Abstract
This review attempts to summarize the information available on emerging trends in the treatment of tuberculosis caused by the bacteria Mycobacterium tuberculosis. Nanostructured biomaterials, liposomes, microparticles and solid lipid nanoparticles have unique physicochemical properties such as particularly small and convenient size, sustained release, great surface area to mass ratio and high reactivity with structure. These properties can be useful in easing the administration of antimicrobial drugs, thereby reducing the number of limitations in long-established antimicrobial therapeutics. In recent years, the encapsulation of antimicrobial drugs in all carrier systems has emerged as an innovative and promising change that increases therapeutic efficiency and reduces undesirable side effects of the drugs.
Introduction
Tuberculosis is a widespread disease, and presents difficulties that transcend the conventional medical approach. Tuberculosis is a very old scourge. Its mortality and morbidity increase because it is a global health problem. It is the most ordinary opportunistic infection in Acquired-Immuno Deficiency Syndrome. Tuberculosis (TB) is a disease caused by a microorganism (Mycobacterium tuberculosis) which invades the lungs and causes the beginning of disease (CitationKaur et al. 2014a).
This communicable disease spreads through air. Mycobacterium has worsened the problem in humans by acquiring various types of resistances like Multi-drug resistance (MDR), Single-drug resistance (SDR), and Extensive drug resistance (XDR).TB is the most common cause of death due to a single infectious agent worldwide in adults. The exact cause of this is unknown, although it is thought that it could be because of the low rate of recovery from TB due to a HIV infection as well as Multiple Drug Resistant Tuberculosis (MDR-TB), due to ineffective management. It is calculated that each year, approximately eight million new cases and two million deaths occur (CitationKaur et al. 2014b). represents the various species of Mycobacteria which cause tuberculosis.
Table I. Species of Mycobacteria that cause tuberculosis.
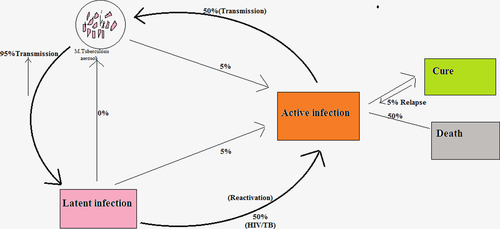
TB is commonly classified as being either latent or active.
Latent TB - The bacteria are motionless but present in the body. The patient has no signs and it is not communicable.
Active TB - The bacteria are active and make the patient sick. Active TB is communicable.
Transmission of tuberculosis
TB infection is initiated when the mycobacterium comes into contact with the alveolar macrophages. Mycobacterium tuberculosis (MTB) is free in its use of multiple cell surface receptors to grow into macrophages. The primary infection replicates within the endosome of macrophages, and then multiplies to the lymph nodes. The primary infection has no symptom in adults. Macrophages, B-lymphocytes, T-lymphocytes and fibroblasts are among the cells that combine to form a granuloma adjoining the infected macrophages (CitationKaur et al. 2014c). Bacteria are not always removed within the granuloma, but can become inactive, causing a latent infection (). Activation of TB again mostly occurs in the lungs but involves other organs also. Secondary TB lesions can grow in the lungs, peripheral lymph nodes, brain, kidneys and bone. All body parts can be affected by the disease, although it rarely affects the heart, pancreas, skeletal muscles and thyroid. The symptoms and signs of the disease are a cough with a duration of more than 2–3 weeks, production of mucus, bloody sputum, tissue destruction, chills, chest pain, fever, fatigue, shortness of breath, severe headache, weight loss, loss of appetite, tiredness, night sweats, or weakness (CitationKaur et al. 2014d).
Epidemiology
Initial data show that the number of TB cases detailed in the United States(US) was 9588, a rate of 3.0 cases per 100,000 population, compared with 3.2 cases per 100,000 population in 2012. The 4 states of California, Texas, New York, and Florida detailed more than half (51.3%) of all TB cases reported in 2013. Even though TB cases among foreign-born persons in the United States continue to decline, the rate of decline in TB incidence since 2012 among foreign-born persons (2.1%) lagged behind the rate of decrease among the U.S. born (8.4%), causing the proportion of TB cases in foreign-born persons to continue to increase .
