?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Quercetin (3,5,7,3’,4’-pentahydroxyflavone) is a natural bio-flavonoid originating from fruits, vegetables, seeds, berries, and tea. The antioxidant activity of quercetin and its protective effects against cardiovascular disorders, anti-cancer, anti-inflammatory, and anti-viral activities have been extensively documented; however, the clinical request of quercetin in cancer treatment is significantly limited due to its very poor delivery features. In order to increase the hydrophilicity and drug delivery capability, we encapsulated quercetin into liposomes. Our data indicated that liposomal quercetin can significantly improve the solubility and bioavailability of quercetin and can be used as an effective antioxidant for ROS protection within the polar cytoplasm, and the nano-sized quercetin encapsulated by liposomes enhanced the cellular uptake (cancer cell human MCF_7). Quercetin has many pharmaceutical applications, many of which arise from its potent antioxidant properties. The present research examined the antioxidant activities of quercetin in polar solvents by a comparative study using reduction of ferric iron in aqueous medium, intracellular ROS/toxicity assays, and reducing DPPH assays. Cell viability and ROS assays demonstrated that quercetin was able to penetrate into the polar medium inside the cells and to protect them against the highly toxic and deadly belongings of cumene hydroperoxide.
The purpose of this study was to determine whether a liposomal formulation of quercetin can suggestively improve its solubility and bioavailability and can be a possible request in the treatment of tumor.
The authors encapsulated quercetin in a liposomal delivery system. They studied the in vitro effects of this compound on proliferation using human MCF-7 carcinoma cells. The activity of liposomal quercetin was equal to or better than that of free quercetin at equimolar concentrations. Our data indicated that liposomal quercetin can significantly improve the solubility and bioavailability of quercetin and can be a potential application in the treatment of tumor.
Introduction
Throughout the life, cells in all biological systems are unprotected to oxidation, which is created in all biological systems and is indication of the creation of free radicals. Free radicals are combinations that contain one or more unpaired electrons. These radicals are often reactive oxygen species (ROS) (CitationLefer and Granger 2000, CitationNelson et al. 2006). The creation of ROS causes central and effective changes in cell membranes (CitationErkoc and Erkoc 2002). One of the source factors that decrease ROS is antioxidant. Antioxidants are chemical combinations that can reduce the reactive radical intermediates that are shaped throughout the oxidative reactions (CitationKlein et al. 2006). Liposomes are spherical lipid vesicles with a bilayered membrane construction consisting of amphiphilic lipid molecules. They have been known as one of the most extensively used carriers for delivering a numerous pharmaceuticals, cosmeticeuticals, and imaging and diagnostics agents (CitationTorchilin 2005). As carriers for anticancer drugs, they have been revealed to decline side effect, such as anthracycline-induced cardiomyopathy (CitationBatist et al. 2001). Liposomes accrue specially at tumor sites for of their ability to extravasate through pores in the capillary endothelium. These pores seem to be an important rapid angiogenesis happening in tumors and are not usually present in organs or normal tissues (CitationYuan et al. 1995). Liposomes can transport both hydrophobic and hydrophilic agents with high effectiveness and defend them from undesired belongings of exterior situations. Antioxidant liposomes, spherical nanoparticles consisting of phospholipid bilayer(s) with an aqueous cubicle, own the unique capability to effectually deliver both water-soluble chemical antioxidants in the aqueous phase and lipid-soluble chemical antioxidants in the lipid bilayer (CitationStone et al. 2002, CitationStone and Smith 2004). Their surface can be freely functionalized with particular ligands that target liposomes and their loads to the locations of action. Furthermore, the size, composition, surface charge, and other formulation possessions of liposomes