Abstract
The present study aimed to develop a kinetically stable nanoemulsion of artemether with improved solubility, stability and oral bioavailability. Nanoemulsion was prepared by ultrasonication technique using internal oil phase (consisted of the drug dissolved in coconut oil and span 80) and external phase (comprising tween 80 and ethanol dissolved in water). The formulations were optimized using various parameters like percentage transmittance, refractive index, drug content, viscosity, zeta potential and release rate. Stability studies were conducted for a period of 90 days using stability chambers. In vivo studies of the developed formulations were conducted on Wistar rats and data were analyzed statistically. The nanoemulsion as observed under transmission electron microscope were found to be spherical in shape with an average size of 79.0 nm and a zeta potential of −15 mV which indicated of good electrokinetic stability of nanoemulsion . Nanoemulsion was found to be clear and transparent in appearance with a percentage transmittance of 98.2. Refractive index of 1.32 of the nanoemulsion indicated the isotropic nature of the drug. Release rate of the drug from the nanoemulsion formulation was found to be quite significant (P < 0.001) as compared to the plain drug. In vivo oral bioavailability of the nanoemulsion formulation was found to be 2.6-fold higher than the plain drug (˜ 40%) as observed from pharmacokinetic studies. Thus it was observed that nanoemulsion proved itself as a promising alternate for improving the bioavailability of artemether.
Introduction
Solubility and intestinal permeability of drug particles are the two important characteristics to achieve the desired concentration of drug in the systemic circulation. To achieve the desired therapeutic response, a drug product must deliver drug particles at an optimal rate and extent to systemic circulation which ultimately depends upon the bioavailability of the drug molecules. It has been reported that only 8% of new drug molecules have high solubility and permeability (CitationMohanachandran et al. 2010). Therefore, low bioavailability of new chemical entities (NCEs) is the major problem during drug development process. The oral bioavailability depends on several factors including aqueous solubility, drug permeability, dissolution rate, first pass metabolism, pre-systemic metabolism and susceptibility of efflux mechanisms. Various approaches are being used for the bioavailability enhancement of the orally administered drugs. One of the useful technique consist of incorporation of the active lipophilic component into the inert lipid vehicles like liposomes (CitationNiu et al. 2012, CitationAllison and Gregoriadis 1974), niosomes (CitationJadon et al. 2009), nanostructure lipid carrier (NLC) (CitationZhuang et al. 2010), solid lipid nanoparticles (SLN) (CitationLuo et al. 2006), nanosuspensions (CitationWang et al. 2010), self-emulsifying drug delivery system (SEDDS) (CitationMin et al. 2010), nanoemulsion (CitationChhabra et al. 2011; CitationMcClements et al. 2007), etc. Among all these methods nanoemulsions seem to have attracted major attention because of their less toxicity and ability to increase the bioavailability of the drugs to many folds (CitationAhmed et al. 2012, CitationKuo et al. 2008). Nanoemulsion are isotropic mixture, kinetically stable, transparent or translucent oil globules dispersed in aqueous phase stabilized by an interfacial film of surfactant and co-surfactant molecules having thedroplet size less than 100 nm (CitationMason et al. 2006; CitationBouchemal et al. 2004). They have the greatest potential for the oral delivery of poorly water-soluble drugs by enhancing the solubility and protecting the active moiety from enzymatic degradation. Use of surfactants and co-surfactants in nanoemulsion formulation helps in changing the intestinal permeability which increases the absorption of the drugs (CitationAboofazeli 2010). One of the unique property of nanoemulsion is to overcome inter and intrasubject variations in patients (CitationJadhav et al. 2006; CitationGhosh and Murthy 2006).
Malaria is the most prevalent parasitic disease in the world, which is caused by the Apicomplex protozoan of the Plasmodium (P.) genus. There were an estimated 225 million cases of malaria worldwide in 2009 (CitationWHO report 2011). An estimated 781,000 people died from malaria in 2009 according to the World Health Organization's 2010 World Malaria Report, accounting for 2.23% of deaths worldwide (CitationWHO report 2011). Ninety percent of malaria related deaths occur in sub-Saharan Africa, with the majority of deaths being young children. P. falciparum, the most severe form of malaria, is responsible for the vast majority of deaths associated with the disease (CitationSnow et al. 2005) Among the cases that occurred outside Africa, 80% occurred in India, Myanmar, Bangladesh, Indonesia, Papua New Guinea and Pakistan (CitationWHO report 2011).
Artemether is a rapidly acting antimalarial agent and is generally classified under class IV drug according to Biopharmaceutic Classification Scheme (BCS). It is a potent and lipid soluble derivative of artemisinin (CitationTayade and Nagarsenker 2010). It is highly effective against the blood schizonts of both malarial parasites P. falciparum and P. vivax (CitationJoshi and Patravale 2008). It is highly lipophilic molecule having a partition coefficient (log P) (octanol/water) value of 3.07. Artemether constrains its therapeutic response due to its low oral bioavailability (∼40%). It has been reported that administration of artemether with fatty meals increases its bioavailability. A study suggested the use of liposomal formulation containing the beta-artemether and analyzed for their encapsulating capacity, chemical stability, leakage, in vitro release and therapeutic efficiency against P. chabaudi infection. The encapsulation efficiency was found to be nearly 100%, the drug being located in the lipid bilayers. This formulation was used to treat mice infected with the virulent rodent malaria parasite P. chabaudi and a 100% cure rate was achieved (CitationChimanuka et al. 2002). Therefore it was observed that lipid based delivery system such as liposomes, nanoemulsion seems to have greatest potential in improving the oral bioavailability of this drug. Parenteral administration of oily formulation of artemether leads to pain and poor patient compliance; however, no such kind of problem occurs on oral administration of artemether in the form of self microemulsifying drug delivery system (SMEDDs) (CitationMandawgade and Sharma 2008).
