 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study utilized the Seahorse Analyzer to examine the effect of the bile acid ursodeoxycholic acid (UDCA), on the morphology, swelling, stability, and size of novel microencapsulated β-cells, in real-time. UDCA was conjugated with fluorescent compounds, and its partitioning within the microcapsules was examined using confocal microscopy. UDCA produced microcapsules with good morphology, better mechanical strength (p < 0.01), and reduced swelling properties (p < 0.01), but lower cell viability (p < 0.05) and cell count per microcapsule (p < 0.01). UDCA reduced the cells’ biochemical activities, mitochondrial respiration, and energy production, post-microencapsulation. This is the first time biological functions of microencapsulated β-cells have been analyzed in real-time.
Introduction
Insulin injections have been used for decades in diabetes treatment (CitationRafaelsen 1964). Type 1 diabetic patients rely on insulin injections due to the lack of insulin-producing pancreatic cells, while about 30% of Type 2 diabetic patients resort to injecting insulin due to either the inability of existing β-cells to produce sufficient insulin and control glycemia, or the absence of functional β-cells as a result of excessive stimulation by anti-diabetic drugs such as sulfonlyureas (CitationBarnett 2010).
Despite strict use of anti-diabetic drugs to control blood glucose levels, hypoglycemic episodes and blood glucose fluctuations remain common among diabetic patients (CitationBonadonna et al. 2006, CitationMaji 2004). Recently, there have been new and robust delivery systems for insulin, such as microchips, that are more sensitive to blood glucose changes and more tailored to patients’ requirements. However, the long term safety and efficacy of these new systems remain questionable (CitationTuch et al. 2011). An ideal treatment for insulin deficiency would be to rejuvenate the damaged β-cells and permanently control glycemia without any adverse effects. This can be achieved by either transplanting stem cells, which will differentiate into functional β-cells, or by transplanting already differentiated β-cells. To date, transplantation of β-cells has not been successful, due to many obstacles (CitationPuri and Hebrok 2012). These obstacles are mainly the immune system of the host, and inflammation (Citationde Vos et al. 2002, Citation2006). In order to achieve significant glycemic control, the transplanted cells have to be in sufficient numbers and exhibit adequate biological activity, without triggering any immune response. Thus, there is a need for suitable capsules that can encapsulate and protect the cells permanently, while allowing their biological activity post-transplantation. This can be achieved by using the artificial cell microencapsulation (ACM) technology, which was pioneered by Thomas Chang at McGill University in Canada in the 1960s, to deliver drugs and cells (CitationChang 1971, Citation1992, CitationChang et al. 1967).
ACM technology utilizes polymers for cellular microencapsulation, and forms a range of microcapsules with various measures of thickness and size (CitationKittel et al. 2013, CitationWhelehan and Marison 2011). In previously published work, we demonstrated the potential applications of bile acids, either alone or combined with other agents, in diabetes therapy (CitationAl-Salami et al. 2007, Citation2008, Citation2009, Citation2012, CitationCalasan et al. 2012, CitationLalic-Popovic et al. 2013a, Citation2013b, CitationMikov et al. 2008, Citation2012). In a recent study, we developed a microcapsule-based platform for β-cells, and we used bile acids as membrane-stabilizing excipients (CitationMooranian et al. 2014a). However, cell viability remained limited, and thus, in this study, we aimed to incorporate a polyelectrolyte mixture into our microcapsules. This mixture comprises ultrasonic gel, poly-l-ornithine, poly-allylamine, and poly-(4styrene)-sulfonate, and will be added into the polymer, sodium alginate. We anticipate the addition of the polyelectrolyte mixture to improve the physical characteristics of the microcapsules, which may result in better cell survival, post-microencapsulation. We also aim to examine the impact of integrating ursodeoxycholic acid (a bile acid with anti-inflammatory effects) (CitationMartinez-Moya et al. 2013) into the microcapsules, and study the microcapsules’ structure, morphology, size, mechanical strength, and stability, as well as cell viability and distribution within the microcapsules, in vitro. We have also examined the partitioning of the bile acids within the microcapsules through its conjugation with fluorescent compounds, followed with studies using spinning disk confocal scanning.