can be well-controlled to encounter the necessities of specific conditions (CitationLian and Ho 2001, CitationZhang et al. 2008, CitationBarenholz 2001). Liposomes are well recognized as drug carriers in current treatment of diseases. They can increase permeation of encapsulated hydrophobic drugs into the cell to let a suitable therapeutic influence. Liposomes as drug carriers have many benefits, such as drugs can be targeted to specific areas using ascribing ligands to the liposome, liposomes are easily engrossed by cells, as drug deliverers they allow potentially lower doses of drug to be used, the degree of drug release may be organized by the choice of liposome, and they reduce side effects and toxicity. Moreover, it is imaginable that gene therapy drugs may be delivered by liposomes (CitationSahoo and Labhasetwar 2003, CitationSharma and Sharma 1997). Quercetin is a flavonoid with anticancer possessions () (CitationPhromnoi et al. 2009). It is one of the most plentiful dietary flavonoids. Quercetin and its derivatives found about 99% of the flavonoids in apple peel (CitationHe and Liu 2008). Many in vitro and animal model studies using quercetin alone or quercetin in mixture with additional bioactive compounds have presented the anticancer activities of the complex (CitationAkbarzadeh et al. 2012a, CitationAkbarzadeh et al. 2012b, CitationAkbarzadeh et al. 2012c, CitationEbrahimnezhad et al. 2013, CitationDavaran et al. 2014a, CitationRezaei-Sadabady et al. 2013, CitationNejati-Koshki et al. 2013, CitationGhasemali et al. 2013). While quercetin has promising anti-cancer activities, its usage in anti-cancer treatments is limited because of its low aqueous solubility, low bioavailability, and variability under conditions met for the period of food/pharmaceutical products processing (temperature, light, and pH) or throughout storing (oxygen and light) (CitationDavaran et al. 2014b, CitationDavaran et al. 2013). Controlled delivery systems, such as nanoparticles, have shown their potential to control the release, defend, and upsurge the action of different bioactive complexes (CitationAhmadi et al. 2014, CitationAkbarzadeh et al. 2012d). Our hypotheses is that liposomal preparations improved the penetration of quercetin in the MCF-7. In the present work, encapsulation capacity of liposome is used. Two samples are created, first having free quercetin and second liposome incorporated quercetin (). In this study, MCF-7 cells were used as an in vitro cell model to study the intracellular efficiency of free and encapsulated quercetin as a protective agent against ROS injury and their behavior in this situation is studied. Extracellular free radical scavenging was assessed by reducing power. In this study, we prepared liposomes from a soybean lecithin and quercetin using the Bangham method (CitationValizadeh et al. 2012), and examined the trapping efficiency. We then investigated the particle size and dispersibility. We also evaluated the effects of liposomal quercetin on proliferation in human MCF-7 cell line. Therefore, the aim of this study was to encapsulate quercetin within liposomes nanoparticles, using a soybean lecithin and characterize their physicochemical and antioxidant properties. Liposomal quercetin has anti-proliferation on breast cancer MCF-7 cells by inhibiting their proliferation. The effects of the liposomal quercetin were a little better than those of liposomes alone and to free quercetin. MTT proliferation/survival assay was performed after 48 h of incubation and was found that liposomal quercetin inhibits the proliferation the breast cancer MCF-7 cells.
Materials and methods
Potassium ferricyanide and ferric chloride were acquired from Merck. Quercetin, trichloroacetic acid (TCA), and cumene hydroperoxide (CHP) were purchased Sigma Chemical Company (St. Louis, MO). Fetal bovine serum was purchased from GIBCO (Grand Island, NY). Antibiotics and sterile plastic ware for cell culture were purchased from Flow Laboratory (Irvine, UK).
Reagent
Quercetin (Q0125) was purchased from Sigma (St. Louis, MO). A stock solution of 50 mM quercetin was prepared in dimethyl sulfoxide (DMSO), aliquoted in single-use portions, and stored at –20°C. Unused portions of any froze aliquots were thrown away. Working dilutions of the stock was prepared in culture medium.