In the present study the bioavailability of Artemether was improved by delivering it in the form of nanoemulsion which was further characterized and evaluated for their efficacy using various parameters. Thus, the present study was focused on alleviating poor bioavailability problems associated with artemether by means of improving its solubility and permeability features.
Materials and methods
Materials
Artemether and coconut oil were obtained as gift samples from IPCA Lab. Pvt. Ltd (Mumbai, India). Giemsa's stain, dialysis bags (MW 12 kDa), methanol (HPLC grade) and eosin yellow dye were procured from Sigma-Aldrich (Mumbai, India). Span 80, tween 80, disodium hydrogen phosphate (AR grade), ethanol and orthophosphoric acid were procured from Central Drug House (CDH, New Delhi, India). Hydrochloric acid, potassium bromide (AR grade) were procured from Himedia laboratories Pvt Ltd. (Mumbai, India).
Preparation of nanoemulsion
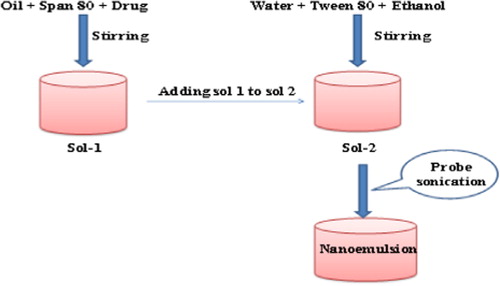
Nanoemulsions of artemether were prepared by ultrasonication method as described by CitationTang and Sivankur with minor modifications (2012). Briefly, solution of artemether (80 mg) in coconut oil (5% v/v) and span 80 (0.5% v/v) was taken in a beaker and mixed under continuous stirring. The resultant solution was added into aqueous phase containing tween 80 (5%) as emulsifier and ethanol (5% v/v) as co-solvent under continuous stirring at 3000 rpm for 3 min. The solution was then sonicated using ultrasonic probe sonicator (D.P. 120, Lark, USA) with 6 s/3 s on/off cycle for 3 min at 40% amplitude resulting in the formation of nanoemulsion. As probe sonication may damage sample so to mitigate the ultrasound thermal effect of probe sonicator, all the samples of pre-emulsions were kept in an ice bath throughout the experiment. The schematic process of nanoemulsion preparation is shown in .
Optimization of nanoemulsion
Nanoemulsion formulations were optimized on the basis of particle size, size distribution, ultrasonication time, concentration of surfactant, co-surfactant and co-solvent. Selection of oil was done on the basis of immediate formation of emulsion by stirring the formulation with glass rod. The formulations were then observed for few days to determine optimized concentration of oil (). Coconut oil was selected as it contains 77% saturated fatty acids content in comparison to soyabean oil which has only 15% saturated fatty acid content. Also coconut oil has good stability, good solvent capacity and is also resistant to oxidation which makes it an ideal candidate for the selection (CitationTalegaonkar et al. 2008). Surfactants were added to the nanoemulsion formulation to provide good solvency for the drug and stability to the formulation. They were further screened and optimized on the basis of the particle size and polydispersibility index (PDI) obtained from the resultant nanoemulsion formulation.
Table I. Selection of optimized concentration of oil.
Characterization of nanoemulsion
nanoemulsion were characterized in terms of various physicochemical parameters like morphology, refractive index, particle size and their distribution, zeta potential, percentage transmittance, dilution test, emulsifying time, dye test, drug content, viscosity and drug release. They were also tested for their stability and various pharmokinetic parameters such as Cmax, Tmax, AUC and mean residence time (MRT).
Morphological characteristics
The surface morphology of artemether loaded nanoemulsion was determined using transmission electron microscope (TEM) (Hitachi H-7500, USA).
Particle size
Globule size and size distribution (PDI) of optimized formulation was determined by photon correlation spectroscopy by using Delsa Nano C Zeta Sizer (Beckman coulter DelsaTM Nano C, USA). For the measurement of globule size and PDI, 2 ml of nanoemulsion was placed into cuvettes of Beckman coulter and measurements were recorded.
Particle charge (zeta potential)
Particle charge determines the physical stability of the nanoemulsion. Particle charge is quantified as zeta potential value which is measured via electrophoretic mobility of particles in an electrical field. Zeta potential of optimized formulation was measured using Beckman coulter Delsa™ Nano C particle analyzer, USA (CitationTamilvanan and Benita 2004).
Percentage transmittance
Percentage transmittance was determined using Shimadzu UV/VIS spectrophotometer (UV-1700 Pharma Spec, Shimadzu, Japan). One milliliter of the formulation was diluted 100 times using double distilled water and analyzed at 210 nm against water as blank.
Refractive index
Refractive index of selected formulations was determined using Abb type refractometer (Bellingham & Stanley Refractometer, RFM840 Type 26-840, USA).
Dilution test
Dilution test was performed in order to observe for the phase inversion of the nanoemulsion. For this 1 ml of optimized nanoemulsion was diluted with 10 ml of water in a test tube and observed for phase inversion (CitationShahnaz et al. 2011).
Dye solubility test (o/w test)
Few drops of water soluble dye (Eosin yellow) was added to 1 ml of nanoemulsion in an eppendrof and mixed properly. The formulation was observed under fluorescent inverted microscope (Olympus CK147, USA) (data not shown) (CitationKhoo et al. 1998).
Emulsifying time
Emulsification time was determined as per the method described by CitationKhoo et al. (1998) with slight modification. This was done by adding 0.3 ml of self-emulsifying oil formulations into a beaker containing 200 ml milli-Q water at 37°C. The sample was stirred and visually monitored to determine the time for complete emulsification.
