Abstract
Malemide polyethylene glycol-conjugated Hb (MP4OX, Sangart Inc.), a high-affinity low-concentration acellular hemoglobin (P50 = 5 mmHg, 4.3 g/dl) solution, has been shown to optimize microvascular perfusion and target oxygen delivery to anoxic tissue. Microvascular perfusion during an acute hypoxic challenge in a transgenic anemic sickle cell disease mouse model was studied with MP4OX and saline. Arterioles were dilated in both groups. Functional capillary density (FCD) was maintained at a higher level with MP4OX. In conclusion, MP4OX treatment reduced the hypoxia-mediated decline in FCD, an effect in part due to higher arterial pressure resulting in increased microvascular perfusion pressures.
Introduction
Sickle cell disease (SCD) is caused by a single amino acid substitution on the β hemoglobin (Hb) subunit, which results in the abnormal hemoglobin S (HbS). Deoxygenation of hemoglobin S leads to Hb protein polymerization and reduced red blood cell (RBC) flexibility, which induces RBC shape modification and changes in blood rheology (CitationDong et al. 1992). Repeated cycles of shape change increase membrane fragility causing RBCs to either become sequestered in the spleen or self-destruct (hemolysis). The disease is also characterized by chronic hemolytic anemia, episodes of severe pain and significant ischemic damage to major organs.
In vivo studies have sought to understand the trigger for the transient vaso-occlusive episodes which are characteristic of SCD. Recently, studies have shown that the vaso-occlusive episodes are not caused only by Hb S polymerization, damaged RBC membrane and changes in blood rheology. There is increasing evidence that the clinical manifestations of SCD are related to ischemia-reperfusion damage leading to an abnormal endothelium (CitationOsarogiagbon et al. 2000, CitationKaul et al. 1996, CitationKaul and Hebbel 2000). A chronic inflammation condition prevails in which there are increased cytokine levels, oxidant production, leukocyte– endothelial interactions, and a number of circulating leukocytes (CitationAslan et al. 2001, CitationKlug et al. 1982, CitationPlatt 2000, CitationVercellotti and Belcher 2014, CitationLi et al. 2014). The vasculature in SCD is believed to have a heightened susceptibility to adhesive and occlusive events due to increased RBC– endothelial interaction caused by the altered RBC rheological properties (Hb polymerization) and an activated vascular wall (chronic inflammation). Both mechanisms, that is, endothelial inflammation and Hb polymerization, are intertwined, rendering it difficult to determine their individual contributions in causing the onset of the vaso-occlusive episode characteristic of SCD.
Enhancement of the oxygen transport properties of blood by the addition of an acellular Hb to the plasma could reduce the RBC deoxy-HbS concentration and thus their tendency to sickle. The small size of the acellular Hb allows it to transport oxygen to regions perfused by plasma but blocked by sickle cells. The oxygen transported by the acellular Hb to these anoxic conditions will offload oxygen to the tissue and to the RBC and, by this means, decrease the deoxy-HbS concentration significantly to delay, prevent, or reverse the HbS polymerization. Thus, access to these regions by the acellular Hb would reduce the extent of ischemia reperfusion injury and diminish vascular inflammation. However, the acellular Hb within the plasma reduces the bioavailability of nitric oxide (NO) (CitationReiter et al. 2002) and this could lead to instability in microvessel tone and negatively affect perfusion, resulting in an increase of ischemic and anoxic areas. Additionally, acellular Hb is a source for OH- formation and could amplify the chronic inflammatory condition already present in SCD. Hypoxia is a component of SCD pathophysiology; therefore, its prevention may limit the incidence of RBC sickling, vascular occlusion, pain and organ failure, thus alleviating some factors leading to the morbidity and mortality of this disease.
The objective of this study was to determine functional capillary density (FCD, number of capillaries with RBC transit per unit area of tissue) and vascular diameter in conditions of systemic hypoxia in knockout transgenic mice, that mimic SCD, after treatment with high oxygen affinity acellular Hb (MP4OX Sangart Inc, San Diego, CA). These mice express human sickle hemoglobin and have moderate anemia (CitationRyan et al. 1997). The protocol was designed to elucidate if the addition of MP4OX would affect vasoconstriction and tissue perfusion.
Materials and methods
All experiments were approved by the Institutional Animal Care and Use Committee at the University of California, San Diego and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (CitationNational Research Council (U.S.) et al. 2011).