In 2010, the reported number of TB cases declined a little from the previous year. In 2010, there were 11,182 detailed TB cases (3.6 cases per 100,000 persons) evaluated to 11,537 reported tuberculosis cases from 2009. Tuberculosis case totals are at present at the lowest number recorded since national reporting began in 1953. Even though detailed TB cases achieved all-time lows in the United States, there are still excessively high rates of TB among ethnic/racial minorities, mainly U.S.-born blacks. Tuberculosis rates are greater for a number of racial and ethnic groups, because a higher proportion of people in these groups have other risk factors for TB. shows the rate of tuberculosis cases by race/ethnicity in United States.
African-Americans or blacks born in the United States presented 40% of TB cases among U.S.-born persons.
Hispanics or Latinos accounted for the highest percentage of total cases of TB of any race/ethnicity (29%). The TB percent range for Asians (22.4 per 100,000) was approximately 3 times higher than that for Latinos or Hispanics (6.5 per 100,000) or African-Americans or blacks (7.0 per 100,000).
Table II. Rate of tuberculosis cases by race/ethnicity in United States.
Conventional drug therapy
An initial severe phase of rifampicin (RIF), isoniazid (INH), pyrazinamide (PYZ), and ethambutol (ETB) administered daily for 2 months.
A continuation phase of RIF and INH for an extra 4 months, daily or 3 (three) times per week, to be administered. INH eliminates most of the rapidly replicating bacilli in the first 2 weeks of treatment, together with streptomycin and ETB. represents the various examples of antitubercular drugs.
Table III. Classification of antitubercular drugs.
Drawback of conventional therapy
Rifampicin is an effective antibiotic used in anti-tuberculosis therapy, but treatment is prolonged, with oral administration of high systemic doses over a period of 4–10 months. Various systemic side effects and poor patient compliance are related after a long duration of anti-tubercular chemotherapy. To overcome these drawbacks, ligands are attached with various drug delivery systems. P-Amino salicylic acid (PAS) conjugated to maleylated bovine serum albumin (MBSA) was taken up competently through high-affinity MBSA binding sites on macrophages. Binding of the radio-labeled conjugate to cultured mouse peritoneal macrophages at 4°C was competed for by MBSA but not by P-Amino salicylic acid (PAS). At 37°C, the radio-labeled conjugate was quickly corrupted by the macrophages and the release of acid-soluble corrupted products in the medium. The drug linkage was about 100 times as effective as free PAS in killing the intracellular mycobacterium in mouse peritoneal macrophages infected in culture with Mycobacterium tuberculosis. discusses various side-effects associated with anti-tubercular drugs.
Table IV. Side effects of Anti TB drugs.
Emerging trends for the treatment of TB
There are several techniques which are used in TB treatment. A number of delivery systems are used for targeted drug delivery. The delivery systems are microparticles, nanoparticles, microspheres liposomes, solid lipid nanoparticles etc. A number of novel implants like micro particulate and a variety of other carrier-based drug delivery systems incorporating the principal anti-tuberculosis agents target the site of tuberculosis infection or reduce the dosing frequency with the aim of improving patient outcomes. represents some salient features of drug carriers which have been successfully used for the treatment of tuberculosis.
Table V. Salient features of drug carriers.