Drug content
Drug content of the nanoemulsion formulation was carried out by dissolving 1 ml of the formulation in 10 ml of methanol. This formulation was then placed in shaking incubator (LSI-2005 RL, Lab Tech Co., Korea) (50 rpm at 37 ± 0.5°C) for 30 min. After 30 min supernatant was collected and analyzed using UV spectrophotometer (UV-1700 Pharma Spec, Shimadzu, Japan) at 210 nm against methanol as blank (CitationShahnaz et al. 2011).
Viscosity
Viscosity of nanoemulsion was determined using 1 ml of the formulation and speed of the spindle was adjusted to 100 rpm and a shear rate of 100 s−1 was applied at 37 ± 0.5°C for 10 min (CitationPal et al. 2007). Viscosity of the optimized nanoemulsion was determined using R/S CPS plus Rheometer (R/S+ Rheometer CPS, Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA).
Release study
In vitro release study
In vitro drug release of the formulation was performed in simulated intestinal fluid (SIF) (pH 6.8) using dialysis membrane (Chowhan and Amoro 1977, CitationYu et al. 2000). The membrane (pore size: 12 KD, Sigma Chemical Co., USA) was activated by keeping it in SIF overnight. It was exposed to running water for few hours to remove glycerin based contents. Nanoemulsion formulation (equivalent to 80 mg of drug) were loaded into the dialysis membrane and placed in a 150 ml of 1 N SIF (pH 6.8) in a shaking incubator (maintained at 50 rpm and 37 ± 0.5°C). Samples were withdrawn at predetermined intervals and replaced with same volume of the fresh medium. The samples were then filtered and assayed for the drug content at 210 nm on UV/Visible spectrophotometer against the blank.
Ex vivo drug release
Ex vivo drug release profile of entrapped drug from nanoemulsion was studied in SIF (pH 6.8) using everted sac method (Chowhan and Amoro 1977). In this technique rats were sacrificed and the abdomen was opened by midline incision. Small intestine was removed by cutting each end. The middle small intestine was obtained from the proximal end. The entire length of the small intestine was washed with saline solution to remove blood and debris. A narrow glass rod was inserted into one end of the intestine. Ligatures were tied over the thickened part of the glass rod and exert the sac by gently pushing the rod through the whole length of the intestine. The rod was then removed and intestine was placed in SIF at room temperature. Intestine of 4 cm length was tied off with thread and cut an open sac from the main length. Second ligature was placed loosely round the open end of the sac and a blunt needle was introduced attached to a syringe. The loose ligature was tightened over the needle and 2 ml of artemether nanoemulsion was injected into the sac; the ligature was tightened and needle was withdrawn. All the ligatures were firm enough to prevent leaks but not tightened so as to damage the tissue. The intestine was then placed in 150 ml of SIF (pH 6.8) in a shaking incubator (maintained at 50 rpm and 37 ± 0.5°C). Samples were then withdrawn at predetermined intervals and replaced with same volume of fresh media. The samples were then filtered and assayed for drug content at 210 nm against the blank using UV/Visible spectrophotometer. Mean results of triplicate measurements and standard deviation were reported (n = 3).
Drug-release kinetics and mechanism of drug release
The release kinetics of the drug from the nanoemulsion is described using four different kinetic models, that is, zero order, first order, Hixson–Crowell Model and Higuchi Model (CitationZellner et al., 1996). All of these models explain the release pattern to be dependent on some individual property. The mechanism of drug release was studied using Peppas equation as given below:
where Mt is the cumulative amount of drug released at time t, M∞ is amount release at infinite time, Km is a constant characteristic of the drug–polymer system, and n is the diffusion exponent suggesting the nature of the release mechanism.
Stability of nanoemulsion
Stability of the optimized nanoemulsion was determined by keeping at accelerated conditions of 40 ± 2°C and 75 ± 5% RH for 3 months (CitationBali et al. 2011). Samples were then withdrawn at the end of 0, 30, 60 and 90 days and analyzed for any change in the droplet size and drug content using UV/VIS spectrophotometer at 210 nm.
Thermal cycling
The physical stability of nanoemulsions was evaluated by subjecting the formulation to stress conditions of freeze thaw cycling and measuring the change in the particle size at definite time interval (CitationShafiq et al. 2007).
Centrifugation
The effect of centrifugation was studied to evaluate potential metastable conditions, including phase separation, creaming and/or drug precipitation. This was done by centrifuging each formulation of nanoemulsion at 2000 rpm for 30 min and visually observed for phase separation and drug precipitation. The appearance of the system was also observed microscopically. Secondly, nanoemulsion formulations were also subjected to stress conditions, temperature cycling consisting of 12 h refrigeration (4°C) followed by 12 h storage at room temperature (25°C) for a period of 1 week (CitationShahnaz et al. 2011; CitationEe et al. 2008). Formulations were then evaluated for phase separation or drug precipitation.
In vivo bioavailability study
The in vivo studies of the optimized formulations were assessed in adult Wister rats (200–250 g) of either sex. Animals were housed in groups of six (n = 6) with free access to food and water. The study protocol as approved by Institutional Animal Ethical Committee of ISF College of Pharmacy was followed. The studies were carried out as per the guidelines of Council for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India. The formulations (nanoemulsions and plain drug) were given orally at a concentration of 4 mg/kg body weight. The rats were anesthetized with ketamine and polyethylene cannula was placed in the jugular vein 5 days before initiation of the experiments. The cannula was exteriorized at the dorsal part of the neck (CitationStepensky et al. 2001). The blood samples were collected (0.2 ml) at 0.5, 1, 2, 4 and 8 h in heparinized eppendorfs and centrifuged at 5000 rpm for 10 min. The plasma was separated and stored in freezer (at −20°C) until further analysis. Plasma samples were collected from rats and were analyzed after adding 45 μg of artemether in each eppendroff using validated UV/VIS spectrophotometric method and drug plasma concentration values were determined from the calibration curve.