Cell lines
Human MCF-7 breast cancer cells were obtained from the American Type Culture Collection (Rockville, MD). MCF-7are estrogen-receptor positive human breast cancer cells. They were grown in RPMI 1640 supplemented with 10% (v/v) fetal bovine serum, penicillin, and streptomycin .These cells were incubated at 37°C with 5% CO2 and were usually sub-cultured every 3 days once.
Cell growth assays
Briefly, the human MCF-7 breast cancer cells grown in 6-well plates were treated with free and encapsulated quercetin for a period of 48 h. Cells harvested after trypsinization were recovered using a quick centrifugation. Immediate staining of (10,000) cells/well with trypan blue enabled rapid and clear identification of dead cells with deep blue staining and the living cells with less uneven staining of the cell membrane. Total numbers of viable cells from each treatment were recorded and were compared with the untreated controls. Three independent set of experiments were performed simultaneously for each treatment.
Study of anti-proliferation assay (MTT bioassay)
The effects of free and encapsulated quercetin on the growth and survival of human MCF-7 cell lines were measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay (CitationAkbarzadeh et al. 2013). Enduringly to monitor cell viability, MCF-7 cells (8.5 × 104 per well) were plated in 0.2 mL medium containing 10% fetal bovine serum in triplicate in 96-well plate, after 24 h medium was removed and then treated with 0.2 mL medium containing the indicated concentrations of free or encapsulated quercetin at 37°C for 48 h. Empty liposomes were added at concentrations equivalent to the lipid concentration found in the liposomal quercetin formulations. At the end of incubation, 0.020 mL of MTT solution (2 mg/mL) was added to each microwell. After 4 h of incubation with the reagent, the supernatant was removed and replaced with 200 μL of DMSO. The absorbance of the MTT formazan was determined at 570 nm in an enzyme-linked immunosorbent assay reader. The relative cell viability was expressed as a percentage of the control that was not treated with free quercetin or liposome-incorporated quercetin.
MTT method for anti-toxicity assay
Yellow MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole], is reduced to purple formazan in the mitochondria of living cells. The absorbance of this colored solution can be quantified by measuring at a certain wavelength (500–600 nm) using a spectrophotometer (CitationAkbarzadeh et al. 2013). Therefore, the request allows measuring the anti-toxicity of quercetin based on viability of cells. When the amount of purple formazan produced by the cells treated with an agent is compared with the amount of formazan created by the untreated control cells, the efficiency of the mediator in causing death of cells can be realized. MCF-7 cells were seeded in 6-well plates until they reached 95–100% meeting, then they were incubated for 30 min with different concentrations of free and encapsulated quercetin (0.0, 1.0, 5,10, or 50 μM). Thereafter, cells persuaded with 5 μl (1:100) CHP as a powerful cytotoxic ROS-generating compound (CitationPourhassan-Moghaddam et al. 2013) in a final volume of 1 ml PBS for 30 min. Control and treated cells were incubated with MTT at a final concentration of 1 mg mL− 1 for 4 h at 37°C. The cells were then scratched off and centrifuged at 1500 rpm for 5 min. The pellet was re-suspended in 250 mL phosphate-buffered saline (PBS) and sonicated on ice for 25 s with an Ultrasonic W-225R, at setting 4, and centrifuged in a microfuge at 13000 rpm for 10 min. The supernatant was cast-off and the water-insoluble formazan assay produced was dissolved in DMSO and measured at 560 nm. A decrease in cell number results in a decrease in the amount of MTT formazan shaped and a reduction in absorbance. Then, the percentages of cell viability were normalized and calculated with the following equation:
Preparation of phosphatidylcholine
The Bangham method was first used to prepare multilamellar liposomes (MLVs) (CitationValizadeh et al. 2012). We dissolved 30 mg of PCin chloroform and methanol and dried the solution in a rotary evaporator to obtain a dry film. The final lipid concentration in this formulation was 30 mg/mL.