Mice
Heterozygous HbAS-Townes transgenic sickle mice at 8–12 weeks of age were used as models for SCD trait. These mice were the result of breeding knockout mice with mutations of the mouse α- and β-globin genes and transgenic mice that expressed human sickle hemoglobin. The offspring mainly synthesize human hemoglobin in adult RBCs and have hemolytic anemia and extensive organ pathology similar to patients with SCD (CitationRyan et al. 1997). The heterozygous HbAs mice were derived by breeding male HbSS-Townes mice (mα−/−; mβ−/−; hα+/+; hβS+/+) with female HbSS-Townes mice (mα−/−; mβ−/−; hα+/+; hβS+/−). These animals were obtained under a license with the University of Alabama and were bred for these studies at Charles River. A normal group, C57Bl/6 mice (Charles River, Hollister, CA), was studied to determine the murine response to the hypoxia challenge protocol.
Window chamber preparation
The murine window chamber model is widely used for microvascular and SCD studies (CitationEmbury et al. 2004, CitationKalambur et al. 2004) in the unanesthetized state. The complete surgical technique for its preparation has been previously described in detail (CitationLehr et al. 1993, CitationCabrales et al. 2005a). This model allows for the study of the microcirculation in an intact subcutaneous tissue and a retractor muscle free from surgical manipulation and exposure to ambient atmospheric conditions.
The window chamber implantation was performed 5 days prior to the study to mitigate the effects of surgery on the tissue. On the day of the study, the animals were reanesthetized and the right carotid artery cannulated (PE-10 tubing); the catheter was tunneled under the skin and then exteriorized at the base of the window chamber. This is a minor procedure and takes about 15 min. The animals were returned to their cage after they fully recovered from the surgical procedure (2 h). Approximately 6 h after recovery, the animals were placed into a restraining tube to begin the experimental protocol.
Inclusion criteria
These animals had to meet the following criteria for their inclusion in the study: a) the microscopic examination of the tissue observed under ×650 magnification did not reveal signs of edema or bleeding and b) mean arterial blood pressure (MAP) > 100 mmHg and heart rate (HR) > 580 beats/min.
Study groups
Animal groups: Transgenic sickle cell (SS) and C57Bl/6 (Normal) mice were used. Treatment groups: Animals were infused with either MP4OX (Malemide polyethylene glycol-conjugated Hb, Sangart Inc., San Diego, CA) or Saline (0.9% NaCl). MP4OX is a semi-hybrid polymer consisting of human hemoglobin modified by conjugation to approximately 6–7 strands of Maleimide-activated polyoxyethylene (5 kDa PEG). Its properties and synthesis have been described (CitationAcharya et al. 1996; CitationVandegriff et al. 2003).
Systemic and laboratory parameters
Mean arterial pressure (MAP) and heart rate (HR) were monitored continuously (MP 150, Biopac System; Santa Barbara, CA) via the carotid catheter except during the infusion of the study solution. Hematocrit (HCT) was measured from a blood sample taken in a heparinized microcapillary tube (40 μl) and then centrifuged. Total Hb and plasma Hb were measured from a drop of arterial blood and plasma, respectively, using a handheld photometer (B-Hemoglobin, HemoCue, Mission Viejo, CA).
Microvascular tone: vessel diameter
Video image-shearing was used to measure the vessel diameter (Image Shearing Monitor, Vista Electronics, San Diego, CA) (CitationIntaglietta and Tompkins 1973). Changes in the arteriolar and venular diameter from baseline were used as indicators of an alteration in vascular tone.
Tissue perfusion – functional capillary density
The number of capillaries which transect the middle of the observation field under 20 × magnification which had RBCs flowing into them over a period of 30 s were tabulated for a distance of 4 mm. An alteration in FCD reflects a change in the microvascular tissue perfusion. The same vessel and FCD fields were observed at each experimental period.
Experimental design
The animals were awake during the procedure and were placed into a restraining tube for the experiment. Warmed air was gently blown (flow rate 2.6 l/min) into the face of the animals through a diffuser connected at the front of the tube. The tube containing the conscious animals was then affixed onto a microscopic stage of an intravital microscope (BX51WI, Olympus, New York, 40X objective, n.a. 0.7 SW). The animals had 30 min to adjust to the tube environment prior to measuring baseline parameters (MAP, HR, HCT). The tissue image was projected onto a CCD camera (4815-2000, COHU, San Diego) and viewed on a monitor. Arterioles and venules, chosen by their visual acuity (2–5 each type), were characterized by their diameter. Vessels chosen at baseline were then followed throughout the experiment.