Liposomes
A liposome is a nano-sized bubble (vesicle). Liposomes have a spherical shape, and are made out of the same material as a cell membrane. Liposomes can be produced from lipid (phospholipids) and cholesterol. Charge, lipid concentration and size (ranging from 20 to 10,000 nm) of liposomes can be varied and these variations powerfully affect their behavior in vivo (CitationGarg and Goyal 2014b). Numerous liposome formulations are quickly taken up by macrophages. They are broken either for macrophage-specific delivery of drugs or for passive drug targets, permitting slow release of the drug over time from these cells. Liposomes can be packed with drugs, and used to deliver drugs for cancer and other diseases (CitationGagandeep et al. 2014). Liposomes hold a core of aqueous solution so phospholipids combine with water at once, which forms a bi-layered bubble because one end of each molecule is water soluble, while the another end is water insoluble. Lipid bubbles that have no aqueous material are called micelles. Furthermore, a targeting device or ligand can be incorporated on the external surface of the liposome in order to obtain targeted drug delivery. Liposomes are mostly classified by their structure: 1) multilamellar liposomes (several bilayers), 2) unilamellar liposomes (one bilayer) (CitationGarg 2014). The properties of liposomes and surfactants which formulate them and are helpful for different applications are:
Show stability of structure on dilution.
Variation in permeability of the bilayer to different molecules.
Capacity to entrap mutually water soluble and insoluble substances and deliver them into desired environments.
Liposomes have also been developed as a cargo carriers for cell- and organ-specific targeting using ligands, including proteins, peptides, polysaccharides, glycolipids, glycoproteins, lectins, and anti-target monoclonal antibodies (CitationGarg and Goyal 2012). There are several liposome formulations that have been commercialized and there are many other liposome formulations. After two decades of development, the in vivo and pharmaceutical action of liposomes is now better understood and outlines the basis for further development of liposome-mediated drug targeting strategies for clinical application (CitationGarg and Goyal 2014a). represents the mode of delivery and outcomes of liposome-based drug delivery systems in the treatment of TB.
Table VI. Outcomes of liposome-based drug delivery systems in the treatment of TB.
Nanoparticles
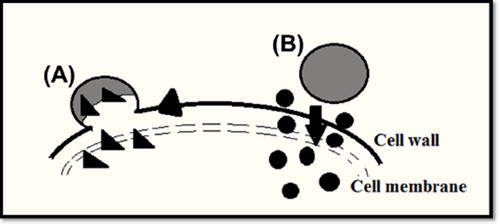
A nanoparticle is one that measures 100 nm or less. The properties of many conventional materials change when shaped from nanoparticles. A nanoparticle is defined as a tiny object that works as a whole unit, with its transport function and properties. It works as transport because a nanoparticle has a larger surface area than a larger particle, which makes it to more reactive to some new molecules (CitationGarg et al. 2013). represents mechanisms of nanoparticle-based antimicrobial drug delivery to microorganisms.
Figure 2. Mechanisms of nanoparticle-based antimicrobial drug delivery to microorganisms: (A) Nanoparticles combine with microbial cell wall or membrane and discharge the transported drugs within the cell wall or membrane; (B) nanoparticles attach to the cell wall and serve as a drug store to continuously release drug particles, which will distribute into the interior of the microorganisms.
represents the mode of delivery and outcomes of nanoparticle-based drug delivery systems in the treatment of TB.
Table VII. Outcomes of nanoparticle-based drug delivery systems in the treatment of TB.
Nanoparticle-based drug delivery systems are used for the treatment of tuberculosis (TB). Nanoparticles originate from biocompatible polymers and are soluble in particle-type carriers. Carriers have also been prepared using ficoll, poly-L-lysine or Sepharose as the most important carriers. Recently, alginate nanoparticles have been described for targeting as antituberculosis drug carriers (CitationGoyal et al. 2014). The benefits of nanoparticles utilized as drug carriers are-
Nanoparticles show high stability
High carrier capability
Possibility of incorporation of both hydrophilic and hydrophobic substances
Possibility of variable routes of administration, including oral application and inhalation.
These properties of nanoparticles make it possible to enhance drug bioavailability and reduce dosing rate (CitationJohal et al. 2014).