Liposome preparation
The required amount of lipid and antioxidants were dissolved in 1 mL of a 9:1 (v/v) chloroform–methanol mixture. The solvents were evaporated off under a high vacuum, and the resulting film was kept at 35°C under vacuum for 6–8 h. The dry lipid films obtained after sample preparation were hydrated by the addition of 30 mL in buffer. The hydration was performed in buffers at 1 pH level 7.2. MLVs were obtained by hydrating the above films with 500 μL of a 0.12-M phosphate buffer solution (pH7.2). The hydrated samples were vortexed and liposomes were obtained using ultrasonication in an ultrasonic bath until the suspension became clear. Liposomes obtained at pH 7.2 were used in the encapsulation efficiency and permeability experiments.
Measurement particle size of liposomes
Size distribution of liposomes encapsulating quercetin was estimated by dynamic light scattering using a FPAR-1000 (Otsuka Electronics Co., Ltd., Japan). All samples were diluted in PBS to achieve a suitable signal in the detector. The light source was a diode-pumped solid state laser with a wavelength of 658 nm as a light source. Results are articulated as volume dissemination and presented as the mean width and diameter. The diffusivity of liposomal suspension (D) was obtained with above measurement, and the average liposome diameter (dhy) was calculated according to Eq.(1) (CitationKouhi et al. 2014)
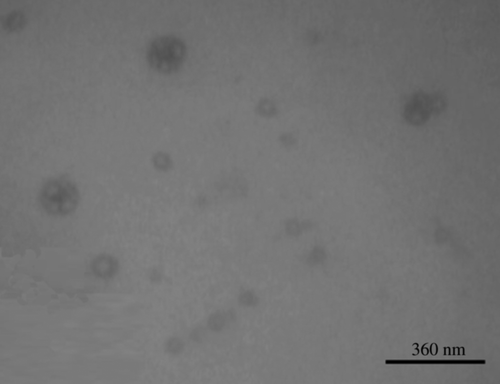
where k is the Boltzmann constant, T is the absolute temperature, Π is the circular constant, and Ƞ is the viscosity. All the prepared liposomal batches were studied with the help of scanning electron microscopy for their size and morphology ().
Zeta potential determination
Surface charges of vesicles were quantitatively analyzed by laser diffraction technique using Zetasizer (nano-series model, Malvern instruments, Worcestershire, UK) at 25°C, and illustrated as zeta potential.
Entrapment efficiency
A disorganization of quercetin loading was measured using ultracentrifugation method. All samples solution were centrifuged using centrifugator at 10,000 rpm for 60 min. Free quercetin in supernatant was determined using UV–visible spectrophotometer (1240, Shimadzu, Japan) at 375 nm. The quercetin entrapment percentage was designed from the correlation:
where EE is entrapment efficiency, Ctotal is total quantity of added quercetin and Cfree is quantity of free quercetin detected from supernatant.
DPPH free radical scavenging assay
The assay is based on a color change (from violet to yellow) of DPPH (2,2-diphenyl-1-picrylhydrazyl), which is a stable organic nitrogen radical. The DPPH test is based on the ability of the stable 2,2-diphenyl-1-picrylhydrazyl free radical to react with hydrogen donors. The DPPH•radical shows an intense UV–visible absorption spectrum. In this test, a solution of radical is decolorized after decrease with an antioxidant (AnH) or a radical (R•) in consensus with the following pattern: DPPH•+ AnH→DPPH•− H+ An•, DPPH•+ R•→DPPH-R (CitationTabatabaei Mirakabad et al. 2014). This method is very rapid and also simple for manual investigation. The reduction in absorbance in the attendance of an antioxidant was shadowed at 517 nm.
Reagent preparation
0.1 mmol-L− 1 solution of radical DPPH (m = 0.00394 g/ 100 mL). First, this amount is dissolved in 50 mL of DMSO and after dissolution made up to a volume of 100 mL with water. The solution can be used for 7 days when stored at 4° C and in the dark.