Materials and methods
Cell culture
As described previously (CitationMooranian et al. 2014a), BRIN-BD11 cells were cultured in T-75 cm2 tissue culture flasks (Thermo Fisher Scientific®, Australia) and fed with RPMI 1640 media (Gibco, Life Technologies, USA) supplemented with 5.5 mmol glucose (Sigma Chemical Co, USA), 10% fetal bovine serum (Thermo Fisher Scientific, Australia), and 5% penicillin-streptomycin (Thermo Fisher Scientific, Australia). The BRIN-BD11 cells were incubated in an environment of 5% CO2 in humidified air at 37°C, using a NuAire NU-8500 Water Jacket CO2 Incubator (NuAire, USA). The trypsinized cells were added to an equivalent volume of freshly prepared media and centrifuged at 1500 rpm for 5 min at 20°C, using a Beckman Coulter Allegra X-12 centrifuge (Beckman Coulter, USA). The cells were resuspended in fresh media, ready for microencapsulation under sterile conditions.
Formulation preparation
Sodium alginate (SA, ≥ 99%), sterile poly-l-ornithine hydrochloride (PLO), and ursodeoxycholic acid (UDCA, 99%) were all purchased from Sigma Chemical Co, USA. Calcium chloride dihydrate (CaCl2.2H20, 98%) was obtained from Scharlab S.L, Australia. Sterilized poly-(4styrene)-sulfonate (PSS) and poly-allylamine hydrochloride (PAA) were both purchased from Sigma Chemical Co, USA. Ultrasonic gel was purchased from Australian Medical Association (Perth, Australia). Stock solutions were prepared using UltraPure™ distilled water (Life Technologies, USA), see .
Table I. The chemical constituents of the two formulations used to microencapsulate BRIN BD11 pancreatic β- cells.
All solutions were mixed well using sterilized laboratory equipment in a Gelaire BH-EN Class II biological safety cabinet (Gelaire Company, Australia), with independently certified sterile working conditions, in a physical containment level 2 laboratory.
Sterilization protocol
All reagents were sterile upon purchase, endotoxin-tested, and suitable for cell culture research. All subsequent solutions and formulations made in our laboratory were sterilized by filtration (0.22 μm) before use.
Microencapsulation by vibrating-jet flow method (VJFM)
Microencapsulation was carried out using a Büchi microencapsulation system (BÜCHI Labortechnik, Switzerland) developed in our laboratory (CitationMooranian et al. 2014b, CitationNegrulj et al. 2013). The droplets formed via the Büchi based vibrational-jet flow technology were collected in a 2% w/v CaCl2 hardening bath (CitationMooranian et al. 2014b, CitationNegrulj et al. 2013). Post-microencapsulation, the microcapsules were stored in T-25 cm2 tissue culture flasks (Thermo Fisher Scientific, Australia) containing 5 ml of media, and incubated under sterile conditions (37°C with 5% CO2 in the incubator) for 24 h prior to commencement of experiments.
Characterization of loaded microcapsules
Optical microscopy (OM)
A fluorescent microscope (Olympus IX-51 inverted fluorescent microscope, Japan) was used to view the samples.
Scanning electron microscopy (SEM) and energy dispersive X-ray (EDXR) spectroscopy
MIRA3 FE SEM (Tescan, Czech Republic) with 2.5 nm calibrated resolution and an accelerating voltage of 3 kV was used to examine the microcapsules’ surface morphology. EDXR (Oxford Instruments, INCA X-Act, USA) was used to examine the microcapsules’ surface, as described in our previous studies (CitationMooranian et al. 2014a).
Spinning disk confocal scanning microscopy of encapsulated islet cells
Encapsulated islet cells were stained with the CellTrace™ carboxyfluorescein succinimidyl ester (CFSE) Cell Proliferation Kit (Life Technologies, USA), post-trypsinization. Confocal scanning was carried out using an UltraVIEW Vox spinning disk confocal microscope (Perkin Elmer, USA) equipped with a Yokogawa CSU-X1 confocal scanning unit (Perkin Elmer, USA) and Hamamatsu ORCA-R2 camera (Hamamatsu Cooperation, Japan) with a 488 nm laser. To ensure optimal viability of the encapsulated islet cells and utmost sterility during imaging, microcapsules were placed within a fully incubated environmental control unit (fully enclosed microscope and stage unit) enriched with CO2 (5%) and temperature controlled (37°C). Image analysis was undertaken using the Velocity Multi-Platform 3D Cellular Imaging and Analysis Software (Perkin Elmer, USA) (CitationMooranian et al. 2014a).
