?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Hydroxyapatite (HA), the main mineral component of bones and teeth, was synthesized by using the reaction between calcium nitrate tetrahydrate Ca(NO3)2∙4H2O and diammonium hydrogen phosphate (NH4)2HPO4 (DAHP) with a chemical precipitation method. The objective of this study is to utilize novel inorganic–organic nanocomposites for biomedical applications. HA is an inorganic component (75% w) and chitosan, alginate and albumin (Egg white) are organic components of nanocomposites (25% w). Nanocomposites were prepared in deionized water solutions, at room temperature, using a mechanical and magnetic stirrer for 48 h. The microstructure and morphology of sintered n-HAP were tested at different preheating temperature and laser sintering speed with scanning electron microscopy (SEM), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR).
Introduction
Bone is a composite consisting of 69% hydroxyapatite (HA; inorganic component), 20% collagen type I (organic matrix) and 9% water (CitationTathe et al. 2010). HA [Ca10(PO4)6(OH)2] is the main mineral component of bones, teeth and Sea corals (CitationRatner et al. 2004, CitationThamaraiselive and Rajeswari 2004). HA ceramic attracted many interest because of its excellent biocompatibility, bioactivity, osteoconductivity and osteoinductivity (CitationDeer and Howie 1985, CitationWang 2003). HA particles have been used extensively for dental and bone repair and tissue engineering (CitationNayak 2010, CitationBouyer et al. 2000, CitationFerraz et al. 2004, CitationSantos et al. 2004, CitationManuel et al. 2003, CitationJarcho et al. 1977, CitationJanackovic et al. 2001, CitationBalamurugan et al. 2006). In addition, HA has been studied as carriers of protein (CitationBalamurugan et al. 2006, CitationManso et al. 2000), antibiotics and antibacterials (CitationBrendel et al. 1992) anticancer (CitationTakahashi et al. 1995), growth factor (CitationManafi and Joughehdoust 2009), etc(1). Several methods have been used for the synthesis of HA including sol–gel approach (CitationSantos and Luklinska 2001, CitationMontazeri and Jahandideh 2011), hydrothermal technique (CitationAthanasiou et al. 2000, CitationSopyan et al. 2007, CitationLee 2005), electrodeposition technique (CitationWeiner et al. 1999), precipitation technique, (CitationMyer 2003, CitationTeoh 2004, CitationYaszemski et al. 1996, CitationNair and Laurencin 2006, CitationDrotleff and Lungwitz 2004) multiple emulsion technique (CitationWang and Li 2007), and spray drying method (CitationOliveiraa et al. 2006). Practically, the HA particles have been used as bone scaffolds to prove an improved bone in-growth and osseointegration (CitationBrendel et al. 1992). However, the brittleness and low strength limited their wider applications in hard tissue implants (CitationLim 1999, CitationPeppas and Mikos 1986, CitationBouwstra and Jungiger 1993). So composites of HA synthesis and natural polymers were prepared and used as dental and bone replacement implants. Elkady et al. have reported the synthesis of HA–polyvinyl alcohol nanocomposites and investigated the in vitro bioactivity test (CitationDeer and Howie 1985). Kazemzadeh et al. reported the fabrication of HA– gelatin composite scaffolds for bone tissue engineering application (CitationThamaraiselive and Rajeswari 2004). Cui et al. have reported the preparation of nano-HA/collagen/PLA composite by biomimetic synthesis and investigated the in vitro and in vivo studies (CitationWang 2003). Reis et al. have reported the synthesis of novel HA–chitosan bilayered scaffold (CitationBouyer et al. 2000). Lavrynenko et al. reported the preparation of HA–chitosan composite made by a one-step co-precipitation method and investigated the in vivo study (CitationFerraz et al. 2004). Zargarian et al. carried out the synthesis of nanofibrous composite scaffold of PCL/HA–chitosan/PVA (CitationSantos et al. 2004). Kalpana et al. have reported the synthesis of chitosan–polygalacturonic acid/HA nanocomposites for in vitro study (CitationManuel et al. 2003). Chitosan is a deacetylation derivative of chitin (CitationFerraz et al. 2004). Chitosan attracted many interest because of its excellent nontoxic, biodegradable, biocompatibility (CitationGranja et al. 2004, CitationVijayalakshmi and Rajeswari 2006). So it has been widely used as biomaterials in pharmaceutical and medical fields such as tissue engineering scaffolding, as well as collagen (CitationSantos et al. 2004). Alginic acid (Alg) is a natural heteropolysaccharide and a linear copolymer composed of two monomeric units, d-mannuronic acid and l-guluronic acid (CitationBarinov et al. 2008, CitationKimura 2007). Alginate attracted many interest because of its excellent nontoxic, biodegradable, biocompatibility (CitationStamatialis and Papenburg 2008, CitationManso et al. 2000). So it has been widely used as biomaterials in pharmaceutical and medical fields such as tissue engineering scaffolding and drug delivery systems (CitationOh et al. 2006, CitationXuan et al. 2009).