The free radical-scavenging activity of the quercetin against 2, 2 Diphenyl-1- Picryl Hydrazyl abridged was resolute. Changing concentrations of the quercetin(1, 5, 10, and 50 μM) were prepared from the stock solution. The reaction consisted of 1 ml of 0.1 mM DPPH in DMSO and 0.5 ml of (1, 5, 10, and 50 μM) quercetin. The tubes were kept in the dark and the absorbance was measured at 517 nm. The decrease in absorbance at 517 nm was continuously recorded in a spectrophotometer for 15 min. The scavenging effect, expressed as the decrease of absorbance at 517 nm, was observed and the percentage of DPPH radical-scavenging ability of the various concentrations were calculated using the following formula. Ascorbic acid and BHA were used as positive reference standards in this study.
where Ac is the absorbance of the control at 15 min, and As is the absorbance of the sample at 15 min. All samples were analyzed in triplicate.
Reducing power
Reducing power assessment was based on the capability of antioxidants to reduce iron(III) to iron(II). The reducing power of antioxidants was determined by analyzing their electron-donating potency according to the method of Oyaizu (CitationKlein et al. 2006). Potassium ferricyanide [K3Fe(CN)6] (1%) and 1:1 volume ratio of phosphate buffer saline (PBS) with different concentrations of quercetin (0.0, 1.0, 5, 10, or 50 μM) were used. The samples were incubated for 20 min at 50°C and then half volume of TCA (10%) was added. After adding 200 μl of ferric chloride (0.1%) to the final volume of 1.5 ml, the absorbance at 700 nm was recorded. The reduction of Fe3 + by antioxidants in the samples led to an increase in absorbance at 700 nm. Hence, the absorbance of the mixture increased with increasing the reducing power of quercetin.
Results
Liposome characterization and drug encapsulation efficiency
Zeta potential was used to determine the charge of the liposomes to propose the physicochemical possessions of the spreading system. Zeta potential specified that these liposomes formulation display negative surface (CitationAngelini et al. 2007, CitationKaul and Amiji 2002).
The physicochemical characteristics measured for quercetin-containing liposomes prepared are presented in .
Table I. Physicochemical characteristics measured for Liposomal quercetin prepared.
Inhibition of cell proliferation by L-Q treatment
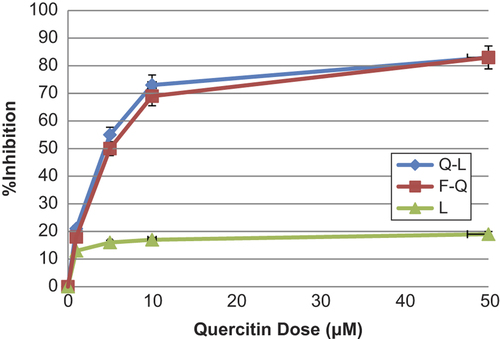
The liposomal quercetin treatment resulted in inhibition of cell proliferation in vitro, which has similar anti-proliferative effect compared with free quercetin. The inhibition effect was dependent on the dose of Q-L. For example, when MCF-7 cells were treated for 48 h, the percentage inhibition of 1, 10, and 50 μM/mL Q-L was 21%, 73%, and 83%, respectively (). Liposomal quercetin has greater anti-oxidant effects than free quercetin onMCF-7 cells. The effects of liposomal quercetin were compared to those of liposomes alone, free quercetin, and liposomal quercetin onMCF-7 cells. The results demonstrate that the growth inhibitory effects of liposomal quercetin were almost equivalent to or better than of free quercetin. Empty liposomes had minimal growth survival effects.