Pharmacokinetic study and statistical analysis
Pharmacokinetic (PK) parameters were calculated by non-compartmental analysis called as model independent analysis using Software Kinetica 5.0. All PK parameters (tmax, Cmax, AUC0–24, AUC0–α and MRT) were calculated individually. The PK data of different formulations were compared for statistical significance by two-way ANOVA (CitationZellner et al. 1996).
Results
The objective of the present study was to explore the potential of nanoemulsion as for the enhancement of bioavailability of artemether. For alleviating poor bioavailability problems associated with artemether by improving its solubility and permeability. Nanoemulsion formulations were prepared by ultrasonication technique and optimized in terms of various physicochemical parameters like morphology, particle size and their distribution, zeta potential, percentage transmittance, dilution test, dye solubility test, drug content, viscosity, in vitro and ex vivo drug release. Use of coconut oil increased the solubility of the drug and also prevented it from oxidation. Another critical component of the formulation was the surfactants since they were responsible to impart stability to the formulation. The use of one surfactant may hardly achieve transient negative interfacial tension and fluid interfacial film; so it is better to incorporate a co-surfactant (CitationBaboota et al. 2007). Generally, co-surfactants are responsible for lowering the interfacial bonding stress and predispose the interfacial film to occupy enough flexibility to assume various curvatures required for nanoemulsion formulation over a wide range of compositions, which can also be obtained by higher concentration of surfactants (CitationKawakami et al. 2002, CitationTalegaonkar et al. 2008). Non-ionic surfactants like tween 80 were used since they are less toxic and possess permeability enhancing property. Lipophilic surfactants such as span 80 were used as an emulsifier in order to provide better emulsification. In the present study surfactants and co-surfactants were optimized in terms of the particle size and PDI of the nanoemulsion obtained. It was found that tween 80 and span 80 give the best results over other surfactants in terms of particle size (192 ± 5.3 nm) and PDI (0.287 ± 0.02) of the nanoemulsion obtained. Optimized combinations of surfactants used in the formulation are shown in .
Table II. Selection of optimized concentration of surfactant (n = 3).
Optimization of sonication time was done in terms of particle size obtained. It was observed that particle size was reduced as the sonication time was increased upto 2 min; however, beyond 2 min the particle size seemed to increase which may be due to the agglomeration of the particles. Thus sonication time of 2 min was found to be sufficient for the formulation of the Nanoemulsion (). As co-solvents are capable of dissolving large quantities of the drugs (CitationNiu et al. 2012), different concentration of ethanol were screened and optimized in term of the particle size, PDI and drug release of the nanoemulsion obtained. Optimized concentration of co-solvent is shown in . It has been shown that co-solvent increase the solvent capacity of the drugs, dissolve large quantities of the hydrophilic surfactant in oil and increase the stability of nanoemulsion by wedging themselves between the surfactant molecules (CitationTalegaonkar et al. 2008). It was found that optimized concentration of ethanol was 5% v/v based on the particle size and PDI.
Table III. Optimization of sonication time.
Table IV. Optimized composition for final formulation.
Morphological evaluation
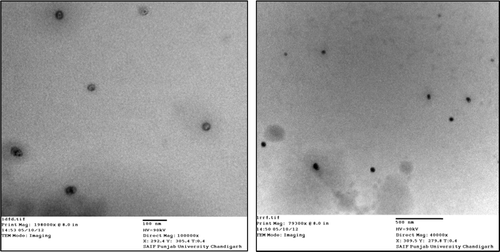
The morphology of the optimized nanoemulsion formulation was performed by TEM. From the TEM images of nanoemulsion (), it was observed that: 1) Particles were uniformly distributed, 2) Globules were spherical in shape with size less than 100 nm and 3) They were discrete and non-aggregated.
Particle size
Both particle size and size distribution (PDI) of nanoemulsion s are important in order to predict physical stability and in vivo rate of the drug as well as the carrier. Small particle size prevents any flocculation, enabling the system to remain dispersed with no separation. Particle size and size distribution (PDI) of the optimized artemether nanoemulsion formulation were determined by photon correlation spectroscopy method using particle size analyzer at room temperature and were found to be 79.0 ± 5.7 nm, 0.220 ± 0.05 and −15.54 ± 0.21 mV, respectively (). This nanometric size remained for long time even after 100 times dilution with water which proved systems compatibility with aqueous fluids. Enhanced absorption of nanoemulsion formulation may be attributed in terms of large specific area due to small particle size of nanoemulsion droplets.
Table V. Optimized formulation along with their parameters (± S.D., n = 3).
Zeta potential
Zeta potential signifies the charge on the particles which is indicative of the degree of repulsion between the “like charged particles” surrounding the adjacent particle. Zeta potential values attribute to the interactive forces between particles at the surfaces of nanoemulsion, which contributes to stability of nanoemulsion at macroscopic scale. In order to obtain electrostatically stabilized nanoemulsion, zeta potential values should be in the range of ± 30 mV (CitationMahajan and Dinger 2011). Here zeta potential value obtained was −15.54 ± 0.21 mV, which was indicative of electrostatically stabilized nanoemulsion. The negative charge of the artemether nanoemulsion is probably due to the anionic groups of the fatty acids and glycols present in the surfactant and co-surfactant. Thus, there are minimal chances of aggregation of nanoemulsion in the biological environment and during its shelf life. Optimized composition for final formulation and optimized formulation of nanoemulsion on the basis of particle size (PS), polydispersibility index (PDI), zeta potential (ZP) and percentage drug release (% DR) are shown in and , respectively.
Percentage transmittance
The percentage transmittance of the optimized artemether nanoemulsion was found to be 98.20 ± 0.7%. A value of percentage transmittance closer to 100% indicates that the optimized formulation was clear and transparent ().
