutic delivery of factor IX in nude haemophilia B mice by encapsulated C2C12 myoblasts: concurrent tumourigenesis. Haemophilia. 7:207–214.
- Karoubi G, Ormiston ML, Stewart DJ, Courtman DW. 2009. Single-cell hydrogel encapsulation for enhanced survival of human marrow stromal cells. Biomaterials. 30:5445–5455.
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. 2010. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA. 107:4872–4877.
- Koo J, Chang TM. 1993. Secretion of erythropoietin from microencapsulated rat kidney cells: preliminary results. Int J Artif Organs. 16:557–560.
- Lim F, Sun AM. 1980. Microencapsulated islets as bioartificial endocrine pancreas. Science. 210:908–910.
- Marshall, E. 2001. Gene therapy. Panel reviews risks of germ line changes.Science. 294:2268–2269.
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 6:483–495.
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. 2011. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 365:2357–2365.
- Orive G, Hernández RM, Gascón AR, Calafiore R, Chang TM, De Vos P, et al. 2003. Cell encapsulation: promise and progress. Nat Med. 9:104–107.
- Orive G, Hernández RM, Rodríguez Gascón A, Calafiore R, Chang TM, de Vos P, et al. 2004. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 22:87–93.
- Orive G, De Castro M, Kong HJ, Hernández RM, Ponce S, Mooney DJ, Pedraz JL. 2009. Bioactive cell-hydrogel microcapsules for cell-based drug delivery. J Control Release. 135:203–210.
- Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, et al. 2008. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 233:901–913.
- Sadler J, Davie E. 1987. The metabolic basis of inherited disease. In Hemophilia A, hemophilia B, and von Willebrand's disease. New York: W.B. Saunders. pp. 575–598.
- Sayyar B, Dodd M, Wen J, Ma S, Marquez-Curtis L, Janowska-Wieczorek A, Hortelano G. 2012. Encapsulation of factor IX–engineered mesenchymal stem cells in fibrinogen–alginate microcapsules enhances their viability and transgene secretion. J Tissue Eng [online]. 3:2041731412462018. Available at: http://www.tej.sagepub.com/content/3/1/2041731412462018.long. Accessed on December 5 2013.
- Sayyar B, Dodd M, Marquez-Curtis L, Janowska-Wieczorek A, Hortelano G. 2013. Cell-matrix interactions of Factor IX (FIX)- engineered human mesenchymal stromal cells encapsulated in RGD-alginate vs. fibrinogen-alginate microcapsules. Artif Cells Nanomed Biotechnol [online]. E-pub ahead of print. Available at: http://www.informahealthcare.com/doi/abs/10.3109/21691401.2013.794354. Accessed on December 5 2013.
- Schoen FJ, Mitchell RN 1996. Biomaterials Science: An Introduction to Materials in Medicine. San Diego: Academic Press.
- Shin H, Jo S, Mikos AG. 2003. Biomimetic materials for tissue engineering. Biomaterials. 24:4353–4364.
- Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. 2006. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 24: 1254–1264.
- Stupack DG, Cheresh DA. 2002. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 115:3729–3738.
- Thakur A, Sengupta R, Matsui H, Lillicrap D, Jones K, Hortelano G. 2010. Characterization of viability and proliferation of alginate-poly-L-lysine-alginate encapsulated myoblasts using flow cytometry. J Biomed Mater Res Part B Appl Biomater. 94: 296–304.
- Wen J, Xu N, Li A, Bourgeois J, Ofosu FA, Hortelano G. 2007. Encapsulated human primary myoblasts deliver functional hFIX in hemophilic mice. J Gene Med. 9:1002–1010.
- Wen J, Vargas AG, Ofosu FA, Hortelano G. 2006. Sustained and therapeutic levels of human factor IX in hemophilia B mice implanted with microcapsules: key role of encapsulated cells. J Gene Med. 8:362–369.
- Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. 2000. Differential roles for alpha(M)beta(2) integrin clustering or activation in the control of apoptosis via regulation of akt and ERK survival mechanisms. J Cell Biol. 151:1305–1320.
- Zvibel I, Smets F, Soriano H. 2002. Anoikis: roadblock to cell transplantation? Cell Transplant. 11:621–630.