Experimental protocol
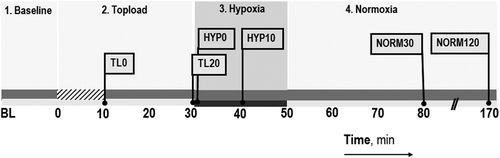
shows the four stages of the experiment timeline. In the first stage, baseline measurements were assessed. Afterwards in the treatment stage, the animals were infused with the study solution (volume equal to 10% of its blood volume, estimated as 6% of the body weight) via the carotid catheter at a rate of 14–15 μl/min over a 10-min period. Immediately following this hypervolemic infusion (TOPLOAD), the catheter was reconnected to the blood pressure transducer (TL0). After a 10-min recovery period, microvascular parameters were reassessed and then blood pressure and heart rate noted (TL20). In the third stage, the animals were subjected to hypoxia (HYPOXIA) by changing the FiO2 from 0.21 to 0.08 (HYP0). After a 10-min exposure (HYP10), microvascular parameters were assessed, followed by blood pressure and heart rate. Then, microvascular measurements began and lasted for another 10 min after the start of hypoxia. In the fourth and last stage, the FiO2 was then returned to 0.21 (NORMOXIA) and after 30 min, parameters were reassessed (NORM30), and again, in a subset of SS animals, after 120 min (NORM120). Observations at NORM120 were exploratory and focused on changes in FCD and microvessel diameter to examine a potential time-dependent increase in the inflammatory level after the hypoxic challenge.
Figure 1. Experimental timeline. Stage 1. Baseline parameters are assessed. Stage 2. Topload is the period in which the study solutions were infused in 10 min (hatched zone on the time line) followed by an assessment of parameters afterwards TL0 and TL20. Stage 3. Hypoxia is induced by changing from FiO2 = 0.21 to 0.08. Data are taken immediately and 10 min after (HYP0 and HYP10). Stage 4. Normoxia, inspired air is switched back (FiO2 = 0.21). Data are taken 30 min later (NORM30) and in some animals 120 min later (NORM120). Parallel slash lines on the x-axis denote a break in the time axis. The flag represents time points where measurements are reported.
Data analysis
All values are shown as mean ± standard deviation (N denotes the number of animals studied systemically; NM denotes the number of animals studied both systemically and in the microcirculation; n denotes the number of vessels studied). Statistics were prepared using Prism version 5.0 for Windows (GraphPad, San Diego, CA). Differences within groups were first tested with one-way ANOVA for repeated measures and for multiple comparisons between groups using Bonferroni post hoc test if significance was obtained. The time points used for the statistical analysis were as follows: Baseline, Topload (TL20), Hypoxia (HYP20), and Normoxia (NORM30). For MAP and HR, the time points analyzed were differentiated on the graphs by color, black versus gray (not used in the statistical analysis but are presented to illustrate the time dependency of the parameters). Changes were considered statistically significant if P < 0.05. The effect of the treatment (MP4OX vs. Saline) was studied by making a comparison of the data at each time point using the unpaired t-test. Some data are presented normalized to baseline in order to focus on the trend of the data rather than its dispersion..
Results
The SS animals (N = 14, average weight = 27.1 ± 3.4 g) had an average Hct and a total Hb content of 39.8 ± 3.3% and 11.2 ± 1.7 g/dl which were statistically significantly lower than the Normal animals (p < 0.1). Normal animals (N = 8, 22.9 ± 3.5 g) had an average Hct and a total Hb content of 46.5 ± 1.4% and 13.7 ± 1.5 g/dl. Topload with MP4OX resulted in a plasma hemoglobin concentration of 0.2 ± 0.1 and 0.3 ± 0.1 g/dl for the SS and Normal groups, respectively.
Systemic parameters: MAP and HR
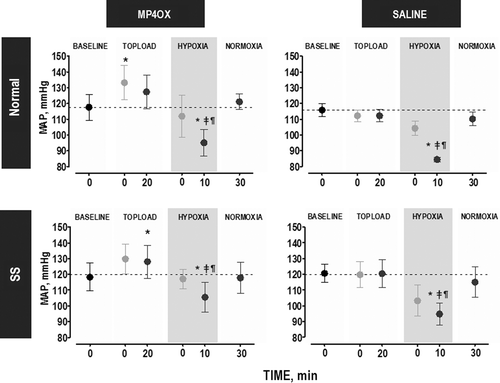
shows the changes in MAP over the experimental time course. MP4OX tended to increase MAP after topload when compared with Saline, but these changes with MP4OX were not different in SS and Normal animals. Animals in each group responded to hypoxia (HYP) with a statistically significant reduction in MAP. The reduction in MAP tended to be greater in Saline-treated animals than in MP4OX-treated animals, and this pattern was observed in both SS animals and Normal animals. SS animals had higher MAP after MP4OX treatment than after Saline treatment at the TL20 and HYP time points.