In this work, HA–natural polymers nanocomposites were successfully synthesized. The structure and morphology of the nanocomposites were investigated by XRD, EDX, FT-IR and SEM. Finally, in vitro cytotoxicity testing on the nanocomposites was investigated.
Materials and methods
Materials
Calcium nitrate tetrahydrate (Ca(NO3)2∙4H2O), diammonium hydrogen phosphate (NH4)2HPO4 (DAHP), ammonia solution (25%) and triethanolamine (TEA) were purchased from Merck (Germany). Chitosan (75% degree of deacetylation) and sodium alginate were purchased from Sigma-Aldrich (St Louis, MO). Ammonium chloride (NH4Cl) was purchased from Fluka (Buchs, Switzerland). The infrared spectra of the nanocomposites were recorded on a Perkin Elmer 983 infrared spectrophotometer (Perkin Elmer, Boston, MA) at room temperature. X-ray powder diffraction using a Rigaku D/MAX-2400 X-ray diffractometer with Ni-filtered Cu Kα radiation and scanning electron microscopy measurements were conducted using a VEGA/TESCAN.
Methods
Synthesis of Nano-Hydroxyapatite (n-HA)
In this work, HA nanoparticles were synthesized using a chemical precipitation method (CitationHarris 1984, CitationKumar 2006). According to this method, 6.5 g of Ca(NO3)2∙4H2O (0.033 mol) and 4.3 g of diammonium hydrogen phosphate (0.035 mol) were dissolved in 50 and 45 mL of deionized water, respectively, with ratio Ca/P = 1.7. Triethanolamine (TEA; 1.8 g) (0.013 mol) was used in conjunction with Ca(NO3)2∙4H2O solution (Ca2+:TEA = 1:0.6). The pH of both calcium nitrate and DAHP solutions was maintained at ∼11–12. (NH4)2HPO4 solution was added drop-wise to the mixture of Ca(NO3)2∙4H2O and TEA, stirred using a mechanical stirrer for 5 h. The pH of the reacting mixture was also maintained at ∼11–12 by adding NH4OH solution. A white gelatinous precipitate was formed, which was filtered by a centrifugal filtration process and washed with deionized water and NH4Cl solution, so dried at 85°C for 20 h (CitationAkbarzadeh et al. 2012c, CitationEbrahimnezhad et al. 2013, CitationAlimirzalu et al. 2014, CitationHosseininasab et al. 2014, CitationRezaei-Sadabady et al. 2013, CitationNejati-Koshki et al. 2013, CitationGhasemali et al. 2013, CitationMollazade et al. 2013, CitationAkbarzadeh et al. 2014, CitationAkbarzadeh et al. 2013a, CitationAhmadi et al. 2014, CitationAlizadeh et al. 2014c).
Preparation of HA–natural polymers nanocomposites
In this part, five samples were prepared using HA (75% w) and natural polymers (25% w) (chitosan, alginate, albumin) as inorganic and organic components of nanocomposites, respectively (CitationFuge and Saltzman 1997, CitationVert et al. 1991, CitationKaye 1989, CitationLanger 1990).
Preparation of sample 1: 0.4 g chitosan was dissolved in 15 ml acetic acid 5% w/v solution using a magnetic stirrer at room temperature for 4 h. HA (3 g) was added slowly to the chitosan solution and stirred with a magnetic stirrer for 36 h. After creating homogeneous suspension, 0.8 g albumin was added to the suspension and stirred using mechanical and magnetic stirrer for 25 h at mentioned conditions. These conditions were established for the synthesis of other samples. Sample 2 was synthesized in the same way. However, in the synthesis of other samples was not used acetic acid solution. The resulting suspension was placed in the frozen overnight. The following shows the components of each sample (CitationPourhassan-Moghaddam et al. 2014, CitationAnganeh et al. 2014, CitationDavoudi et al. 2014, CitationAkbarzadeh et al. 2012b, CitationValizadeh et al. 2012, CitationAkbarzadeh et al. 2013b, CitationPourhassan-Moghaddam et al. 2013, CitationKouhi et al. 2014, CitationSadat Tabatabaei Mirakabad et al. 2014, CitationEatemadi et al. 2014b, CitationAbbasi et al. 2014b, Citation2014c).
Table I. The components of each sample.
Results and discussion
Characterization
The infrared spectra were recorded using a Fourier transform infrared spectrophotometer (FT-IR, Nicolet NEXUS 670; Thermo Scientific, Waltham, MA), and the sample and KBr were pressed to form a tablet. Powder X-ray diffraction (Rigaku D/MAX-2400 X-ray diffractometer with Ni-filtered Cu Kα radiation) was used to investigate the crystal structure of the nanocomposites. The infrared spectra of the nanocomposites were recorded on a Perkin Elmer 983 infrared spectrometer (Perkin Elmer) at room temperature. The size and shape of the nanocomposites were determined using a scanning electron microscope (VEGA/TESCAN), whereby a sample was dispersed in ethanol and a small drop was spread onto a 400 mesh copper grid.