Refractive index
Refractive index of the optimized nanoemulsion was found to be 1.320 ± 0.05, which was an indication of the isotropic nature of the nanoemulsion . Also no significant difference (P > 0.05) was observed in the refractive index values ().
Dilution test
Dilution test was performed on the optimized nanoemulsion (o/w) formulation and was observed for the phase inversion. Optimized artemether nanoemulsion (o/w) did not show any sign of phase inversion and any kind of precipitation. Thus, this test confirmed that the optimized formulation was stable.
Dye solubility test
In this test water soluble dye eosin yellow was added to the nanoemulsion and observed under the microscope. It was found that the continuous aqueous phase was labeled with eosin yellow dye while the dispersed oily phase remained unlabeled. This test confirmed that nanoemulsion formed was o/w type.
Emulsification time
The rate of emulsification was too fast (within a few seconds) to be accurately measured. The high rate of emulsification precluded determination of a dissolution profile, as the formulations showed 70% drug release within the earliest time point measured (3 min) (). All formulation displayed a Newtonian flow pattern.
Drug content
Drug content of the artemether nanoemulsion was found to be 98.42 ± 0.87% which showed good drug loading capacity of the nanoemulsion, which was an essential requirement for the nanoemulsion ().
Table VI. Stability studies of artemether nanoemulsion under accelerated stability conditions.
Viscosity
Viscosity is an important criterion for nanoemulsion as it measures the physical stability of nanoemulsion. Viscosity of the optimized formulation was low and was found to be 17 ± 1.8 cPs ().
Drug release study
In vitro drug release of the plain drug and nanoemulsion were carried out in SIF (pH 6.8) and it was found that artemether (plain drug) owing to its crystalline nature, lipophilicity and low solubility in water showed maximum in vitro drug release of 37.5 ± 1.2% in 24 h while artemether nanoemulsion led to an increase in the dissolution rate with maximum drug release of 91.7 ± 1.8% in 24 h. This could be attributed to small droplet size which provided large surface area for the release of drug. Thus, this showed that nanoemulsion provided much better release profile (P < 0.001) as compared to the plain drug release ().
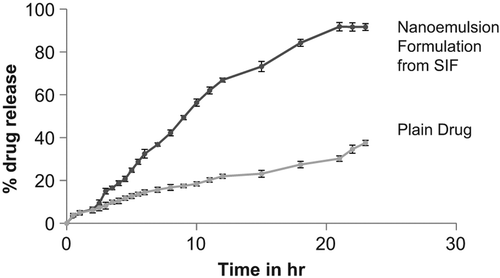
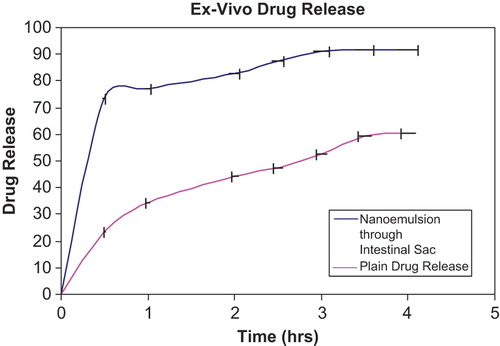
Ex vivo drug release profile from nanoemulsion was carried out in SIF (pH 6.8) using intestinal sac method and it was found that nanoemulsions exhibited 91.6 ± 0.9% in 4 h with a burst release of 73 ± 0.8% in first 30 min as compared to plain drug which was just 42.21 ± 0.23% after 4 h (). This initial burst effect observed may be accounted due to small globule size, and eventually higher surface area in case of nanoemulsion, which permit faster rate of drug release. On comparing the in vitro and ex vivo release profile of artemether nanoemulsion it was observed that approximately 91% of the drug was released within first 3 h in ex vivo (intestinal sac) studies whereas only 15% drug release was found in case of in vitro studies (from the dialysis bag).
Study of drug-release kinetics and mechanism of drug release
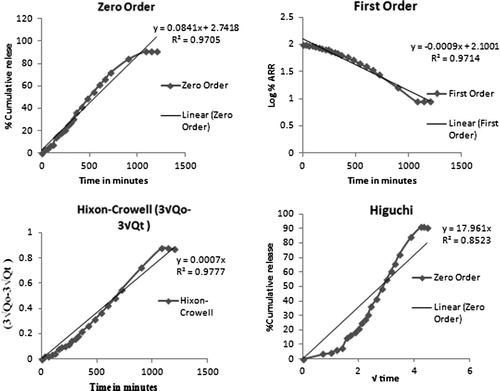
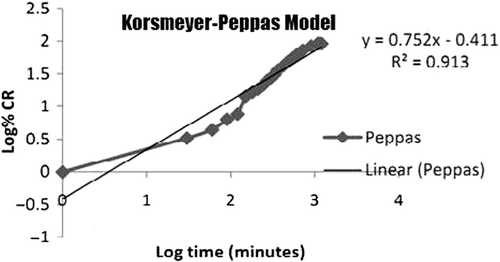
The release kinetics of the drug from the nanoemulsion is described using four different kinetic models. These models are used to evaluate the kinetic and mechanism of drug release from the nanoemulsion . The model that best fits the release data is selected based upon the correlation coefficient (r) value in various models. The model that gives high ‘r’ value is considered to be the best fit of the release data. In order to completely understand the kinetics of the drug release it was essential to apply the different kinetic models including zero order, first order, Hixson–Crowell and Higuchi curves. All these models for the optimized formulation are shown in . It was observed that optimized nanoemulsion followed Hixson–Crowell release kinetics (0.9777) which describes that the drug release occur by dissolution process and changes with the changes in the surface area and diameter of the particles occurred due to diffusion. Mechanism of drug release was studied using Korsmeyer–Peppas Model (). This model explains that whether release pattern follows Fickian diffusion or not and this can only be judged on the basis of the value of n where “n” is estimated from linear regression. If the value of n is 0.45 then release pattern follow Fickian diffusion. If the value of n is in between 0.45 and 0.89, then the release pattern follow non-Fickian diffusion (i.e., combination of both diffusion and erosion controlled release takes place) whereas if the value of n is greater than 0.89 it is indicative of case-2 relaxation or super case transport-2. It was observed that artemether nanoemulsion followed Korsmeyer–Peppas Model and the value of release exponent (n) obtained for the formulations was found to be 0.7529, which showed that the release pattern followed non-Fickian diffusion.