Figure 2. Change in MAP over the time course of the experiment. Statistical analyses were performed at the time points denoted in black which represent the steady state level during each of the four stages (symbols in gray illustrate the changes at intermediate time points and were not used in statistical analyses): Topload (T20, 20 min after completion of topload infusion), Hypoxia (HYP10, 10 min after changing FiO2 from 0.21 to 0.08), and Normoxia (NORM30, 30 min after returning to normal FiO2 of 0.21). P < 0.05 (within the same group), relative to: *, Baseline; ‡, T20; and ¶, NORM30. The pair-wise comparisons at specific time points are used to understand the effect of the treatment. These statistics are not marked on the graphs but are tabulated here for each animal group. Normal group, compare treatments. Time point (P value): TL20 (0.06), HYP (0.08), NORM (0.02). SS group, compare treatments. Time point (P value): TL20 (0.88), HYP(0.40), NORM(0.73). Light gray shading, FiO2 = 0.08.
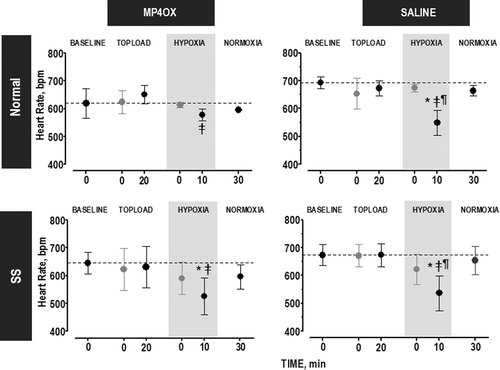
During the time course of the experiment, there was no difference in HR when MP4OX or Saline treatment was done on both animal groups (). All animals responded to the hypoxia with a lower HR. Normal Group: In saline-treated animals, the lower HR during hypoxia was statistically significant from baseline, TL20, HYP10, and NORM30. In the MP4OX-treated animals, the lower HR was only different between hypoxia and TL20, but this change was not statistically significant. SS Group: During hypoxia, a statistically significant reduction in HR was obtained for Saline-(Baseline, TL20 and NORM30) and MP4OX-treated animals (Baseline and TL20).
Figure 3. Change in HR over the time course of the experiment. Statistical analyses were performed at the time points denoted in black which represent the steady state level during each of the four stages (symbols in gray illustrate the changes at intermediate time points, but were not used in statistical analyses): Topload (T20, 20 min after the completion of the topload infusion), Hypoxia (HYP10, 10 min after changing FiO2 from 0.21 to 0.08), and Normoxia (NORM30, 30 min after returning to normal FiO2 of 0.21). P < 0.05 (within the same group), relative to: *, Baseline; ‡, T20; and ¶, NORM. No statistical differences were found across groups at the same time point. Gray shading, FiO2 = 0.08.
Microvascular parameters: vessel diameter and FCD
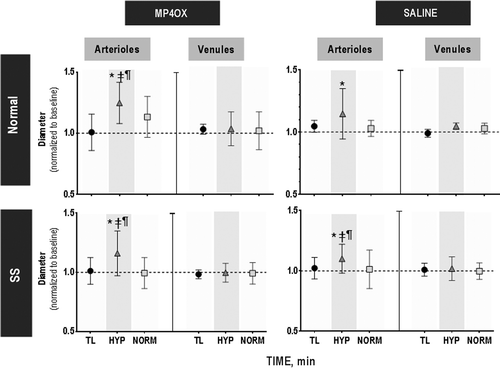
Changes in microvascular diameter for arterioles and venules at each stage are shown in . Topload with Saline or MP4OX did not alter the arteriolar or venular diameter from baseline. Hypoxia caused a significant increase in the arteriolar diameter relative to baseline, TL and NORM in Normal/MP4OX, SS/MP4OX and SS/Saline groups. For Normal/Saline, there is a trend showing an increased diameter relative to baseline during hypoxia (P < 0.10). Venules did not show any statistically significant changes in the vascular diameter. The sampling of venular vessels is small; however, the magnitude of the standard deviations suggests that there was little variability in response by venules during the four stages of the study.