Fourier transform infrared spectroscopy (FT-IR)
Fourier transform infrared spectroscopy was used to show the structure of HA (), HA/chitosan–albumin nanocomposite (), HA/alginate–albumin nanocomposite () and HA/albumin nanocomposite ().
Figure 1. Fourier transform infrared spectroscopy (a) structure of HA, (b) HA/chitosan–albumin nanocomposite, (c) HA/alginate–albumin nanocomposite, and (d) HA/albumin nanocomposite.
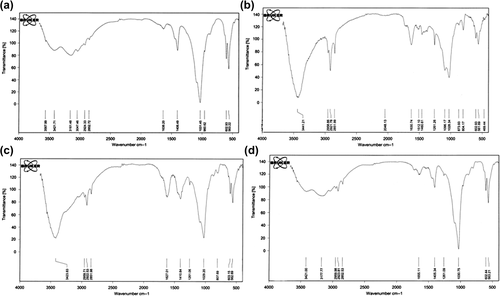
From the infrared spectra shown in , the absorption peaks at 3422 cm‐1 correspond to the stretching vibration of H–O bonds in HA. Comparing the infrared spectra in , HA–natural polymers showed absorption peak at 3440 attributable to the stretching vibrations of H–O bonds in HA and chitosan, N–H bonds in chitosan and albumin (), stretching vibration of H–O bonds in HA and alginate, N–H bonds in albumin (), stretching vibration mode of O–H bonds in HA and N–H bonds in albumin (). A peak at 1631 cm‐1 (), 1627 cm‐1 () and 1655 cm‐1 () corresponds to stretching vibration of carbonyl groups in natural polymers. The absorption peaks at 1512–1461 cm‐1 and 1030, 603, 563 cm‐1 attributable to the CO3 − 2 and PO4 − 3 groups in HA, respectively () (CitationAlizadeh et al. 2014b, CitationNejati-Koshki et al. 2014a, CitationGhalhar et al. 2014, CitationKarnoosh- Yamchi et al. 2014, CitationAlizadeh et al. 2014a, CitationZohre et al. 2014).
X-ray diffraction patterns
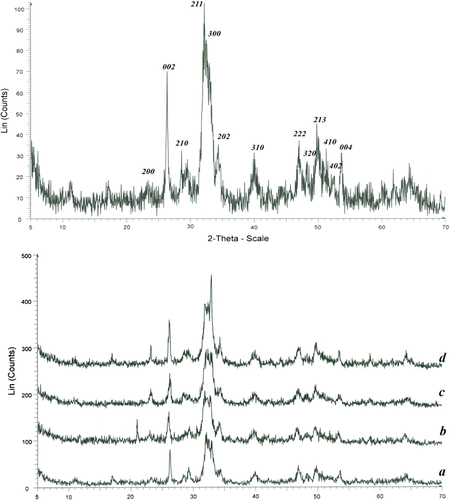
shows the X-ray diffraction patterns for the pure HA (a) and HA–natural polymers nanocomposites (b–d). It can be concluded from that the diffraction peaks of sintered n-HAP under different preheating temperature are basically the same in position and number with that of initial n-HAP, which means n-HAP remains undecomposed after preheated and sintered (CitationKost and Langer 1984, CitationMirth 1980, CitationDash and Cudworth 1998). The diffraction peaks become higher and the peak (2 1 1) became narrower after sintered, which indicated that n-HAP grains have grown up and the degree of crystalline increases with the increase of preheating temperature. The results are consistent with the analysis of FT-IR and SEM. The width B of a diffraction peak is the comprehensive effects of grain size and strain broadening. So the Hall–Williamson method (Eq. (1)) was adopted to calculate grain size (CitationNejati-Koshki et al. 2014b, CitationFekri Aval et al. 2014, CitationAbbasi et al. 2014a, CitationEatemadi et al. 2014a, CitationEbrahimi et al. 2014).
Figure 2. Shows the X-ray diffraction patterns for the pure HA (a) and HA–natural polymers nanocomposites (b–d).
Here β is the strain in the crystallites, D represents the size of the crystallites, the constant k is typically close to unity and ranges from 0.8 to 1.39, θ is the Bragg angle, and λ is the X-ray wavelength, which equals to 1.54056 Å (CitationApicella and Hopfenberg 1978, CitationFlooladi 1978, CitationBanker 1984, CitationBenedtti et al. 1990).
The absorption peaks at (0 0 2), (2 1 1) and (3 0 0) are the main crystal phases of HA. The diffraction peaks of nanocomposites shows the main peaks of HA, so it can be concluded that the main crystal phases of the HA and nanocomposites were just HA. The diffraction peaks of HA became weaker than that of HA–natural polymers nanocomposites, which proved that the crystallinity of HA was lower than that of HA–natural polymers nanocomposites. The low crystallinity may lead HA to the low stability of the obtained material. On the other hand, the low crystallinity indicates that the bioactivity of the material is high, which is favorable to its nano bioeffects (CitationAdlar et al. 1960, CitationLando and Morawezt 1963, CitationKuzuya and Kondo 1991).