Spinning disk confocal scanning microscopy of TRITC-UDCA chemical conjugate
To determine the partitioning of the bile acid UDCA within all the layers of the microcapsule, a conjugation reaction using a fluorescent compound (tetramethylrhodamine isothiocyanate; TRITC) was performed, based on the work of Sherman and Fisher (CitationSherman and Fisher 1986). The microcapsules were then analyzed for their morphology and bile acid distribution/partitioning within the microcapsule matrix, using an UltraVIEW Vox spinning disk confocal microscope (Perkin Elmer, USA) equipped with a 532 nm laser. As no islet cells were stained and no other substance displayed any fluorescent activity, fluorescent visualization of the microcapsules at 532 nm was determined to be specific to the TRITC-UDCA conjugate.
Microencapsulated cell count and the microencapsulation efficiency
To determine the total number of live cells used for microencapsulation following re-suspension post-centrifugation, a 40 μl aliquot was removed and placed in a 65 μl Eppendorf tube (Eppendorf South Pacific, Australia) to which 10 μl of 0.4% trypan blue solution was added (Sigma Chemical CO, Australia). The mixture was mixed well, and two lots of 10 μl were removed from the mixture and each placed on opposite sides of a Countess cell counter chamber slide (Invitrogen, Korea), and the slide was then placed into the slide port of a Countess Automated Cell Counter (Invitrogen, Korea) for analysis, as described in our previous studies (CitationMooranian et al. 2014a).
At the end of this protocol, no β-cells remained attached to the surface of the flask, or remained within the microcapsule core, confirming that all cells were gradually released from the microcapsules. The microencapsulation efficiency was calculated according to the following equation:
MTT assays (cell mitochondrial activity assessment)
A validated method was used, based on the work of Uludag and Sefton, with slight modifications (CitationUludag and Sefton 1990, Citation1992, CitationUludag et al. 1994). MTT was prepared as a 5 mg/ml stock solution (Sigma Chemical CO, USA) in phosphate buffer saline of pH 7.4 (Thermo Fisher Scientific, Australia), and the undissolved residues were removed by sterile filtration. The stock solution was stored in a sterile environment at 4°C in the dark, and used within 7 days of preparation. The MTT assay was carried out as described previously (CitationMooranian et al. 2014a).
Microencapsulated β-cell respiration and metabolic activity (glycolysis)
Evaluation of mitochondrial activities of cells within the microcapsules, including oxygen consumption rate (OCR), extracellular acidification rate (ECAR), and proton production rate (PPR) were undertaken in real-time, using a method developed in-house, using a Seahorse Flux Analyzer XF 96 (Seahorse Bioscience, USA). Various other parameters were also measured: Basal Respiration (BR), ATP production (ATPP), Maximum Respiration (MR), Coupling Efficiency (CE), Non-Mitochondria-OCR (NM-OCR), and Glycolysis (G).
The instrument measures mitochondrial biomarkers by sensing changes in oxygen and proton (pH) content via a fluorescent biosensor (CitationNicholls et al. 2010, CitationGerencser et al. 2009). Measurements of key mitochondrial activities are done in a non-invasive manner, such that the microcapsule structure is not damaged during the process, and cell viability is measured before and after the run, to ensure accurate results that are based on the biological activities of viable cells (CitationWu et al. 2007). The experiments were designed to determine microencapsulated cellular respiration at a glucose concentration of 2.5 mM (CitationMalmgren et al. 2009). For an analysis of mitochondrial respiration and activity to take place, a series of injections (20 min apart) from a multichannel injector port system were required (CitationGerencser et al. 2009, CitationNicholls et al. 2010). The first injection was of media (no glucose), which served to equilibrate experimental conditions. The second injection was of the ATP coupler oligomycin (2 μM) that inhibits ATP synthesis (CitationMalmgren et al. 2009, CitationBrand and Nicholls 2011, CitationWikstrom et al. 2012). This allows the determination of the percentage of oxygen consumption devoted to ATP synthesis, and the proportion of oxygen needed to overcome the proton leak across the mitochondrial membrane (CitationWu et al. 2007, CitationDranka et al. 2010, CitationWikstrom et al. 2012). Prior to running the Seahorse XF stress-testing assays, a final cell count was undertaken in order to measure total viable cell count used. The microencapsulated β-cells of Formula 1 and Formula 2 were prepared and treated in exactly the same way as per our in-house method (CitationWu et al. 2007). All microcapsules were examined visually after the end of mitochondrial stress-testing assays, to determine what morphological impact the procedure had on the microcapsules and their encapsulated cellular contents. Data were presented graphically over the duration of the assays (CitationWikstrom et al. 2012, CitationMalmgren et al. 2009).