Figure 4. Changes in microvascular diameter. There were no statistical differences between groups with treatment during topload (TL, circle) and normoxia (NORM, square) relative to baseline. During hypoxia (HYP, triangle), arterioles dilated while venules were unchanged relative to baseline. Data are presented (mean ± sd) relative to baseline (1.0), changes higher or lower represent vasodilation and vasoconstriction, respectively (i.e., 1.1 would indicate a 10% vasodilation relative to the vessels caliper at baseline). Within each study group, P < 0.05, relative to: *, Baseline; ‡, TL; and ¶, NORM. There were no statistically significant differences among the groups at each time point. Gray shading, FiO2 = 0.08. Average diameters for the arteriolar and venular vessels studied among all groups were 61 ± 23 and 87 ± 35 μm, respectively. The number of vessels studied (n) was Normal/MP4OX (A, n = 17 and V, n = 8); Normal/Saline (A, n = 15 and V, n = 8); SS/MP4OX (A, n = 33 and V, n = 18); SS/Saline (A, n = 29 and V, n = 11). There were no statistical differences in the size of arterioles and venules among study groups.
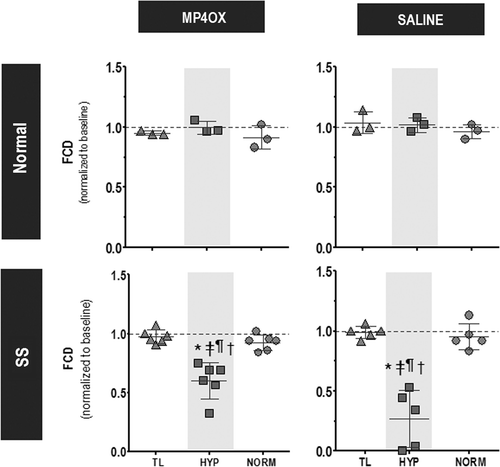
shows the FCD during the different stages of the experimental protocol. FCD was statistically unchanged during the entire protocol for the normal group with either MP4OX or Saline. In the SS group, FCD in the MP4OX and Saline groups was statistically significantly reduced during hypoxia relative to all time points. During hypoxia, the reduction in FCD for the SS/Saline group was significantly greater than for the SS/MP4OX group (P < 0.05). The differences between the Normal and SS groups during hypoxia were also statistically significant. FCD levels in all groups and treatments returned to baseline after 30 and 120 min of normoxia.
Figure 5. Changes to functional capillary density (FCD). Data are presented relative to baseline (1.0), changes higher or lower represent changes in microvascular perfusion. Within each study group, P < 0.05, relative to *, Baseline; ‡, TL; and ¶, NORM. Among all groups, there were no statistical differences at TL and N30. Normal group: no statistical differences between all time points for MP4OX versus Saline treatment. SS group: †P < 0.05 at HYP between MP4OX versus Saline treatment.
FCD and arteriolar diameter were studied at 120 min following reoxygenation in five SS animals (SS/Saline, NM = 3 and SS/MP4OX, NM = 3) to determine if there were delayed effects of hypoxia or MP4OX. FCD was 0.89 ± 0.16 and 0.97 ± 0.06 of baseline for SS/Saline and SS/MP4OX, respectively. The arteriolar diameter was 1.08 ± 0.10 (n = 15) and 1.00 ± 0.09 (n = 21) of baseline for SS/Saline and SS/MP4OX, respectively. The changes were not statistically different from baseline or NORM30 within each treatment group.
Discussion
The most salient findings of this study are that a hypervolemic infusion of MP4OX did not cause vasoconstriction in knockout transgenic sickle cell animals and that microvascular perfusion in terms of FCD was higher in hypoxic animals treated with MP4OX than in those treated with Saline.
MP4OX with its low P50 has the ability to target oxygen delivery to anoxic regions (CitationTsai et al. 2003) and thus establish an environment ideal for preventing deoxygenation of Hb and thus RBC sickling. An in vitro filtration study in which MP4OX was added to human sickle cells and exposed to changes in pO2 demonstrated that MP4OX improved the filtration and, therefore, decreased sickling of red blood cells (CitationOliva 2005). Those results suggest that oxygen transport by MP4OX to red cells was responsible for the improved deformability and filtration of red cells during deoxygenation. In this study, oxygen levels were not measured and thus it is difficult to determine the role that oxygen targeting by MP4OX, per se, would play in maintaining FCD since hemodynamic effects of MP4OX are also evident in this model. However, similar effects of oxygen transport by MP4OX are probably operative in this study. Hemodynamic changes evident with MP4OX have been shown at the microvascular level using the hamster skinfold model. Topload infusion of MP4OX increased MAP due to a transient increase in circulating blood volume because of its markedly higher colloid osmotic pressure (CitationTsai et al. 2006). Exchange transfusion done with MP4OX showed a better transmission of hydraulic pressure from the systemic to the peripheral circulation than the one done with other oxygen carrying and nonoxygen carrying plasma expanders (CitationCabrales et al. 2004, Citation2005b). A higher perfusion pressure at the periphery is directly correlated with capillary perfusion and thus FCD. These factors may help explain the higher FCD with MP4OX relative to Saline during the hypoxic challenge in this study. The finding of a significant improvement of tissue perfusion with MP4OX treatment was also obtained in a recent study with the same transgenic mice where dosing prior to an anoxic challenge reduced venular stasis upon reoxygenation (CitationBelcher et al. 2013).