Size, morphology, and core–shell structure of nanocomposites
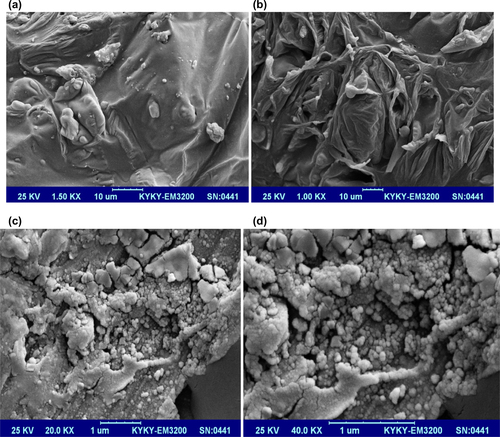
Scanning electron micrographs of pure HA are shown in and HA/chitosan–albumin, HA/alginate–albumin and HA/albumin are shown in , respectively.
In vitro cytotoxicity study
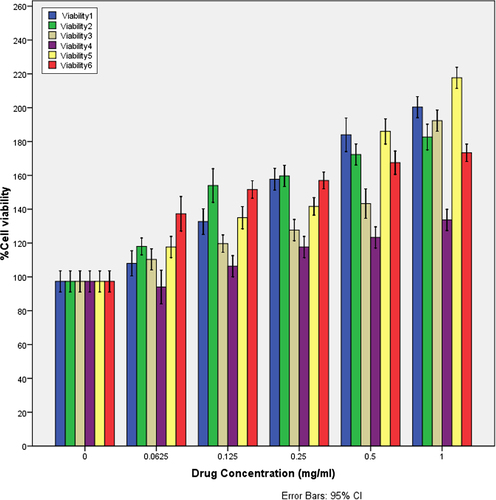
The MTT assay is an important method for evaluating the cytotoxicity of biomaterials in vitro. The crystal size of HA plays a major role in bone tissue engineering for nutrient supplementation and cell attachment. A highly porous and nanocrystal structure is a prerequisite to ensure that the biological environment is conductive for cell attachment, proliferation, tissue growth and adequate nutrient flow. The cytotoxicity effects of derived HA crystals at different components were investigated by MTT assay. The HA crystals showed no cytotoxicity in the MG-63 cell line as seen in (CitationStauffer and Peppas 1992, CitationPeppas and Khare 1993, CitationKaetsu et al. 1993).
In this work, we have characterized the in vitro behavior of HA–natural polymers nanocomposites for biomedical applications. n-HA was prepared with a chemical precipitation method. Ceramic matrix nanocomposites were synthesized by using hydroxyapatite as inorganic component and (chitosan, alginate and albumin) as organic components of nanocomposites. Five samples were prepared with hydrogen bonding between inorganic and organic components at room temperature, using mechanical and magnetic stirrer for 48 h, in the deionized water solution, so freeze drying at − 70°C for 24 h. Fourier transform infrared spectroscopy was used to show the structure of HA nanoparticles and HA–natural polymers nanocomposites. The X-ray powder diffraction data only showed peaks attributable to inorganic component. The size, morphology, and core–shell structure of the synthesized HA and nanocomposites were analyzed by scanning electron microscopy (CitationAkbarzadeh et al. 2012a, CitationAkbarzadeh et al. 2012d).
Conclusion
n-HA was synthesized by the reaction between calcium nitrate tetrahydrate and diammonium hydrogen phosphate (DAHP) solutions with a chemical precipitation method using a mechanical stirrer for 5 h, at room temperature and pH level ∼11–12. The precipitating agent was TEA. Novel inorganic–organic nanocomposites were prepared by HA as inorganic component of nanocomposites (75% w) and natural polymers (chitosan, alginate and albumin) as organic components of nanocomposites (25% w). Nanocomposites were prepared at room temperature, deionized water solution, stirred with mechanical and magnetic stirrer for 48 h, so frozen at − 70°C overnight. These nanocomposites were used for in vitro cytotoxicity study. The resulting nanocomposites were characterized by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR).
Authors’ contributions
JHC conceived the study and participated in its design and coordination. YKK participated in the sequence alignment and drafted the manuscript. AA, MA, SD, MM, GA, MS, KHK, and TYK helped in drafting the manuscript. All authors read and approved the final manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2008-0062282).
References
- Abbasi E, Akbarzadeh A, Kouhi M, Milani M. 2014a. Graphene: synthesis, bio-applications, and properties. Artif Cells Nanomed Biotechnol 43:1–7.