Zeta-potential and size analysis
To determine the electrokinetic stability and uniformity in the size of the microcapsules in the dispersion system, the zeta potential and size distribution for the microencapsulated formulations were measured using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK), and by the Mie and Fraunhofer scattering technique using a Mastersizer 2000 (Malvern Instruments, Malvern, UK), as described in our previous studies (CitationMooranian et al. 2014a, Citation2014b). All determinations were performed in triplicate. Results are reported as mean ±SD.
Swelling studies
To determine the swelling properties of the microcapsules, 50 mg of dry microcapsules were weighed and placed in 20 ml of phosphate buffer pH 7.4, at a temperature of 37°C, for 6 h, and measurements were carried out as per previously published work (CitationMooranian et al. 2014a, Citation2014b). All experiments were done in triplicate. The swelling index of the microcapsules was calculated from the following formula (CitationPal and Nayak 2012, CitationAwasthi and Kulkarni 2013):
Mechanical resistance
In order to test the mechanical stability of the microcapsules, mechanical resistance testing was carried out using a Boeco Multishaker PSU 20 (Boeco Company, Germany). Vials containing 20 microcapsules in 20 ml of phosphate buffer (pH 7.4) were placed in a shaker and vibrated/agitated back and forth, at a frequency of 150 rpm for a period of 24 h. At various time intervals, the number of fractured or damaged microcapsules was counted. The mechanical strength of the microcapsules was then calculated using the following equation (CitationJiin 2000):
Stability studies
The stability test was carried out by placing predetermined amounts of freshly prepared microcapsules onto sterile petri dishes (30 microcapsules in each), and storing them in thermostatically-controlled ovens (environmental stability chambers) at − 20°C, 5°C, 25°C, and 40°C, with relative humidity set at 35%, for 3 days (72 h). The experiment was conducted using a stability chamber (Angelantoni Environmental and Climatic Test Chamber, Italy) (CitationMooranian et al. 2014b).
Statistical analysis
The results were expressed as mean ± standard deviation, n = 3. For statistical analysis, two-way ANOVA with Tukey post-hoc analysis was used, setting the level of significance at p < 0.05. All the statistical analysis was performed using GraphPad Prism version 6.0 (GraphPad Software, Inc., USA). The results for the p value were only reported where significance was noted.
Results and discussion
Optical microscopy
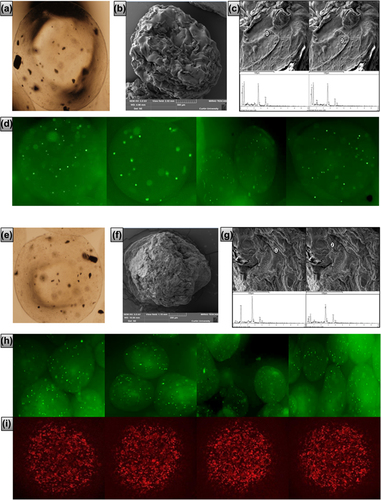
shows β-cell microcapsules without UDCA (F1, control) while shows β-cell microcapsules with UDCA (F2, test). There is no obvious difference in shape or size. There are small agglomerations visible, which we believe to be β-cell populations.
Figure 1. Micrographs of F1 and F2 microcapsules. a: Optical image of F1, b: SEM image of F1, c: Surface sites with corresponding EDXR spectral analysis of F1, d: Confocal images of stained cells of F1 microcapsules, e: Optical image of F2, f: SEM image of F2, g: Surface sites with corresponding EDXR spectral analysis of F2, h: Confocal images of stained cells of F2, and i: Confocal images of fluorescent UDCA images of F2 microcapsules. a and e: Scale 40 ±. d and h: Scale 10 3.