Anti-toxic potency of quercetin
MTT method was used to evaluate the antitoxic properties of free quercetin and liposomal quercetin against cytotoxic and lethal effects of CHP. The reduction of yellow MTT to purple formazan takes place only when mitochondrial reductase enzymes are active, and so the amount of adaptation can be directly accounted for the percentage of viable cells (CitationAkbarzadeh et al. 2013). shows that in the absence of CHP, 100% of cells are viable that reduces MTT compound. While, CHP is a highly toxic and lethal compound for MCF-7cells that causes 75% of cells to die. Presence of free quercetin and liposomal quercetin in samples almost suppressed the lethal effects of CHP. As a powerful stressor molecule, CHP causes the generation of different intracellular ROS, HO° and H2O2, resulting in lethal effects. The results demonstrated that the growth inhibitory effects of liposomal quercetin were approximately equal or better than that of free quercetin.
Table II. Effect of free quercetin and encapsulated quercetin on viability of MCF-7 cells exposed to cumene hydroperoxide (CHP).
After a 30-min treatment of cells with different concentrations of free quercetin and Encapsulated quercetin (0, 1, 5, 10, and 50 μM) the cells were induced with 5 ml (1:100) CHP.
Viability was measured using the MTT assay.
Reducing power
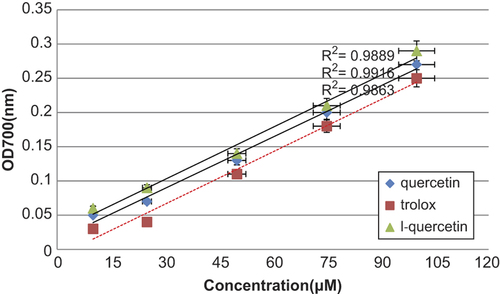
Dissimilar studies have displayed that the electron donation capacity of bioactive compounds is related with antioxidant activity. The ability of acompound to reduce Fe3 + to Fe2+ was resolute and compared to that of trolox, which is known to be a strong reducingagent. displays a good linear relationship between quercetin concentrations and reduced amounts of iron (III) ions in polar solution. It also indicates that quercetin has almost some degree of electron donation capacity in a concentration-dependent dose, but the capacity was inferior to that of trolox under the same condition. Similar relations between iron (III) reducing activity and total antioxidant content have been reported in the literature; however, the correlation may not be always linear. Quercetin that showed comparable absorbance reading with trolox is considered to have high reducing power.
Scavenging activity on 1,1-diphenyl-2-picrylhydrazyl radicals(DPPH radical)
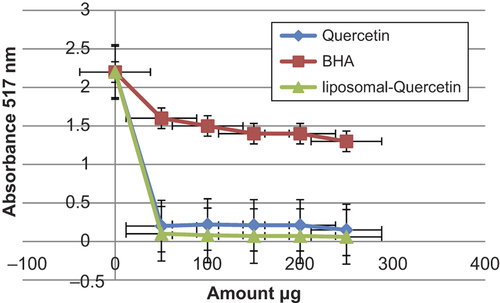
DPPH is a free radical that receives an electron or hydrogen radical to become a stable molecule. The decreaseability of DPPH radical was determined by the reduction in absorbance influenced by antioxidants (CitationLee et al. 2003). Quercetin and liposomal quercetin showed almost equal DPPH scavenging activity; however, significantly are higher than that of BHA. The scavenging effect of BHA, quercetin and liposomal quercetin on the DPPH radical decreased in the order of L-quercetin > quercetin > BHA, respectively (). BHA was used as positive standard with a maximum absorption at 517 nm that can readily undergo scavenging as an antioxidant, this pure antioxidant has been widely used to evaluate the antiradical activity of various samples. It was created that the radical-scavenging activities of all the samples increased with increasing concentration.
Conclusions
In this study, the classical thin-layer evaporation method for liposomal preparation was used to prepare liposomal quercetin, and it yielded vesicle sizes smaller than 100 nm, with a narrow size distribution. Our data indicated that liposomal quercetin can significantly improve the solubility and bioavailability of quercetin and can be a potential application in the treatment of tumor. Quercetin appears to be associated with little toxicity when administered orally or intravenously. Further research will be required to outline the types of malignancy most likely to benefit from this relatively non-toxic therapy. The pharmacological activity of these carriers should be evaluated in the future study. Though, there are still many controversies concerning the mechanisms involved and the role that antioxidants play.