- Abbasi E, Fekri Aval S, Akbarzadeh A, Milani M, Tayefi Nasrabadi H, Joo SW, et al. 2014b. Dendrimers: synthesis, applications and properties. Nanoscale Res Lett. 9:247
- Abbasi E, Milani M, Fekri Aval S, Kouhi M, Akbarzadeh A, Tayefi Nasrabadi H, et al. 2014c. Silver nanoparticles: synthesis, properties, bio-applications and limitations. Crit Rev Microbiol. 1–8.
- Adlar G, Ballamtin D, Baysal B. 1960The mechanism of free radical polymerization in the solid state. J Polym Sci. 48:195–206.
- Ahmadi A, Shirazi H, Pourbagher N, Akbarzadeh A, Omidfar K 2014. An electrochemical immunosensor for digoxin using core-shell gold coated magnetic nanoparticles as labels. Mol Biol Rep. 41:1659–1668.
- Akbarzadeh A, Mikaeili H, Asgari D, Zarghami N, Mohammad R, Davaran S. 2012a. Preparation and in-vitro evaluation of doxorubicin-loaded Fe3O4 magnetic nanoparticles modified with biocompatible copolymers. Int J Nanomedicine. 7:511–526.
- Akbarzadeh A, Mohamad S, Davaran S. 2012b. Magnetic nanoparticles: preparation, physical properties and applications in biomedicine Nanoscale Res Lett.7:144–157.
- Akbarzadeh A, Nejati-Koshki K, Soghrati MM, Alimohammadi S, Ghamari MF, Davaran S. 2013a. In vitro studies of NIPAAM-MAA-VP copolymer-coated magnetic nanoparticles for controlled anticancer drug release J Encapsulation Adsorpt Sci.3:108–115.
- Akbarzadeh A, Rezaei A, Nejati-Koshki K, Alimohammadi S, Davaran S. 2014. Synthesis and physicochemical characterization of biodegradable star-shaped poly lactide-co-glycolide– β-cyclodextrin copolymer nanoparticles containing albumin Adv Nanoparticles3:14–22.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. 2013b. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 8:102.
- Akbarzadeh A, Samiei M, Joo SW, Anzaby M, Hanifehpour Y, Nasrabadi HT, Davaran S. 2012c. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line J Nanobiotechnol. 10:46.
- Akbarzadeh A, Zarghami N, Mikaeili H, Asgari D, Goganian AM, Khiabani HK, Davaran S. 2012d. Synthesis, characterization and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Int J Nanotechnol Sci Environ. 5:13–25
- Alimirzalu S, Akbarzadeh A, Abbasian M, Alimohammadi S, Davaran S, Hanifehpour Y, et al. 2014. Synthesis and study of physicochemical characteristics of Fe3O4 magnetic nanocomposites based on poly(Nisopropylacrylamide)for anti-cancer drugs delivery. Asian Pac J Cancer Prev. 15:49–54.
- Alizadeh E, Akbarzadeh A, Zarghami N, Eslaminejad MB, Hashemzadeh S, Nejati-Koshki K. 2014a. Up-regulation of liver enriched transcription factors (HNF4a and HNF6) and liver specific microRNA (miR-122) by inhibition of Let-7b in mesenchymal stem cells. Chem Biol Drug Design(in press)
- Alizadeh E, Zarghami N, Eslaminejad MB, Akbarzadeh A, Barzegar A, Mohammadi SA. 2014b. The effect of dimethyl sulfoxide (DMSO) on hepatic differentiation of mesenchymal stem cells. Artif Cells Nanomed Biotechnol 43:1–8.
- Alizadeh E, Zarghami N, Eslaminejad MB, Akbarzadeh A, Jahangir S, Barzegar A, et al. 2014c. The effect of dimethyl sulfoxide on hepatogenic differentiation of mesenchymal stem cells. Int J Pediatrics (2):57–58.
- Anganeh MT, Sadat Tabatabaei Mirakabad F, Izadi M, Zeighamian V, Badrzadeh F, Salehi R, et al. 2014. The comparison between effects of free curcumin and curcumin loaded PLGA-PEG on telomerase and TRF1 expressions in calu-6 lung cancer cell line. Int J Biosci. 4:134–145.
- Apicella A, Hopfenberg HB. 1978. The effects of thermal treatment, residual solvent, and preswelling on the thermal properties and sorption behavior of poly(2,6-dimethyl, 1,4-phenylene oxide)- polystyrene blends. P olym Eng Sci. 18:1006–1011.
- Athanasiou KA, Zhu CF, Lanctot DR, Agrawal CM, Wang X. 2000. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 6:361–381.
- Balamurugan A, Michel J, Faure J, Benhayoune H, Wortham L, Sockalingum G, et al. 2006. Synthesis and structural analysis of sol gel derived stoicheometric monophasic hydroxyapatite. Ceramics-Silikaty. 50:27–31.
- Banker GS. 1984. Medical Application of Controlled Release. Boca Raton, FL: CRC Press Inc.