Microspheres or Microparticles
In 1976, the first polymer-based drug delivery system was worked out by Langer and Folkman for macromolecule delivery (CitationJoshi et al. 2014). Microspheres originate from biocompatible polymers and are soluble in the particle-type carriers. Carriers have also been prepared using ficoll, dextrans, poly-L-lysine or Sepharose as the most important carrier. In recent times, alginate microparticles have been described for targeting as antituberculosis drug carriers. Nanoparticles are smaller (0.2–0.5 μm) than microspheres (30–200 μm) and have a smaller drug loading capability than the soluble polymers (CitationKataria et al. 2014), at the inner core and at the surface of the particles. Formulation of drugs can occur in nanoparticles, depending on the physicochemical character of the drug. The site of drug absorption extensively influences its release rate from the particle. Deliberately induced immunological tolerance of this protein against beta-lacto globulin in a poly-lactic–glycolide microsphere formulation has been achieved (CitationMarwah et al. 2014). represents the mode of delivery and outcomes of microparticle/microsphere-based drug delivery systems in the treatment of TB.
Table VIII. Outcomes of microparticle/microsphere-based drug delivery systems in the treatment of TB.
Use of sustained release drug delivery systems recovers patient compliance in tuberculosis chemotherapy. As sustained release carrier systems, poly (DL-lactide-co-glycolide) (PLG) micro particles containing a combination of isoniazid and rifampicin were developed. A single dose of PLG microparticles showed a sustained release of isoniazid and rifampicin in vivo for up to 7 and 6 weeks, respectively (CitationSharma et al. 2014a). One dose of PLG microparticles cleared bacteria more efficiently from the lungs and liver, in an experimental murine model of tuberculosis after low-dose PLG combination drug therapy and in liver after high-dose PLG combination drug therapy, as compared with a daily administration of the free drugs. Therefore, the improvement in tuberculosis chemotherapy using PLG microparticles offers advantages over the conventional treatment (CitationSharma et al. 2014b).
Solid lipid nanoparticles
A solid lipid nanoparticle is spherical with a standard diameter between 10 and 1000 nanometers (nm). Solid lipid nanoparticles have a solid lipid core matrix that can dissolve http://en.wikipedia.org/wiki/Solubility in lipophilic molecules (CitationGoyal et al. 2013a). The lipid core is formed by added emulsifiers (surfactants). The lipid is used here in an extensive sense and consists of triglycerides like tristearin, diglycerides like glycerol behenate, monoglycerides like glycerol monostearate, fatty acids like stearic acid, steroids like cholesterol, and waxes like cetyl palmitate. Surfactants have been applied to stabilize the lipid dispersion. It has been found that the mixture of emulsifiers avoids particle agglomeration more competently (CitationGoyal et al. 2013b). Increment of solid lipid nanoparticles is one of the rising fields of lipid nanotechnology http://en.wikipedia.org/wiki/Solid_lipid_nanoparticle - cite_note-6 with numerous latent applications in drug delivery, research and clinical medicine. Due to their exclusive size-dependent properties, lipid nanoparticles present the option to develop new therapeutics. Nanocarriers have the ability to encapsulate drugs and offer a new example in drug delivery that holds large promise for achieving improvement in bioavailability, along with controlled and site-specific drug delivery. Solid lipid nanoparticles have in recent times appeared as a novel approach to parenteral and oral drug delivery systems (CitationSingh et al., 2014b). SLNs unite the advantages of polymeric nanoparticle systems and lipid emulsion while overcoming the chronological and in vivo stability issues that trouble the polymeric nanoparticles as well as conventional drug delivery approaches. It has been suggested that SLNs unite numerous advantages over the other colloidal carriers, i.e., possible absorption of hydrophilic and lipophilic drugs, no carrier toxicity, avoidance from organic solvents, chances of controlled drug release and drug targeting, raised drug stability and no difficulties with respect to bulk level production (CitationSingh et al. 2014a). represents the mode of delivery and outcomes of solid lipid nanoparticle based drug delivery systems in the treatment of TB.
Table IX. Outcomes of solid lipid nanoparticle based drug delivery systems in the treatment of TB.