Microvascular studies have demonstrated a strong relationship between sickling and microvascular function (CitationKaul and Fabry 2004), showing 1) depressed response to reactive hyperemia (CitationLipowsky et al. 1987); 2) increased flowmotion (CitationRodgers et al. 1990); 3) attenuated vascular response to oxygen (CitationKaul and Hebbel 2000); and 4) reduced flow-mediated vasodilation. Studies that focused on understanding the role of inflammation in triggering the vaso- occlusive episodes using different strains of knockout transgenic sickle cell mice that were not anemic found vaso-occlusion episodes and significantly increased leukocyte adhesion taking place hours after the anoxic stimulus (CitationBelcher et al. 2003, CitationEmbury et al. 2004). In the present investigation using HbSS-Townes anemic sickle cell mice, vascular changes were measured during hypoxia and immediately after reoxygenation. In a few animals, the observation period was extended to 2 h after the reoxygenation time point. Microvascular tone and FCD at either time point of reoxygenation were not statistically different from those obtained at baseline.
Investigations using oxygen therapeutics for SCD have been limited and therefore, mechanisms, such as changes to blood rheology and/or reduction in ischemic tissue regions, have been speculated upon. An in vitro study with a polyoxyethylene Hb conjugate suggested that it could prevent sickling of cell and it was speculated that this would reverse capillary occlusion (CitationYabuki et al. 1988). Infusion of oxygenated perfluorcarbon emulsion reversed vascular occlusion blockage by deoxygenated human sickle cell when the rat mesocecum was used (CitationKaul et al. 2001). Studies using cell-free hemoglobin (CFH) solutions have been performed on SCD patients, but their scope has been very limited (CitationGonzalez et al. 1997, CitationFeola et al. 1992) and their efficacy as a treatment has not been proved yet. A CFH with low-oxygen-affinity (HRC 101, Hemosol; Mississauga, Ontario, Canada) prevented sickle-related mortality in transgenic SCD mice during exposure to acute, severe hypoxic stress (CitationCrawford et al. 2007). The mechanism proposed for the improved outcome with CFH was its early release of oxygen prior to deoxygenation of the sickle RBC. In this study, the working mechanism of the low oxygen affinity CFH is the elimination of hypoxic regions by targeted off-loading of oxygen to anoxic tissue ameliorating and prevention of polymerization of the HbS. The use of an oxygen-carrying therapeutic agent to prevent or reverse sickling is appealing, since deprivation of oxygen must be the root cause of pathophysiology in patients with sickle cell anemia. Indeed, an older formulation of PEG-modified hemoglobin decreased sickling when tested in vitro (CitationYabuki et al. 1988), but the safety of that formulation was never established in human clinical trials, and it was not successfully introduced for clinical use.
MP4OX differs from previously developed hemoglobin-based oxygen carriers in that while it binds NO normally (CitationTsai et al. 2006, CitationVandegriff et al. 2004), it is not vasoactive in animal models (CitationVandegriff et al. 2004, CitationDrobin et al. 2004) and can support life in the near-absence of red blood cells (CitationWinslow et al. 2004). The presumed mechanism of action of this CFH is the preservation of FCD and the targeted release of oxygen in hypoxic tissue (CitationTsai et al. 2003) by the avoidance of autoregulatory mechanisms (CitationWinslow et al. 2002).
Preservation of capillary perfusion and the transport of O2 in the microcirculation would seem the ideal approach to treat sickle cell crisis, but caution is necessary, since NO scavenging by CFH has also been invoked as a mechanism for local ischemia in patients with SCD (CitationGladwin et al. 2004). Furthermore, some evidence has accumulated to suggest that transgenic sickle cell mice may have altered microcirculatory autoregulatory mechanisms (CitationEmbury et al. 1999). Although different formulations of hemoglobin-based oxygen carriers have been tested in sickle cell anemia patients (CitationFeola et al. 1992, CitationGonzalez et al. 1997, CitationOrringer et al. 1995), clinical trials in these patients with a hemoglobin-based product must be deliberate and methodical, since all of the physiological effects of these compounds have not yet been documented. A recent study using MP4OX mutant mice with SCD also show its ability to reduce venular stasis following an hypoxia stimulus (CitationBelcher et al. 2013). In these studies, PEG-Hb saturated with carbon monoxide (MP4CO) was found to be more effective than MP4OX, but the findings of the present study corroborate the observations made about reduced vaso-occlusion with MP4OX in that study.