- Barinov SM, Komlev VS, Smirnov VV, Fadeeva IV, Fomin AS. 2008. Advanced nanoceramics for bone tissue engineering powder. Metall Prog. 8:331–335.
- Benedtti LM, Toppa EM, Stella VJ. 1990. Microspheres of hyaluronic acid esters—Fabrication methods and in vitro hydrocortisone release. J Control Release. 13:33.
- Bouwstra JA, Jungiger HE. 1993. Encyclopedia of Pharmaceutical Thecnology. New York: Marcel Dekker, 7:441.
- Bouyer E, Gitzhofer F, Boulos MI. 2000. Morphological study of hydroxyapatite nanocrystal suspension. J Mater Sci Mater Med. 11:523–531.
- Brendel T, Engel A, Russel C. 1992. Hydroxyapatite coating by polymeric route J Mater Sci Mater Med. 3:175–179.
- Dash AK, Cudworth GC. 1998. Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol Methods. 40:1–12.
- Davoudi Z, Akbarzadeh A, Rahmatiyamchi M, Movassaghpour AA, Alipour M, Nejati-Koshki K, et al. 2014. Molecular target therapy of Akt and NF-kB signaling pathways and multidrug resistanceby specific cell penetrating inhibitor peptides in HL-60 cell line. APJCP. 9:4353–4358.
- Deer WA, Howie RA 1985. An Introduction to the Rock Forming Minerals. Longman: Harlow, 504–509.
- Drotleff S, Lungwitz U, Breunig M, Dennis A, Blunk T, Tessmar J, Gopferich A. 2004. Biomimetic polymers in pharmaceutical and biomedical sciences. Eur J Pharm Biopharm. 58:385–407.
- Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A, et al. 2014a. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. (in press)
- Eatemadi A, Daraee H, Zarghami N, Melat Yar H, Akbarzadeh A, Hanifehpour Y. 2014b. Nanofiber: synthesis and biomedical applications. Artif Cells Nanomed Biotechnol. 1:1–11
- Ebrahimi E, Abbasi E, Akbarzadeh A, Khandaghi AA, Davaran S. 2014. Novel drug delivery system based on doxorubicin-encapsulated magnetic nanoparticles modified with PLGA-PEG1000 copolymer. Artif Cells Nanomed Biotechnol(in press)
- Ebrahimnezhad Z, Zarghami N, Keyhani M, Amirsaadat S, Akbarzadeh A, Rahmati M, et al. 2013. Inhibition of hTERT gene expression by silibinin-loaded PLGA-PEG-Fe3O4 in T47D breast cancer cell line. Bioimpacts. 3:67–74.
- Fekri Aval S, Akbarzadeh A, Yamchi MR, Barkhordari A, Mohamadzadeh N, Nejati-Koshki K, et al. 2014. Gene silencing effect of SiRNA-magnetic copolymers on hTERT gene expression in lung cancer cell line Artif Cells Nanomed Biotechnol(in press)
- Ferraz MP, Monteri FJ, Manuel CM. 2004. Hydroxyapatite nanoparticles: a review of preparation methodologies. J Appl Biomater Biomech. 2:74–80.
- Flooladi M. 1978. Synthesis and Charactrization and Release Study of the Polymer with Pendent Pesticide. Ph.D. Thesis.
- Fuge KF, Saltzman WM. 1997. Polymeric implants for cancer chemotherapy. Adv Drug Deliv Rev. 26:209–230.
- Ghalhar MG, Akbarzadeh A, Rahmati M, Mellatyar H, Dariushnejad H, Zarghami N. 2014. Comparison of inhibitory effect of 17-AAG nanoparticles and free 17-AAG in HSP90 gene expression in breast cancer. APJCP. (in press)
- Ghasemali S, Akbarzadeh A, Alimirzalu S, Rahmati Yamchi M, Barkhordari A, Tozihi M, Kordi SH, et al. 2013. Study of inhibitory effect of β-cyclodextrin-helenalincomplex on HTERT gene expression in T47D breast cancer cell line by real time quantitative PCR (q-PCR) APJCP. 14:6949–6953.
- Granja PL, Silva AIN, Borges JP, Barrias CC, Amaral IF. 2004. Preparation and Characterization of Injectable Chitosan-Hydroxyapatite Microspheres. Key Eng Mat. 254–256:573–576.
- Harris FW. 1984. Medical Application of Controlled Release. Boca Raton, Florida: CRC Press Inc.
- Hosseininasab S, Pashaei‐Asl R, Khandaghi AA, Nasrabadi HT, Nejati‐Koshki K, Akbarzadeh A, et al. 2014. Synthesis, characterization, and in vitro studies of PLGA‐PEG nanoparticles for oral insulin delivery. Chem Biol Drug Desi. 84:349–357.
- Janackovic D, Petrovic-Prelevic I, Kostic-Gvozdenovic L, Petrovic R, Jokanovic V, Uskokovic D. 2001. Influence of synthesis parameters on the particle sizes of nanostructured calciumhydroxyapatite. Key Eng Mater. 192–195:203–206.
