Abstract
Objective: Nanoethosomal carriers of valsartan have been previously prepared, characterized and optimized. A gel formulation of valsartan vesicular lipid carriers was composed of Carbopol® (1% w/w), polyethylene glycol-400 (15% w/w) and triethanolamine (0.5% w/w). The influence of the valsartan nanoethosomal formulation developed on the blood pressure of experimental hypertensive rats, and its potential for skin irritation, are presented in this report. Materials and methods: The experimental rats were divided into three groups; the control group received no treatment (Group A). Group B was administered methyl prednisolone acetate (20 mg/kg/week) for two weeks (hypertensive control). Group C received methyl prednisolone acetate, followed by administration of the valsartan ethosomal formulation. The blood pressure of the rats was measured using a non-invasive rat blood pressure instrument based on the tail-cuff technique. The statistical analysis was performed using GraphPad InStat 3 software. Results and discussion: The treatment group showed a significant (P < 0.05) and constant fall in blood pressure, for up to 48 h. The valsartan ethosomal formulation was found to be effective, with a 34.11% reduction in blood pressure. The formulation's potential for skin irritation was assessed by the Draize irritation score test, which ruled out the possibility of any skin irritation caused by application of the formulation in rats. Conclusion: Our results suggest that nanoethosomes are efficient carriers for transdermal delivery of valsartan, for the management of hypertension.
Introduction
Hypertension seems to be one of the disorders chosen for considering the development of a transdermal therapeutic system, because it requires long and continuing therapy. A good number of anti-hypertensive drugs undergo hepatic first-pass metabolism, and this leads to poor bioavailability via the oral route and requires frequent dosing. Hence, such drugs are excellent candidates for the development of the transdermal system. Transdermal therapy could be the answer for circumventing the above problem (CitationAhad et al. 2011).
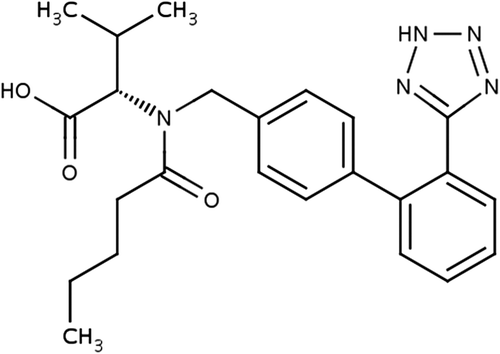
The major use of valsartan () is for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents. Thus, the drug is generally administered for a longer duration of time. This requires a daily dosing schedule and inconveniences the patient. The absolute bioavailability of valsartan is approximately 25% after oral dosing. The low bioavailability is primarily due to incomplete absorption, and partly due to pre-systemic metabolism. Peak plasma concentrations of valsartan occur 2–4 h after an oral dose. It is between 94% and 97% bound to plasma proteins. Valsartan is not significantly metabolized, and is excreted mainly via the bile as unchanged drug. The terminal elimination half-life is about 5 to 9 h. Following an oral dose, about 83% is excreted through the feces and 13% through the urine (CitationAhad et al. 2014c).
Due to its low bioavailability after oral administration, and the inconveniences related to parenteral administration, the development of new dosage forms for valsartan has reasonable importance. In this context, a transdermal delivery system could be a new interesting pharmaceutical dosage form that could improve the treatment by providing higher comfort (lower dosing frequency and no traumatic administration) and consequently improving patient compliance (CitationAhad et al. 2014b). This route allows for controlled release of the drug at rates approaching zero-order, simulating those provided by intravenous infusion.
The transdermal route is an attractive route for drug administration, because it can avoid the first-pass hepatic metabolism of drugs intended for systemic action. Major advantages provided by transdermal route include improved bioavailability, more uniform plasma levels, longer duration of action resulting in less dosing frequency, reduced side effects, and improved therapy due to the maintenance of plasma levels (CitationAhad et al. 2013a, Citation2014a, Citation2014b).
The skin is a complex and dynamic layered organ with outstanding barrier properties when compared with other biological membranes. The low permeability of the skin is the main problem encountered using this route for drug delivery (CitationSingh et al. 2014). One way to reduce this problem and enhance the transdermal delivery after the topical application of a drug is to incorporate a penetration enhancer in the formulation (CitationAhad et al. 2009, CitationAqil et al. 2007, CitationBabu and Pandit 2005, CitationSinha and Kaur 2000, CitationWilliams and Barry 2004).
A major obstacle to dermal and transdermal drug delivery is the permeation characteristic of the stratum corneum, which limits drug transport, frequently making this route of administration ineffective for medical use (CitationMura et al. 2013). Several strategies have been developed to overcome the skin's resistance, including the use of prodrugs, ion pairs, chemical permeation enhancers, iontophoresis, microneedles, ultrasound, and the vesicular system such as liposomes, transfersomes, and ethosomes (CitationAhad et al. 2009, Citation2010).
Classic liposomes remain confined to the upper surface, with little penetration into the stratum corneum. Hence a new class of liposomes, also called ethosomes or ethanolic liposomes, they have been developed by Touitou et al. (CitationTouitou et al. 1998), which are composed of phospholipid, ethanol, and water. Ethosomes were reported to enhance the skin permeation of drugs due to the interdigitation effect of ethanol on the lipid bilayer of liposomes and increasing the fluidity of stratum corneum lipids (CitationElsayed et al. 2006, CitationSong et al. 2012).