A recent study has showed the use of solid lipid nanoparticles for oral delivery of the nutrients, minerals and iron, by adding the hydrophilic molecule ferrous sulfate (FeSO4) in a lipid matrix compiled of stearic acid (CitationKaur et al. 2014a).
Dendrimers
Dendrimers are spherical, three-dimensional, highly branched macromolecules that encompass from the central core and disclose symmetric, organized patterns of branching, with diameters that range from 10 to 100 nm. Dendrimers represent striking applications for the encapsulation and pulmonary delivery of anti-TB drugs, due to their unique structure. Dendrimers with the carboxylic or hydroxyl terminal are compatible (CitationGarg et al. 2012b). CitationKumar et al. (2007) formulated RIF-containing 4G and 5G PEG-PPI dendrimers to achieve the sustained delivery of RIF. The percentage of entrapment of drug in 4G and 5G PEGylated-PPI (polypropyleneimine) dendrimers was enhanced from 28% to 39% and 47% to 61%, respectively. Cell compatibility assays demonstrated that PEG-PPI dendrimers reduced the intrinsic hemolytic toxicity of nanocarrier materials from 14% to 3% (CitationKumar et al. 2007).
Conclusion
In summary, many antimicrobial drugs are complicated to administer because of the low water-solubility, quick degradation, strong cytotoxicity to tissues, and clearance in the blood circulation. Their antimicrobial activities are not in favor of intracellular microbes and are also strictly limited by poor membrane transfer ability. Generally, studies have shown that nanoparticles, liposomes, polymeric nanoparticles, solid lipid nanoparticles, microspheres and microparticles are able to overcome these problems and facilitate easy antimicrobial delivery to microbial infection sites. While most of these carrier based antimicrobial drug delivery systems are currently in the preclinical development stage, several have been approved for clinical use. With the continuing efforts in this field, there is no doubt that targeted carrier based drug delivery systems will continue to provide better treatment to bacterial infections, especially in life-threatening infections such as tuberculosis and other infections.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for thecontent and writing of the paper.
References
- Ahmad Z, Sharma S, Khuller GK. 2005. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int J Antimicrob Agents. 26:298–303.
- Barrow EL, Winchester GA, Staas JK, Quenelle DC, Barrow WW. 1998. Use of microsphere technology for targeted delivery of rifampin to Mycobacterium tuberculosis-infected macrophages. Antimicrob Agents Chemother. 42:2682–2689.
- El-Ridy MS, Mostafa DM, Shehab A, Nasr EA, Abd El-Alim S. 2007. Biological evaluation of pyrazinamide liposomes for treatment of Mycobacterium tuberculosis. Int J Pharm. 330:82–88.
- Gabor F, Bogner E, Weissenboeck A, Wirth M. 2004. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv Drug Deliv Rev. 56:459–480.
- Gagandeep, Garg T, Malik B, Rath G, Goyal AK. 2014. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–16.
- Gangadharam PR, Ashtekar DA, Ghori N, Goldstein JA, Debs RJ, Duzgunes N. 1991. Chemotherapeutic potential of free and liposome encapsulated streptomycin against experimental Mycobacterium avium complex infections in beige mice. J Antimicrob Chemother. 28:425–435.
- Garcia-Contreras L, Fiegel J, Telko MJ, Elbert K, Hawi A, Thomas M, et al. 2007. Inhaled large porous particles of capreomycin for treatment of tuberculosis in a guinea pig model. Antimicrob Agents Chemother. 51:2830–2836.
- Garg T. 2014. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol. 1–8.
- Garg T, Goyal AK. 2012. Iontophoresis: Drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett. 2:270–280.
- Garg T, Goyal AK. 2014a. Biomaterial-based scaffolds–current status and future directions. Expert Opin Drug Deliv. 11:767–789.
- Garg T, Goyal AK. 2014b. Liposomes: Targeted and controlled delivery system. Drug Deliv Lett. 4:62–71.
- Garg T, Goyal AK, Arora S, Murthy R. 2012a. Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett. 2:251–261.