Stability studies on optimized nanoemulsion
Stability studies of the optimized formulations were carried out according to International Conference on Harmonization (ICH) guidelines. Selected formulations were stored at 40 ± 2°C and 75 ± 5% RH for a period of 3 months. Particle size and drug content of the optimized nanoemulsion were carried out at predetermined time intervals of 0, 30, 60 and 90 days. It was found that there was no significant change in the particle size, drug content and PDI of the nanoemulsions during the given time period () which shows that nanoemulsion formulations were stable and also remained clear and transparent.
In vivo bioavailability studies
To evaluate oral bioavailability of artemether nanoemulsion, formulations were administered to Wistar rats and studied for different pharmacokinetic parameters using UV/Vis spectrophotometer at 210 nm. Bioanalytical method was developed and validated using various parameters. Linear responses were obtained in the range of 45–115 μg/ml with correlation coefficient r2 = 0.9982. Results inferred that Beer's law was followed in these concentration ranges. Limit of detection and limits of quantification were found to be 40.51 and 13.2 μg/ml, respectively. Also the intra and interday precision were expressed in terms of percent relative standard deviation (% RSD) and was found to be 1.151 and 0.795, respectively for artemether nanoemulsion formulation. Thus all these results indicated that the developed bioanalytical method was simple, sensitive, specific, precise and reproducible and it could be used for in vivo bioavailability studies of the developed artemether nanoemulsion. Thus, all pharmacokinetic parameters were determined using above validated bioanalytical method. Results indicated that Tmax and Cmax of optimized formulation were 120 min and 0.986 μg/ml, respectively as compared to the plain drug which were 60 min and 0.46 μg/ml, respectively (). The difference in Tmax of the optimized nanoemulsion and plain drug were analyzed using two ways ANOVA and was found to be highly significant (P < 0.01). Similarly, the difference in Cmax of the optimized formulation was also significant (P < 0.001) as compared to the plain drug ().
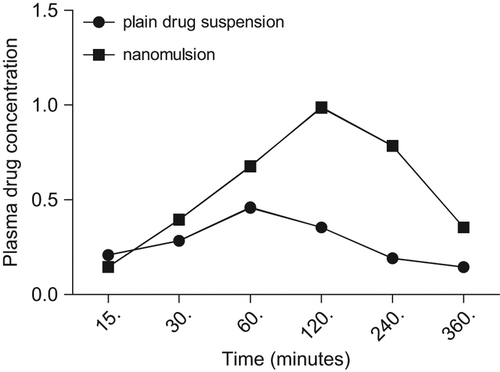
Table VII. Pharmacokinetic parameters of artemether nanoemulsions as well as for plain drug after oral administration*(n = 6).
Thus it was shown that plasma drug concentration profile of artemether nanoemulsion (0.986 μg/ml) was significantly higher than that obtained for the plain drug (0.46 μg/ml) ().
The improved oral bioavailability of the artemether may attribute to the following effects:
a) The drug present in the solubilized form as nanolipid globules provides large surface area between oil globules and intestinal epithelium of the villi which provides a direct contact with absorbing membranes of the gut and release the drug at the site of absorption avoiding its passage to the distal segments where the drug was non-absorbable.
b) The presence of surfactant and co-surfactant in nanoemulsion system in the gastrointestinal track (GIT) (air) might have caused changes in the intestinal permeability, which could have led to an enhancement of the oral absorption of the drug.
All these parameters proved the effectiveness of nanoemulsion in improving the bioavailability of the drug, artemether. Thus we can conclude that nanoemulsion would be a better alternative to overcome the bioavailability problems of artemether.