- Jarcho M, Kay JF, Gumar KI, Doremus RH, Drobeck HP. 1977. Tissue, cellular and subcellular events at a bone ceramic hydroxyapatite interface. J Biosci Bioeng. 1:79–92.
- Kaetsu I, Uchida K, Sutani K, Tankaa S, Okada K. 1993. Controlled Realease Society,Lincolnshire, IL. p 352.
- Karnoosh-Yamchi J, Mobasseri M, Akbarzadeh A, Davaran S, Ostad-Rahimi AR, Hamishehkar H, et al. 2014. Preparation of pH sensitive insulin-loaded Nano hydrogels and evaluation of insulin releasing in different pH conditions. Mol Biol Rep. (in press)
- Kaye TM. 1989. Sales From Focus Polymers, High Performance Fibers From Focus Polymer.9:5–6.
- Kimura I. 2007. Synthesis of hydroxyapatite by interfacial reaction in a multiple emulsion. Res Lett Mater Sci. Article ID 71284, 1–4.
- Kost J, Langer R. 1984. Controlled release of bioactive agents. Trends Biotechnol. 2:47.
- Kouhi M, Vahedi A, Akbarzadeh A, Hanifehpour Y, Joo SW. 2014. Investigation of quadratic electro-optic effects and electro absorption process in GaN/AlGaN spherical quantum dot. Nanoscale Res Lett. 9:1–6.
- Kumar CSSR. 2006. Biological and Pharmaceutical Nanomaterials. Wiley: Hoboken.
- Kuzuya M, Kondo S. 1991The Nature of Hydrolysis of Novel Methacryloyl Polymeric Prodrugs Prepared by Mechanochemical Solid State Polymerization. Chem Pharm Bull. 39:3018–3022.
- Lando JB, Morawezt HJ. 1963. Polymerization in the crystalline state. IV. Calcium acrylate and barium methacrylate. Polym Sci Polym Chem Ed. 19:789.
- Langer R. 1990. New methods of drug delivery. Science. 249:1527–33.
- Lee KL. 2005. Design parameters of polymers for tissue engineering applications. MacromolRes. 13:277–284.
- Lim YB. 1999. A self destroying polycationic polymer: biodegrable polyester, poly (4-hydroxyl-L-proline ester). J Am Chem Soc. 121:5633.
- Manafi SA, Joughehdoust S. 2009. Synthesis of hydroxyapatite nanostructure by hydrothermal condition for biomedical application. Iranian J Pharm Sci.5:89–94.
- Manso M, Jimenez C, Morant C, Herrero P. 2000. Electrodeposition of hydroxyapatite coatings in basic conditions. Biomaterials. 21:1755–1761.
- Manuel CM, Ferraz MP, Monteiro FJ. 2003. Synthesis of hydroxyapatite and tri calcium phosphate nanoparticles. Preliminary Studies Key Eng Mater. 240–242:555–558.
- Mirth DB. 1980. The use of controlled and sustained release agents in dentistry: a review of applications for the control of dental caries. Pharmacol Ther Dent. 5:59.
- Mollazade M, Nejati-Koshki K, Akbarzadeh A, Hanifehpour Y, Zarghami N, Joo SW. 2013. PAMAM dendrimers arugment inhibitory effect of curcumin on cancer cell proliferation: possible inhibition of telomerase. APJCP. 14:6925–6928.
- Montazeri N, Jahandideh R, Biazar E. 2011. Synthesis of fluorapatite– hydroxyapatite nanoparticles and toxicity investigations. Int J Nanomedicine. 6:197–201.
- Myer K. 2003. Standard Handbook of Biomedical Engineering and Design. McGRAW-HILL: New York.
- Nair LS, Laurencin GT. 2006. Polymers as biomaterials for tissue engineering and controlled drug delivery. Adv Biochem Eng B iotechnol. Adv biochem Eng Biotechnol. 102:47–90.
- Nayak AK. 2010. Hydroxyapatite synthesis methodologies: an overview. Int J ChemTech Res. 2:903–907. ISSN: 0974-4290.
- Nejati-Koshki K, Akbarzadeh A, Pourhasan-Moghadam M, Joo SW. 2013. Inhibition of leptin and leptin receptor gene expression by silibinin- curcumin combination. APJCP. 14:6595–9.
- Nejati-Koshki K, Akbarzadeh A, Pourhassan-Moghaddam M, Joo SW, Hanifepour Y. 2014a. Curcumin inhibit leptin gene expression and secretion in breast cancer cells by estrogen receptors. Cancer Cell Int(in press).
- Nejati-Koshki K, Mesgari M, Ebrahimi E, Abhari A, Fekri Aval S, Khandaghi AA, Akbarzadeh A. 2014b. Synthesis and in-vitro study of cisplatin-loaded Fe3O4 nanoparticles modified with PLGA-PEG6000 copolymers in treatment of lung cancer. J Microencapsul. (in press).