Scanning electron microscopy (SEM)
shows SEM surface images of F1 and F2 β-cell microcapsules respectively. F1 and F2 microcapsules were spherically shaped, with similar sizes. F1 microcapsules () exhibit an uneven surface, which is rough and abundant with ridges, compared with F2 microcapsules (). This is in line with our previous studies that showed membrane-stabilizing effects of bile acids on the microcapsules (CitationTakka and Cali 2012).
Energy dispersive X-ray spectroscopy (EDXR) analysis
EDXR analysis of β-cells containing F1 and F2 microcapsule surfaces revealed characteristic elemental compositions expected for microcapsules, formed via ionic gelation chemical reactions in the hardening bath (CitationNegrulj et al. 2013, CitationSteele et al. 2013). Specifically, prominent elemental atoms Ca, O, and C were abundant on the chemical spectra without interference. These atoms are representative of the calcium alginate membrane () and the bile acid UDCA () (CitationNegrulj et al. 2013, CitationKuhajda et al. 2006). Atoms unique to the polyelectrolytes PAA, PSS, and PLO (such as N and S), were not visible on the membrane surface, suggesting that they are in the inner core. This is in line with our previously published work (CitationMooranian et al. 2014a).
Spinning disk confocal scanning microscopy of encapsulated islet cells
shows confocal microscopic images of F1-stained cells, while shows confocal microscopic images of F2-stained cells. There is no obvious difference between viable cell distribution within the microcapsules of F1 and F2 formulations, suggesting that UDCA did not affect viable cell distribution within the microcapsules.
Spinning disk confocal scanning microscopy of TRITC-UDCA chemical conjugate
shows confocal microscopic images of F2 fluorescent conjugated-UDCA microcapsules. UDCA is clearly partitioned throughout all parts of the microcapsules including the surface, and this was supported by the EDXR analysis ().
Microencapsulation efficiency and cell count
shows lower viable cell count per microcapsule in the presence of UDCA (p < 0.01), while the efficiency of the microencapsulation process remains similar. This suggests that UDCA either reduced cell proliferation or enhanced cell death, post-microencapsulation. A possible explanation is that the presence of UDCA resulted in less porous microcapsules, as shown in , which brought about less oxygen and nutrient permeation through the membrane, and eventual cell hypoxia and death.
Figure 2. Microencapsulated β-cell viability within intact microcapsules without (control) and with (test) UDCA. Pre-MC: pre-microencapsulation β-cells, F1 (without UDCA) and F2 (with UDCA) are empty microcapsules, F1: β-cells microcapsules (without UDCA) and F2: β-cells microcapsules (with UDCA). Values are mean ± SD and n = 3.
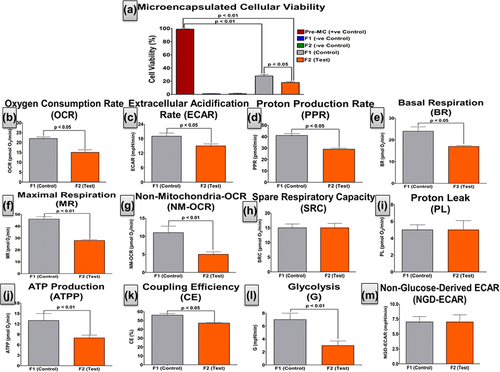
Table II. Microencapsulated live cell count and the microencapsulation efficiencies for both formulations (F1 and F2).
Microcapsule size analysis and zeta potential measurements
shows that UDCA did not adversely affect the size of β-cell-containing microcapsules. However, UDCA reduced the values of Zeta potential (p < 0.05) of the dispersion system, possibly due to its ionic interaction with the alginate system, which is in line with our previous published work (CitationMooranian et al. 2014a, Citation2014b).
Table III. Microcapsule size and Zeta potential of F1 and F2 formulations.