Authors’ contributions
RRS conceived of the study and participated in its design and coordination. NZ participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank Department of Medical Nanotechnology, and Biotechnology Faculty of Advanced Medical Science of Tabriz University for all supports provided. The authors would like to thank Drug Applied Research Center of Tabriz University of Medical Sciences, Tabriz, Iran, for their financial support.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmadi A, Shirazi H, Pourbagher N, Akbarzadeh A, Omidfar K. 2014. An electrochemical immunosensor for digoxin using core-shell gold coated magnetic nanoparticles as labels. Mol Biol Rep. Epub Ahead of Print.
- Akbarzadeh A, Mikaeili H, Asgari D, Zarghami N, Mohammad R, Davaran S. 2012a. Preparation and in-vitro evaluation of doxorubicin-loaded Fe3O4 magnetic nanoparticles modified with biocompatible copolymers. Int J Nanomedicine. 7:511–526.
- Akbarzadeh A, Mohamad S, Davaran S. 2012d. Magnetic nanoparticles: preparation, physical properties and applications in biomedicine. Nanoscale Res Lett. 7:144–157.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. 2013. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 8:102.
- Akbarzadeh A, Samiei M, Joo SW, Anzaby M, Hanifehpour Y, Nasrabadi HT, Davaran S. 2012c. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J Nanobiotechnol. 10:46–58.
- Akbarzadeh A, Zarghami N, Mikaeili H, Asgari D, Goganian AM, Khiabani HK, Davaran S. 2012b. Synthesis, characterization and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Int J Nanotechnol Sci Environ. 5:13–25.
- Angelini G, Boncompagni S, De Maria P, De Nardi M, Fontana A, Gasbarri C, Menna E. 2007. Layer-by-layer deposition of shortened nanotubes or polyethylene glycol- derivatized nanotubes on liposomes:A tool for increasing liposome stability. Carbon. 45:2479–2485.
- Barenholz Y. 2001. Liposome application: problems and prospects. Curr Opin Colloid Interface Sci. 6:66–77.
- Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, et al. 2001. Reducedcardiotoxicity and preserved antitumor efficacy ofliposome-encapsulated doxorubicin and cyclophosphamidecompared with conventional doxorubicinand cyclophosphamide in a randomized, multicentertrial of metastatic breast cancer. J Clin Oncol. 19:1444–1454.
- Davaran S, Akbarzadeh A, Nejati-Koshki K, Alimohammadi S, Ghamari MF, Soghrati MM, et al. 2013. In vitro studies of NIPAAM-MAA-VP copolymer-coated magnetic nanoparticles for controlled anticancer drug release. JEAS. 3:108–115.
- Davaran S, Alimirzalu S, Nejati-Koshki K, Nasrabadi HT, Akbarzadeh A, Khandaghi AA, et al. 2014a. Physicochemical characteristics of Fe3O4 magnetic nanocomposites based on poly(Nisopropylacrylamide) for anti-cancer drugs delivery. Asian Pac J Cancer Prev. 15:49–54.
- Davaran S, Rezaei A, Alimohammadi S, Khandaghi AA, Nejati-Koshki K, Nasrabadi HT, Akbarzadeh A. 2014b. Synthesis and physicochemical characterization of biodegradable star-shaped poly lactide-co-glycolide– β -cyclodextrin copolymer nanoparticles containing albumin. Adv Nanoparticles. 3:14–22.
- Ebrahimnezhad Z, Zarghami N, Keyhani M, Amirsaadat S, Akbarzadeh S, Rahmati M, et al. 2013. Inhibition of hTERT gene expression by silibinin-loaded PLGA-PEG-Fe3O4 in T47D breast cancer cell line. BioImpacts. 3:67–74.