Discussion
Enhancement of the bioavailability and solubility of the poorly soluble drug is the main requirement for the design of new drug delivery system, which leads to increase in the efficiency of the existing drug. Currently, more than 40% of existing drug molecules suffer from problems of low solubility which leads to poor bioavailability, high intra/intersubject variability and lack of dose proportionality (CitationTang et al. 2008). Therefore, development of new delivery system to improve the solubility and bioavailability of the poorly soluble drug is the main requirement of these days. Lipid based drug delivery systems provide an advanced approach to enhance drug solubilization in the GIT thus to improve the oral bioavailability of poorly soluble drugs (CitationBaboota et al. 2007, CitationAmidon et al. 1995). Nanoemulsion is one of the most commonly used systems to overcome the solubility and bioavailability problem of the drugs. nanoemulsions increase the transparency, bioavailability and shelf life of many pharmaceutical drugs. Basically, nanoemulsions are clear, thermodynamically stable, isotropic liquid mixtures of oil, water, surfactant and co-surfactant (CitationKreilgaard et al. 2000). nanoemulsions are transparent or translucent preparations, having the droplet size of less than 100 nm with ultra low interfacial tension and long term physical stability (CitationAboofazeli 2010). Nanoemulsion provides a promising tool for increasing the aqueous solubility of poorly water-soluble drugs. nanoemulsions have many advantages like high drug solubility, good thermodynamic stability and ease of manufacturing (CitationPorecha et al. 2009). Thus design and development of nanoemulsions have been aimed for improving required bioavailability of the artemether. Nanoemulsion was selected as the choice of system for enhancing the bioavailability of the drug, artemether because of the compatibility with the manufacturing method and perceived benefits of the nanoemulsion in overcoming the bioavailability related issues. The preformulation study showed that the procured drug, artemether was in pure form and suggested the lipophilic nature of the drug. Nanoemulsion was prepared by ultrasonication method and optimized using various parameters. The optimized formulation comprised 4 mg/ml of drug with a stabilizer and emulsifier concentration of 5% v/v and 0.5%, v/v, respectively. The particle size of the optimized formulation was found to be 79.0 ± 5.7 nm with zeta potential −15.54 ± 0.21 mV. This nanometric size leads to enhanced absorption of nanoemulsion formulation which may be attributed to large specific area of the particles. Drug release of 91.7 ± 1.8% in 24 h attributed to small droplet size which provided large surface area for the release of drug. Ex vivo study showed that nanoemulsions showed 91.6 ± 0.9% drug release in 4 h with a burst release of 73 ± 0.8% in first 30 min. This initial burst release may be accounted due to their small droplet size, and eventually higher surface area which permit faster rate of drug release. The ex vivo model proved to be much better in comparison to in vitro, while selecting a model for in vivo study as it can mimic the release of drug in better way. It was observed that artemether nanoemulsion followed Korsmeyer–Peppas Model and the value of release exponent (n) showed that the release pattern followed non-Fickian diffusion. Stability study showed that all the formulations maintained stability both during the centrifugation and refrigeration/warming cycle protocols. This may be a clued that this formulation strategy is superior to macroemulsions or colloidal formulations. Oral bioavailability of the artemether nanoemulsion was evaluated by UV/VIS spectrophotometer. The developed method was simple, linear, precise and reproducible. The plasma concentration time profile of artemether from nanoemulsion represented greater improvement of drug absorption than the simple drug suspension. Oral bioavailability of the artemether in the nanoemulsion drug delivery system was increased by 2.6-folds as compared to bioavailability of plain drug which was just near to 40%. Thus the present study established nanoemulsion formulation as promising alternatives to traditional oral formulations of artemether to improve its bioavailability.
Conclusion
Nanoemulsions have great potential for the oral delivery of poorly water-soluble drugs by enhancing their solubility and protecting the active moiety from the enzymatic degradation. They possess unique advantage over other micellar solutions such as higher solublization capacity, non-toxic and non-irritant nature, and they do not damage human or animal cells. They have the capability to improve solubility, bioavailability of the hydrophobic drugs to a greater extent (CitationMason et al. 2006). Use of surfactants and co-surfactants in nanoemulsion aids to change the intestinal permeability of the drug which increases the absorption of the drugs. One of the unique property of nanoemulsion is their ability to overcome inter and intrasubject variations. The present work was aimed to enhance the bioavailability of artemether by formulating it into nanoemulsion form. The absorption of artemether from nanoemulsion form resulted in 2.6-fold increase in bioavailability as compared to plain drug. The results indicated that nanoemulsion provided a promising tool to enhance the oral bioavailability of artemether while avoiding the side effects like poor systemic availability. Results proved that the nanoemulsion could be used for the delivery of hydrophobic compounds with higher drug loading capacity. Present study ensured the significant potential of nanoemulsion, in improvising bioavailability of artemether. Thus nanoemulsion may pave a way for enhancement in the bioavailability, subsequent decrease in side effects and an improvement in patient compliance.
Acknowledgement
The authors are thankful to Chairman of I.S.F College of Pharmacy for their constant encouragement, support and inspiration to carry out this study.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aboofazeli R. 2010. Nanometric-scaled emulsions (Nanoemulsions). Iran J Pharm Res. 9:325–326.
- Ahmed K, Li YJ, McClements D, Xiao H. 2012. Nanoemulsion and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 132:799–807.
- Allison AC, Gregoriadis G. 1974. Liposomes as immunological adjuvants. Nature. 252:2.
- Amidon GL, Lennernas H, Shah VP, Crison JR. 1995. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 12:413–420.
- Baboota S, Shakeel F, Ahuja A, Ali J, Shafiq S. 2007. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 57: 315–332.
- Bali V, Ali M., Ali J. 2011. Nanocarrier for the enhanced bioavailability of a cardiovascular agent: In vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int J Pharm. 403:46–56.
- Bouchemal K, Briançon S, Perrier E, Fessi H. 2004. Nanoemulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int J Pharm. 280:241–251.
- Chhabra G, Chuttani K, Mishra AK, Pathak K. 2011. Design and development of nanoemulsion drug delivery system of amlodipine besilate for improvement of oral bioavailability. Drug Dev Ind Pharm. 8:907–916.
- Chimanuka B, Gabriels M, Detaevernier MR, Plaizier-Vercammen JA. 2002. Preparation of beta-artemether liposomes, their HPLC-UV evaluation and relevance for clearing recrudescent parasitaemia in Plasmodium chabaudi malaria-infected mice. J Pharm Biomed Anal. 28:13–22.
- Chowhan ZT, Amoro AA. 1997. Everted rat intestinal sacs as an in vitro model for assessing absorptivity of new drugs. J Pharm Sci. 66: 1249–1253.
- Ee SL, Duan X, Liew J, Nguyen QD. 2008. Droplet size and stability of nano-emulsions produced by the temperature phase inversion method. Chem Eng J. 140:626–631.
- Ghosh PK, Murthy RS. Microemulsions: a potential drug delivery system. 2006. Curr Drug Deliv. 3:167–180.
- Jadhav KR, Shaikh IM, Ambade KW, Kadam VJ. 2006. Applications of micro emulsion based drug delivery system. Curr Drug Deliv. 3:267–273.