MTT and Seahorse mitochondrial respiration and metabolic activity analyses
A calorimetric assay of cellular viability for β-cells within intact microcapsules was investigated using an MTT assay (). This was complemented by a real-time analysis of cellular microenergetics, mitochondrial activity, and cellular respiration () using the Seahorse analyzer. To the best of our knowledge, this is the first time these parameters have been measured, in real-time, for microencapsulated β-cells. The results were consistent and shows that UDCA significantly reduced cell viability, post-microencapsulation (p < 0.05). It also shows that UDCA significantly reduced β-cells’ OCR (p < 0.05), ECAR (p < 0.05), PPR (p < 0.05), NM-OCR (p < 0.05), BR (p < 0.05), MR (p < 0.01), NM-OCR (p < 0.01), ATPP (p < 0.01), CE (p < 0.05), and cellular glycolysis (p < 0.01). This suggests a strong and consistent negative impact of UDCA on the β-cells’ biological activity and mitochondrial respiration, 48 h post-microencapsulation. This may be explained by UDCA causing a significant reduction in the porosity of the microcapsule membrane, and thereby hindering oxygen/nutrient exchange and resulting in β-cell hypoxia and apoptosis.
shows that UDCA caused a significant decrease in OCR, suggesting reduced mitochondrial respiration (CitationWikstrom et al. 2012, CitationDranka et al. 2010). This indicates that there are fewer O2 molecules serving as electron acceptors and facilitating activity within the electron transport chain, with a proportional decrease in oxidative phosphorylation (CitationBrand and Nicholls 2011, CitationMalmgren et al. 2009, CitationGerencser et al. 2009). This suggests lower BR and less oxidation of pyruvate to produce NADH, which in turn generates a proton (H+) gradient. The gradient is utilized by ATP synthase to generate ATP (ATPP) from ADP and facilitate less β-cell activity (CitationWu et al. 2007). For pancreatic β-cells, the ATP:ADP ratio is of high importance as it triggers glucose-stimulated insulin secretion (CitationMalmgren et al. 2009). However, insulin was not measured during this assay, which is a limitation to our findings. Glycolysis stress-testing revealed that UDCA resulted in less ECAR and PPR, possibly due to reduced cellular energy production via the glycolysis pathway, and resulting in a subsequent decrease in MR and CE. This also resulted in a reduction in the NM-OCR and G activity of the cells, which complements the MTT assay results ().
Previously published data have shown that bile acids enhance β-cell viability via their antioxidant effects (CitationNegrulj et al. 2013, CitationPerez and Briz 2009). This is contrary to our findings. However, our findings were obtained using microencapsulated cells rather than free ones. This suggests that different formulations of bile acids will have different effects on cell viability and biological functionality, post-microencapsulation.
Swelling and mechanical strength
Studies on swelling and mechanical strength were carried out in order to confirm that UDCA significantly increased the strength of the microcapsules. shows that UDCA significantly reduced the swelling properties of the β-cell microcapsules (p < 0.01), and also significantly enhanced the mechanical strength of these microcapsules when exposed to vibrational stress for 24 h (p < 0.01).
Figure 3. Swelling characteristics of β-cell-containing microencapsulated formulas F1 and F2 (a) and mechanical strength testing of β-cell-containing microcapsules F1 and F2.
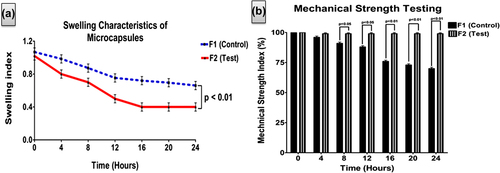
The swelling and mechanical strength data support the SEM data, showing reduced porosity of the microcapsules as a result of UDCA addition (). The even distribution of UDCA throughout all layers of the microcapsules resulted not only in a stronger microcapsule, but also reduced viable cell count (), while maintaining the same microcapsule size (). Thus, the combination of UDCA with the polyelectrolytes (USG, PAA, and PSS) resulted in microcapsules that are too strong to maintain cell viability.
Stability studies
At a fixed humidity of 35%, the change in temperature exerted a significant effect on the morphology, appearance and size of the microcapsules. At − 20°C and 5°C, both types of microcapsules (control and test) maintained their original shape, morphology, color (pale-ivory), and texture (soft and flexible). At 25°C and 40°C, both microcapsules experienced a significant reduction in size (30%), and a change in color (yellow-orange) and texture (hard and brittle). This suggests that UDCA does not have an effect on the size, morphology, or color of the microcapsules, especially at higher temperature.
Conclusion
The addition of UDCA and the polyelectrolyte mixture resulted in stronger β-cell microcapsules, but cell survival and biological functions were compromised. Thus, future studies will explore different polyelectrolyte-bile acid formulations in order to obtain microcapsules with good physical properties as well as better cell viability and long-term survival, which may prove to be a viable delivery platform for β-cells for the treatment of diabetes.