- Oh S, Oh N, Appelford M, Ong JL. 2006. Bioceramics for tissue engineering applications-a review. Am J Biochem Biotechnol. 2:49–56.
- Oliveiraa JM, Rodrigues MT,Silva SS, Malafaya PB, Gomes ME, Viegas CA, et al. 2006. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials. 27:6123–6137.
- Peppas NA, Khare AR. 1993. Preparation, Structure and Diffusional Behavior of Hydrogels in Controlled Release. Adv Drug Del Rev. 11:1.
- Peppas NA, Mikos AG. 1986. Hydrogels in Medicine and Pharmacy. Boca Raton, FL: CRC Press. 1:1–27.
- Pourhassan-Moghaddam M, Rahmati-Yamchi M, Akbarzadeh A, Daraee H, Nejati-Koshki K, Hanifehpour Y, Joo SW. 2013. Protein detection through different platforms of immuno-loop-mediated isothermal amplification. Nanoscale Res Lett. 8:485.
- Pourhassan-Moghaddam M, Zarghami N, Mohsenifar A, Rahmati-Yamchi M, Gholizadeh D, Akbarzadeh A, et al. 2014. Watercress-based gold nanoparticles: biosynthesis, mechanism of formation and study of their biocompatibility in vitro. Micro Nano Lett 9:345–350.
- Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. 2004. Biomaterials Science, An Introduction to Materials in Medicine. 2nd ed. London: Academic Press, pp. 162.
- Rezaei-Sadabady R, Zarghami N, Barzegar A, Eidi A, Akbarzadeh A, Rezaei-Tavirani M. 2013. Studies of the relationship between structure and antioxidant activity in interesting systems, including tyrosol, hydroxytyrosol derivatives indicated by quantum chemical calculations. Soft. 2:13–18.
- Sadat Tabatabaei Mirakabad F, Akbarzadeh A, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S. 2014. PLGA-based nanoparticles as cancer drug delivery systems Asian Pac J Cancer Prev. 15:517–535.
- Santos C, Luklinska ZB. 2001. Hydroxyapatite as a filler for dental composite materials: mechanical properties and in vitro bioactivity of composites. J Mater Sci. 12:565–573.
- Santos MH, Oliveira M, Freitas Souza P, Mansur HS, Vasconcelos WL. 2004. Synthesis control and characterization of hydroxyapatite prepared by wet precipitation process. Mater Res. 7:625–630.
- Sopyan I, Mel M, Ramesh S, Khalid KA. 2007. Porous hydroxyapatite for artificial bone applications. Sci Technol Adv Mat. 8:116–123.
- Stamatialis DF, Papenburg BJ, Girones M, Saiful S, Bettahalli SNM. 2008. Medical applications of membranes: drug delivery, artificial organs and tissue engineering. J Memb Sci. 309:1–34.
- Stauffer SR, Peppas NA. 1992Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer 33:3932–3936.
- Takahashi H, Yashima M, Kakihana M, Yoshimura M. 1995. Synthesis of stoichiometric hydroxyapatite by a gel route from the aqueous solution of citric and phosphoneacetic acids. Eur J Solid State Inorg Chem. 32:829–835.
- Tathe A, Ghodke M, Nikalje AP. 2010. A brief review: biomaterials and their application. Int J Pharm Pharmaceutical Sciences. 2:19–23. ISSN- 0975-1491.
- Teoh SH. 2004. Engineering Materials for Biomedical Applications. Hackensack, NJ: World Scientific Pub.
- Thamaraiselive TV, Rajeswari S. 2004. Biological evaluation of bioceramic materials: a review. Trends Biomater Artif Organs. 18:9–17.
- Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, et al. 2012. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 7:276.
- Vert M, Li S, Garreau H. 1991. More about the degradation of LA/GA-derived matrices in aqueous media. J Control Release. 16:15–26.
- Vijayalakshmi U, Rajeswari S. 2006. Preparation and characterization of microcrystalline hydroxyapatite using sol gel method. Trends Mater Artif Org. 19:57–62.
- Wang L, Li CZ. 2007. Preparation and physicochemical Properties of a novel hydroxyapatite/chitosan-silk fibroin composite. Carbohydr Polym. 68:740–745.
- Wang M. 2003. Developing bioactive composite materials for tissue replacement. Biomaterials. 24:2133–2151.
- Weiner S, Wolfie T, Wagner HD. 1999. Lamellar bone: structure- function relations. J Struct Biol. 126:241–255.
- Xuan C, Hua T, Xinyu S. 2009. Preparation and characterization of homogeneous chito-san–polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 7:2693–2703.
- Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. 1996. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 17: 175–185.
- Zohre S, Akbarzadeh A, Kazem NJ, Nosratollah Z, Mohammad R, Aliakbar M, et al. 2014. Enhanced expression of klf4 and apoptosis in ovarian and lung cancer by histone deacetylase inhibitor: Trichostatin A. APJCP. 2014:9.p.