In summary, the results from the present study shows that free hemoglobin in plasma due to the infusion of MP4OX into transgenic sickle cell mice does not affect capillary perfusion, a response not different from saline. Furthermore during hypoxia, FCD was significantly higher than saline.
Acknowledgments
The authors wish to thank Mr. Froilan Barra and Ms. Cynthia Walser for putting their surgical skills to use in the preparation of the window and catheter implantations.
Research contributions: AGT, performed the experiments, analyzed data, designed the study, and wrote a paper; PC, analyzed data; MAY, analyzed data, designed the study, and contributed to the writing of the paper; RMW, analyzed data and contributed to the writing of the paper; and MI, analyzed data and contributed to the writing of the paper.
Declaration of interest
M.I. and R.M.W. hold patents related to the work that is described in the present study. A.G.T., P.C. and M.A.Y. report no declaration of interest. Research was supported in part from grants NIH P01 HL 110900, NIH R01 HL052684 and USAMRMC Contract W81XWH-11-2-0012. The knockout transgenic mice and MP4OX were provided by Sangart through a collaborative research partnership funding from NIH R24 HL064395.
References
- Acharya AS, Manjula BN, Smith P, . 1996. Hemoglobin crosslinkers. US Patent number. 5:585–484.
- Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, et al. 2001. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. PNAS. 98:15215–15220.
- Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, et al. 2003. Transgenic sickle mice have vascular inflammation. Blood. 101:3953–3959.
- Belcher JD, Young MA, Chen C, Nguyen J, Burhop K, Tran P, Vercellotti GM. 2013. MP4CO, a pegylated hemoglobin saturated with carbon monoxide, is a modulator of HO-1, inflammation, and vaso-occlusion in transgenic sickle mice. Blood. 122:2757–2764.
- Cabrales P, Tsai AG, Frangos JA, Intaglietta M. 2005a. Role of endothelial nitric oxide in microvascular oxygen delivery and consumption. Free Radic Biol Med. 39:1229–1237.
- Cabrales P, Tsai AG, Intaglietta M. 2004. Microvascular pressure and functional capillary density in extreme hemodilution with low and high plasma viscosity expanders. Am J Physiol. 287:H363–H373.
- Cabrales P, Tsai AG, Winslow RM, Intaglietta M. 2005b. Effects of extreme hemodilution with hemoglobin based O2 carriers on microvascular pressure. Am J Physiol. 288:H2146–H2153.
- Crawford MW, Shichor T, Engelhardt T, Adamson G, Bell D, Carmichael FJ, Kim PC. 2007. The novel hemoglobin-based oxygen carrier HRC 101 improves survival in murine sickle cell disease. Anesthesiology. 107:281–287.
- Dong C, Chadwick RS, Schechter AN. 1992. Influence of sickle hemoglobin polymerization and membrane properties on deformability of sickle erythrocytes in the microcirculation. Biophys J. 63:774–783.
- Drobin D, Kjellstrom BT, Malm E, Malavalli A, Lohman L, Vandegriff KD, et al. 2004. Hemodynamic response and oxygen transport in pigs resuscitated with maleimide-polyethylene glycol-modified hemoglobin (MP4). J Appl Physiol. 96:1843–1853.
- Embury SH, Matsui NM, Ramanujam S, Mayadas TN, Noguchi CT, Diwan BA, et al. 2004. The contribution of endothelial cell P-selectin to the microvascular flow of mouse sickle erythrocytes in vivo. Blood. 104:3378–3385.
- Embury SH, Mohandas N, Paszty C, Cooper P, Cheung AT. 1999. In vivo blood flow abnormalities in the transgenic knockout sickle cell mouse. J Clin Invest. 103:915–920.
- Feola M, Simoni J, Angelillo R, Luhruma Z, Kabakele M, Manzombi M, Kaluila M. 1992. Clinical trial of a hemoglobin based blood substitute in patients with sickle cell anemia. Surg Gynecol Obstet. 174:379–386.
- Gladwin MT, Crawford JH, Patel RP. 2004. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med. 36:707–717.
- Gonzalez P, Hackney AC, Jones S, Strayhorn D, Hoffman EB, Hughes G, et al. 1997. A phase I/II study of polymerized bovine hemoglobin in adult patients with sickle cell disease not in crisis at the time of study. J Investig Med. 45:258–264.