Acknowledgments
The authors acknowledge the CHIRI at Curtin University, and the Curtin-seeding grant for the support, and also acknowledge the use of equipment, scientific and technical assistance of the Curtin University Electron Microscope Facility, which has been partially funded by the University, State, and Commonwealth Governments. The authors also acknowledge the Pharmaceutical Technology Laboratory for their valuable assistance (Curtin School of Pharmacy).
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al-Salami H, Butt G, Tucker I, Fawcett PJ, Golocorbin-Kon S, Mikov I, Mikov M. 2009. Gliclazide reduces MKC intestinal transport in healthy but not diabetic rats. Eur J Drug Metab Pharmacokinet. 34:43–50.
- Al-Salami H, Butt G, Tucker I, Golocorbin-Kon S, Mikov M. 2012. Probiotics decreased the bioavailability of the bile acid analog, monoketocholic acid, when coadministered with gliclazide, in healthy but not diabetic rats. Eur J Drug Metab Pharmacokinet. 37:99–108.
- Al-Salami H, Butt G, Tucker I, Mikov M. 2008. Influence of the semisynthetic bile acid MKC on the ileal permeation of gliclazide in vitro in healthy and diabetic rats treated with probiotics. Methods Find Exp Clin Pharmacol. 30:107–113.
- Al-Salami H, Kansara H, King J, Morar B, Jayathilaka B, Fawcett PJ, Mikov M. 2007. Bile acids: a bitter sweet remedy for diabetes. NZ Pharm J. 27:17–20.
- Awasthi R, Kulkarni GT. 2013. Development of novel gastroretentive drug delivery system of gliclazide: hollow beads. Drug Dev Ind Pharm. 1–11.
- Barnett R. 2010. Historical keyword: diabetes. Lancet. 375:191.
- Bonadonna RC, Cucinotta D, Fedele D, Riccardi G, Tiengo A. 2006. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care. 29: 2701–2707.
- Brand M, Nicholls D. 2011. Assessing mitochondrial dysfunction in cells. Biochem J. 435:297–312.
- Calasan J, Al-Salami H, Mikov M. 2012. Bile acids and probiotics could help treating diabetes. FEBS J. 279:267–267.
- Chang TM. 1971. The in vivo effects of semipermeable microcapsules containing L-asparaginase on 6C3HED lymphosarcoma. Nature. 229:117–118.
- Chang TM. 1992. Hybrid artificial cells: microencapsulation of living cells. ASAIO J. 38:128–130.
- Chang TM, Johnson LJ, Ransome OJ. 1967. Semipermeable aqueous microcapsules. IV. Nonthrombogenic microcapsules with heparin-complexed membranes. Can J Physiol Pharmacol. 45:705–715.
- de Vos P., Faas MM, Strand B, Calafiore R. 2006. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 27:5603–5617.
- de Vos P., Hamel A, Tatarkiewicz K. 2002. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 45:159–173.
- Dranka BP, Hill BG, Darley-Usmar VM. 2010. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radic Biol Med. 48:905–914.
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, et al. 2009. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 81:6868–6878.
- Jiin WY. 2000. Development of new polycations for cell encapsulation with alginate. Mater Sci Eng. 1:59–63.
- Kittel A, Falus A, Buzas E. 2013. Microencapsulation technology by nature: Cell derived extracellular vesicles with therapeutic potential. Eur J Microbiol Immunol (Bp). 3:91–96.
- Kuhajda K, Kandrac J, Kevresan S, Mikov M, Fawcett JP. 2006. Structure and origin of bile acids: an overview. Eur J Drug Metab Pharmacokinet. 31:135–143.
- Lalic-Popovic M, Paunkovic J, Grujic Z, Golocorbin-Kon S, Al-Salami H, Mikov M. 2013a. Diabetes and hypertension increase the placental and transcellular permeation of the lipophilic drug diazepam in pregnant women. BMC Pregnancy Childbirth. 13:188.
- Lalic-Popovic M, Vasovic V, Milijasevic B, Golocorbin-Kon S, Al-Salami H, Mikov M. 2013b. Deoxycholic acid as a modifier of the permeation of gliclazide through the blood brain barrier of a rat. J Diabetes Res. 2013:598603.
- Maji D. 2004. Prevention of microvascular and macrovascular complications in diabetes mellitus. J Indian Med Assoc. 102: 426, 428, 430 passim.