- Jadon PS, Gajbhiye V, Jadon RS, Kavita R, Gajbhiye KR, Ganesh N. 2009. Enhanced oral bioavailability of Griseofulvin via Niosomes. AAPS Pharm Sci Tech. 10:1186–1192.
- Joshi MS, Patravale V. 2008. Formulation and evaluation of nanostructured lipid carrier (NLC) based gel of valdecoxib. Drug Dev Ind Pharm. 32: 911–918.
- Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, Masuda K. 2002. Microemulsion formulation for enhanced absorption of poorly soluble drugs, I. Prescription design. J Control Release. 81:65–74.
- Khoo SM, Humberstone AJ, Porter CJ, Edwards GA, Charman WN. 1998. Formulation design and bioavailability assessment of lipidicselfemulsifying formulations of halofantrine. Int J Pharm. 167: 155–164.
- Kreilgaard M, Pedersen EJ, Jaroszewski JW. 2000. NMR characterization and transdermal drug delivery potential of microemulsion systems. J Controled Release. 69:421–433.
- Kuo F, Subramanian B, Kotyla T, Wilson TA, Yoganathan S, Nicolosi RJ. 2008. Nanoemulsion of an anti-oxidant synergy formulation containing gamma tocopherol has enhanced bioavailability and anti-inflammatory properties. Int J Pharm. 363:206–213.
- Luo Y, Chen D, Ren L, Zhao X, Qin J. 2006. Solid lipid nanoparticles for enhancing vinpocetine's oral bioavailability. J Control Release. 114:53–59.
- Mahajan HS, Dinger SB. 2011. Design and In vitro evaluation of nanoemulsion for nasal delivery of artemether. IJNDD. 3:272–277.
- Mandawgade SD, Sharma S. 2008. Development of SMEDDS using natural lipophilic: application to beta-artemether delivery. Int J Pharm Sci Rev Res. 362:179–183.
- Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM. 2006. Nanoemulsion: formation, structure and physical properties. J Phy Cond Matt. 18:636–664.
- McClements DJ, Decker EA, Weiss J. 2007. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 72: R109–R124.
- Min S, Gao Y, Guo C, Cao F, Zhimei S, Xi Y, et al. 2010. Enhancement of transport of curcumin to brain in mice by poly (N-butylcyanoacrylate) nanoparticle. J Nanopart Res. 12: 3111–3122.
- Mohanachandran PS, Sindhumol PG, Kiran TS. 2010. Enhancement of solubility and dissolution rate: an overview. Int J Comp Pharm. 1:1–10.
- Niu M, Lu Y, Hovgaard L, Guan P, Tan Y, Lian R, et al. 2012. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: The effect of cholate type, particle size and administered dose. Eur J Pharm Biopharm. 81:265–272.
- Pal K, Banthia AK, Majumdar DK. 2007. Preparation and characterization of polyvinyl alcohol gelatin hydrogel. Membranes for Biomedical Applications. AAPS Pharm Sci Tech. 8:21.
- Porecha S, Shah T, Jogani V, Naik S, Misra A. 2009. Microemulsion based intranasal delivery system for treatment of insomnia. Drug Deliv. 16:128–134.
- Shafiq S, Faiyaz S, Sushma T, Farhan JA, Khar RK, Ali M. 2007. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 66:227–243.
- Shahnaz G, Hartl M, Barthelmes J, Leithner K, Sarti F, Hintzen F, et al. 2011. Uptake of phenothiazines by the harvested chylomicrons ex vivo model: Influence of self-nanoemulsifying formulation design. Eur J Pharmaceut Biopharmaceut. 79:171–180.
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 434:214–217.
- Stepensky D, Friedman M, Sroura W, Raz I, Hoffman A. 2001. Preclinical evaluation of pharmacokinetic-pharmacodynamic rational for oral CR metformin formulation. J Control Release. 71:107–115.
- Talegaonkar S, Azeem A, Ahmad FJ, Khar RK, Pathan SA, Khan ZI. 2008. Microemulsions: a novel approach to enhanced drug delivery. Recent Pat Drug Deliv Formul. 2:238–257.
- Tamilvanan S, Benita S. 2004. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur J Pharm Biopharm. 58: 357–368.
- Tang SY, Sivankur M. 2012. Anti-inflammatory and analgesic activity of novel oral aspirin-loaded nanoemulsion and nano multiple emulsion formulation formulation generated using Ultrasound cavitation. Int J Pharm. 430:299–306.
- Tang B, Chang G, Gu J, Xu CH. 2008. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov Today. 13:606–612.
- Tayade NG, Nagarsenker MS. 2010. Development and evaluation of artemether parenteral microemulsion. Indian J Pharm Sci. 72 :637–640.
- Wang Y, Zhang D, Liu Z, Liu G, Duan C, Jia L, et al. 2010. In vitro and In vivo Evaluation of silybin nanosuspension for oral and intravenous delivery. Nanotechnology. 21:155–161.
- World Malaria Report summary. World Health Organization. Retrieved 5 November 2011.
- Yu WP, Wong JP, Chang TMS. 2000. Sustained Drug Release Characteristics of Biodegradable Composite Poly (d,l) Lactic Acidpoly(I)Lactic Acid Microcapsules Containing Ciprofloxacin. Artif Cells Blood Substit Immobil Biotechnol. 28:39–55.
- Zellner D, Frankewitsch T, Simon S, Keller F. 1996. Statistical analysis of heterogeneous pharmacokinetic data from the literature. Eur J Clin Chem Clin Biochem. 34:585–589.
- Zhuang CY, Li N, Wang M, Zhang XN, Pan WS, Peng JJ, et al. 2010. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLCs) for improved oral bioavailability. Int J Pharm. 394:179–185.