- Erkoc F, Erkoc S. 2002. Theoretical investigation of flavonoids naringenin and genistein. Theochem. 583:163–167.
- Ghasemali S, Nejati-Koshki K, Akbarzadeh A, Tafsiri E, Zarghami N, Rahmati-Yamchi M, et al. 2013. Study of inhibitory effect of β-cyclodextrin-helenalin complex on HTERT gene expression in T47D breast cancer cell line by real time quantitative PCR (q-PCR). Asian Pac J Cancer Prev. 14:6949–6953.
- He X, Liu RH. 2008. Phytochemicals of apple peels: isolation, structure elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem. 56:9905–9910.
- Klein E, Matis M, Lukes V, Cibulkova Z. 2006. The applicability of AM1and PM3semi-empirical methods for the study of N-H bond dissociation enthalpies and ionization potential of amine type antioxidants. Polym Degrad Stab. 91:262–270.
- Kouhi M, Vahedi A, Akbarzadeh A, Hanifehpour Y, Joo SW. 2014. Investigation of quadratic electro-optic effects and electro absorption process in GaN/AlGaN spherical quantum dot. Nanoscale Res Lett. 9:131–136.
- Kaul G, Amiji M. 2002. Long-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular delivery. Pharm Res. 19:1061–1067.
- Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH. 2003. Screening of medicinal plant extracts for antioxidant activity. Life Science73:167–117.
- Lefer DJ, Granger DN. 2000. Oxidative stress and cardiac disease. Am J Med. 109:315–323.
- Lian T, Ho RJ. 2001. Trends and developments in liposome drug delivery systems. J Pharm Sci. 90:667–680.
- Nejati-Koshki K, Akbarzadeh A, Pourhasan-Moghadam M, Joo SW. 2013. Inhibition of leptin and leptin receptor gene expression by silibinin- curcumin combination. Asian Pac J Cancer Prev. 14: 6595–6599.
- Nelson SK, Bose SK, Grunwald GK, Myhill P, Mc Cord JM. 2006. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radic Biol Med. 40:341–347.
- Phromnoi K, Yodkeeree S, Anuchapreeda S, Limtraku P. 2009. Inhibition of MMP-3 activity and invasion of the MDAMB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol Sin. 30:1169–1176.
- Pourhassan-Moghaddam M, Rahmati-Yamchi M, Akbarzadeh A, Daraee H, Nejati-Koshki K, Hanifehpour Y, Joo SW. 2013. Protein detection through different platforms of immuno-loop-mediated isothermal amplification. Nanoscale Res Lett. 8:485.
- Rezaei-Sadabady R, Zarghami N, Barzegar A, Eidi A, Akbarzadeh A, Rezaei-Tavirani M. 2013. Studies of the relationship between structure and antioxidant activity in interesting systems, including tyrosol, hydroxytyrosol derivatives indicated by quantum chemical calculations. Soft. 2:13–18.
- Sahoo SK, Labhasetwar V. 2003. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 15:1112–1120.
- Sharma A, Sharma US. 1997. Liposomes in drug delivery: progress and limitations. Int J Pharm. 154:123–140.
- Stone WL, Mukherjee S, Smith M, Das SK. 2002. Therapeutic uses of antioxidant liposomes. Methods Mol Biol. 199:145–161.
- Stone WL, Smith M. 2004. Therapeutic uses of antioxidant liposomes. Mol Biotechnol. 27:217–230
- Tabatabaei Mirakabad FS, Akbarzadeh A, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S. 2014. PLGA-cased nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev. 15: 517–535.
- Torchilin VP. 2005. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 4:145–160.
- Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, et al. 2012. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 7:276.
- Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. 1995. Vascular permeabilityin a human tumor xenograft: molecular sizedependence and cutoff size. Cancer Res. 55:3752–3756.
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. 2008. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 83:761–769.