- Malmgren S, Nicholls DG, Taneera J, Bacos K, Koeck T, Tamaddon A, et al. 2009. Tight coupling between glucose and mitochondrial metabolism in clonal β-cells is required for robust insulin secretion. J Biol Chem. 284:32395–32404.
- Martinez-Moya P, Romero-Calvo I, Requena P, Hernandez-Chirlaque C, Aranda CJ, Gonzalez R, et al. 2013. Dose-dependent antiinflammatory effect of ursodeoxycholic acid in experimental colitis. Int Immunopharmacol. 15:372–380.
- Mikov, M, Al-Salami H, Golocorbin-Kon G. 2012. Potentials and Limitations of Bile Acids and Probiotics in Diabetes Mellitus. pp. 365–402
- Mikov M, Al-Salami H, Golocorbin-Kon S, Skrbic R, Raskovic A, Fawcett JP. 2008. The influence of 3alpha,7alpha-dihydroxy-12-keto-5beta-cholanate on gliclazide pharmacokinetics and glucose levels in a rat model of diabetes. Eur J Drug Metab Pharmacokinet. 33:137–142.
- Mooranian A, Negrulj R, Arfuso F, Al-Salami H. 2014a. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic β-cells. Artif Cells Nanomed Biotechnol. (Epub ahead of print).
- Mooranian A, Negrulj R, Mathavan S, Martinez J, Sciarretta J, Chen-Tan N, et al. 2014b. Stability and release kinetics of an advanced gliclazide-cholic acid formulation: the use of artificial-cell microencapsulation in slow release targeted oral delivery of antidiabetics. J Pharm Innov. 9:150–157.
- Negrulj R, Mooranian A., Al-Salami H. 2013. Potentials and Limitations of Bile Acids in Type 2 Diabetes Mellitus: Applications of Microencapsulation as a Novel Oral Delivery System. J Endocrinol Diabetes Mellitus. 1:49–59.
- Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA. 2010. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. 46.
- Pal D, Nayak AK. 2012. Novel tamarind seed polysaccharide-alginate mucoadhesive microspheres for oral gliclazide delivery: in vitro-in vivo evaluation. Drug Deliv. 19:123–131.
- Perez MJ, Briz O. 2009. Bile-acid-induced cell injury and protection. World J Gastroenterol. 15:1677–1689.
- Puri S, Hebrok M. 2012. Diabetic beta Cells: To Be or Not To Be? Cell. 150:1103–4.
- Rafaelsen OJ. 1964. Glycogen content of rat diaphragm after intraperitoneal injection of insulin and other hormones. Acta Physiol Scand. 61:314–322.
- Sherman I, Fisher M. 1986. Hepatic transport of fluorescent molecules: in vivo studies using intravital TV microscopy. Hepatology. 6:444–449.
- Steele JA, Halle JP, Poncelet D, Neufeld RJ. 2013. Therapeutic cell encapsulation techniques and applications in diabetes. Adv Drug Deliv Rev.
- Takka S, Cali AG. 2012. Bile salt-reinforced alginate-chitosan beads. Pharm Dev Technol. 17:23–29.
- Tuch BE, Hughes TC, Evans MD. 2011. Encapsulated pancreatic progenitors derived from human embryonic stem cells as a therapy for insulin-dependent diabetes. Diabetes Metab Res Rev. 27:928–932.
- Uludag H, Horvath V, Black JP, Sefton MV. 1994. Viability and protein secretion from human Hepatoma (HepG2) cells encapsulated in 400‐μm polyacrylate microcapsules by submerged nozzle–liquid jet extrusion. Biotechnol Bioeng. 44:1199–1204.
- Uludag H, Sefton MV. 1990. Colorimetric assay for cellular activity in microcapsules. Biomaterials. 11:708–712.
- Uludag H, Sefton MV. 1992. Metabolic activity of CHO fibroblasts in HEMA–MMA microcapsules. Biotechnol Bioeng. 39:672–678.
- Whelehan M, Marison IW. 2011. Microencapsulation using vibrating technology. J Microencapsul. 28:669–88.
- Wikstrom JD, Sereda SB, Stiles L, Elorza A, Allister EM, Neilson A, et al. 2012. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PLoS One. 7:e33023.
- Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, et al. 2007. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 292:C125–C136.