- Intaglietta M, Tompkins WR. 1973. Microvascular measurements by video image shearing and splitting. Microvasc Res. 5:309–312.
- Kalambur VS, Mahaseth H, Bischof JC, Kielbik MC, Welch TE, Vilback A, et al. 2004. Microvascular blood flow and stasis in transgenic sickle mice: utility of a dorsal skin fold chamber for intravital microscopy. Am J Hematol. 77:117–125.
- Kaul DK, Fabry ME. 2004. In vivo studies of sickle red blood cells. Microcirculation. 11:153–165.
- Kaul DK, Fabry ME, Nagel RL. 1996. The pathophysiology of vascular obstruction in the sickle syndromes. Blood Rev. 10:29–44.
- Kaul DK, Hebbel RP. 2000. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 106:411–420.
- Kaul DK, Liu X, Nagel RL. 2001. Ameliorating effects of fluorocarbon emulsion on sickle red blood cell-induced obstruction in an ex vivo vasculature. Blood. 98:3128–3131.
- Klug PP, Kaye N, Jensen WN. 1982. Endothelial cell and vascular damage in the sickle cell disorders. Blood Cells. 8:175–84.
- Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. 1993. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 143:1055–1062.
- Li J, Kim K, Hahm E, Molokie R, Hay N, Gordeuk VR, et al. 2014. Neutrophil AKT regulates heterotypic cell-cell interactions during vascular inflammation. J Clin Invest. 124:1483–1496.
- Lipowsky HH, Sheikh NU, Katz DM. 1987. Intravital microscopy of capillary hemodynamics in sickle cell disease. J Clin Invest. 80:117–127.
- National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition.Washington, DC: The National Academies Press. 2011.
- Oliva ME. 2005. The effect of Oxy-MP4, CO-MP4 and DPTA on the filterability of sickle cell red blood cells during continuous deoxygenation [Thesis]. M.S., University of California, San Diego.
- Orringer E, Gonzalez P, Hackney A, Jones SK, Hoffman EB, Jacobs EE, Hughes GS. 1995. A phase I study of polymerized bovine hemoglobin (PBH) in adult patients with sickle cell disease not in crisis at the time of study. In: Beuzard Y, Lubin B, Ross J, Eds. Sickle Cell Disease and Thalassemias: New Trends in Therapy. Montrogue, France: Colloque INSERM/John Libbey Eurotext Ldt., pp. 301–302.
- Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. 2000. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 96:314–320.
- Platt OS. 2000. Sickle cell anemia as an inflammatory disease. J Clin Invest. 106:337–338.
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO III, Schechter AN, Gladwin MT. 2002. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 8: 1383–1389.
- Rodgers GP, Schechter AN, Noguchi CT, Klein HG, Nienhuis AW, Bonner RF. 1990. Microcirculatory adaptations in sickle cell anemia: reactive hyperemia response. Am J Physiol Heart Circ Physiol. 258:H113–120.
- Ryan TM, Ciavatta DJ, Townes TM. 1997. Knockout-transgenic mouse model of sickle cell disease. Science. 278:873–876.
- Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M. 2006. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 108:3603–3610.
- Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. 2003. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol. 285:H1411–H1419.
- Vandegriff KD, Bellelli A, Samaja M, Malavalli A, Brunori M, Winslow RM. 2004. Kinetics of NO and O2 binding to a maleimide poly(ethylene glycol)-conjugated human haemoglobin. Biochem J. 382:183–189.
- Vandegriff KD, Malavalli A, Woodridge J, Lohman J, Winslow RM. 2003. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 43:509–516.
- Vercellotti GM, Belcher JD. 2014. Not simply misshapen red cell: multimulecular and cellular events in sickle vasoocclusion. J Clin Invest. 124:1462–1465.
- Winslow RM, Intaglietta M, Tsai AG, Vandegriff KD, Wettstein R. 2002. Autoregulation and vasoconstriction: foundation for a new generation of blood substitutes. Blood. 100:210.
- Winslow RM, Lohman J, Malavalli A, Vandegriff KD. 2004. Comparison of PEG-modified albumin and hemoglobin in extreme hemodilution in the rat. J Appl Physiol. 97:1527–1534.
- Yabuki A, Matsushita M, Malchesky P, Iwasaki K, Iwashita Y, Nose Y. 1988. In vitro evaluation of a pyridoxalated hemoglobin polyoxyethylene conjugated in reversing cell sickling. ASAIO Trans. 773–777.