- Garg T, Singh O, Arora S, Murthy R. 2012b. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Gelperina S, Kisich K, Iseman MD, Heifets L. 2005. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 172:1487–1490.
- Goyal AK, Rath G, Garg T. 2013a. Nanotechnological approaches for genetic immunization. DNA and RNA Nanobiotechnologies in Medicine: Diagnosis and Treatment of Diseases 67–120.
- Goyal G, Garg T, Malik B, Chauhan G, Rath G, Goyal AK. 2013b. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv.
- Goyal G, Garg T, Rath G, Goyal AK. 2014. Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst. 31:89–119.
- Johal HS, Garg T, Rath G, Goyal AK. 2014. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 1–14.
- Joshi D, Garg T, Goyal AK, Rath G. 2014. Advanced drug delivery approaches against periodontitis. Drug Deliv. 1–15.
- Kataria K, Sharma A, Garg T, Goyal AK, Rath G. 2014. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 4:79–86.
- Kaur M, Garg T, Rath G, Goyal AK. 2014a. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31:49–88.
- Kaur M, Malik B, Garg T, Rath G, Goyal AK. 2014b. Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv.
- Kaur R, Garg T, Das Gupta U, Gupta P, Rath G, Goyal AK. 2014c. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol. 1–6.
- Kaur R, Garg T, Malik B, Gupta UD, Gupta P, Rath G, Goyal AK. 2014d. Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv. 1–6.
- Klemens SP, Cynamon MH, Swenson CE, Ginsberg RS. 1990. Liposome-encapsulated-gentamicin therapy of Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 34:967–970.
- Kumar PV, Agashe H, Dutta T, Jain NK. 2007. PEGylated dendritic architecture for development of a prolonged drug delivery system for an antitubercular drug. Curr Drug Deliv. 4:11–19.
- Lu D, Garcia-Contreras L, Xu D, Kurtz SL, Liu J, Braunstein M, et al. 2007. Poly (lactide-co-glycolide) microspheres in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen 85B. Pharm Res. 24:1834–1843.
- Marwah H, Garg T, Goyal AK, Rath G. 2014. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 1–15.
- Pandey R, Khuller GK. 2005a. Antitubercular inhaled therapy: opportunities, progress and challenges. J Antimicrob Chemother. 55:430–435.
- Pandey R, Khuller GK. 2005b. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis (Edinb). 85:227–234.
- Pandey R, Sharma A, Zahoor A, Sharma S, Khuller GK, Prasad B. 2003. Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J Antimicrob Chemother. 52:981–986.
- Pandey R, Sharma S, Khuller GK. 2005. Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis (Edinb). 85: 415–420.
- Parnami N, Garg T, Rath G, Goyal AK. 2013. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol.
- Qurrat ul A, Sharma S, Khuller GK, Garg SK. 2003. Alginate-based oral drug delivery system for tuberculosis: pharmacokinetics and therapeutic effects. J Antimicrob Chemother. 51:931–938.
- Sharma A, Garg T, Aman A, Panchal K, Sharma R, Kumar S, Markandeywar T. 2014a. Nanogel-an advanced drug delivery tool: Current and future. Artif Cells Nanomed Biotechnol. 1–13.
- Sharma A, Sharma S, Khuller GK. 2004. Lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J Antimicrob Chemother. 54:761–766.
- Sharma R, Singh H, Joshi M, Sharma A, Garg T, Goyal AK, Rath G. 2014b. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst. 31:187–217.
- Singh B, Garg T, Goyal AK, Rath G. 2014a. Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol. 1–10.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014b. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol.
- Suarez S, O’Hara P, Kazantseva M, Newcomer CE, Hopfer R, Mcmurray DN, Hickey AJ. 2001. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: screening in an infectious disease model. Pharm Res. 18:1315–1319.
- Vyas SP, Kannan ME, Jain S, Mishra V, Singh P. 2004. Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. Int J Pharm. 269:37–49.