Ethosomes are promising nanocarriers for non-invasive transdermal delivery (CitationAn et al. 2011, Citation2013). The high flexibility of vesicular membranes from the added ethanol permits the elastic vesicles to squeeze themselves through the pores, which are much smaller in size than their diameters; thus, ethosomal systems are considerably more efficient in delivering substances to the skin in terms of quantity and depth than either conventional liposomes or hydroalcoholic solution (CitationDubey et al. 2007a, Citation2007b, CitationTouitou et al. 2000, Citation2001). Also, ethosomes are colloidal carriers which are easily accumulated in the leaky synovial tissue, which leads to peripheral targeting. Ethosomes also act as a depot, resulting in a controlled drug delivery (CitationVerma and Pathak 2010, Citation2012). Besides the advantages of the good affinity with the skin, innocuity, and safety, the priorities associated with ethosomes were their high flexibility and high skin penetration. The actives transfer across the skin from the epidermis into the dermis, and finally into the lymphatic vessels and blood vessels, and possess a therapeutic effect (CitationAhad et al. 2014e).
From our laboratory, we have already reported the development, characterization, and in vitro permeation pattern of a transdermal ethosomal system of valsartan (CitationAhad et al. 2013b). The objective of the present investigation is to assess the in vivo pharmacodynamic performance of an already optimized ethosomal system in rats.
Materials and methods
Valsartan was received as a gratis sample from Ranbaxy Research Laboratories Ltd, Gurgaon, India. Carbopol® 940, Polyethylene glycol-400, Ortho-phosphoric acid, and Triethanolamine were purchased from S.D. Fine Chemicals, India. Methylprednisolone acetate (MPA) Depo-Medrol was purchased from Pfizer, Mumbai, India. Potassium dihydrogen orthophosphate was purchased from Merck India Ltd., India. Methanol and chloroform were purchased from Spectrochem Pvt. Ltd., India. Absolute ethanol was purchased from Merck (Darmstadt, Germany). Double distilled water was used for all experiments.
Preparation of valsartan ethosomal gel formulations
Valsartan ethosomal formulations were prepared using the Box-Behnken design by the conventional thin layer evaporation technique. Precisely, Phospholipon® 90G and valsartan were taken in a clean, dry, round bottom flask (100 mL), and the lipid mixture was dissolved in chloroform/methanol at a ratio of 2/1 v/v. The organic solvent was removed by rotary evaporation. The prepared lipid film was hydrated with ethanolic- phosphate-buffered saline (35/65) mixture, by rotating at 60 rpm for 1 h at room temperature. To formulate smaller vesicles, large multilamellar vesicles were probe-sonicated. The readers are directed to a comprehensive research by us on the formulation, characterization and optimization of the valsartan ethosomal system (CitationAhad et al. 2013b). Further, the valsartan ethosomal dispersion was converted into a gel. Carbopol 940 (1% w/w) was added into the water and kept overnight for complete humectation of polymer chains. Valsartan ethosomal formulation was added to the hydrated Carbopol solution, with stirring. Other ingredients, like 15% w/w polyethylene glycol-400 and triethanolamine (0.5% w/w), were added to get a homogeneous dispersion of gel, and this gel formulation was used for the in vivo pharmacodynamic study.
Protocol for anti-hypertensive activity
Procurement, identification, and housing of animals
Albino Wistar rats (6–8 weeks/100–125 g) were supplied by the Central Animal House of Jamia Hamdard University, and were housed under standard laboratory conditions in a 12-h light/dark cycle at 25 ± 2o C. The animals were nourished with a pellet diet (Lipton, India) and water, ad libitum. The animals were received after the study was duly approved by the University Animal Ethics Committee, and the CPSCEA (Committee for the Purpose of Control and Supervision on Experiments on Animals), Government of India. They were marked with picric acid solution for easy identification.
Conditioning/training of animals
To reduce spontaneous variations in blood pressure, animals were adjusted to the restrainer (rat holder) by bringing them into the restrainer 3–4 times before the start of the experiment, for a period of 30–60 min. The restrainer had only one side open for the entry/exit of the animal, with proper ventilation at all other sides. As the rats were unaccustomed to remaining in the restrainer in a calm and non-aggressive manner for a long period, they were trained for their stay in the restrainer, because a slight movement or aggression by the animal would have led to variation in blood pressure reading. For this reason, the rats were restrained in the restrainer headlong until the whole body was conveniently accommodated inside. The restrainer was locked by screwing the open side of the apparatus, leaving the tail outside. The exercise was repeated before the actual experiment, until the animals learned to stay in the restrainer without aggression and were familiar with the experimental conditions (CitationAqil et al. 2004b, CitationShams et al. 2010).
Measurement of initial blood pressure of rats
The initial blood pressure of all the rats was recorded using a small noninvasive animal tail blood pressure system, NIBP200A (Biopac System, Inc. Goleta, CA, USA), based on the tail-cuff technique. The restrainer carrying the rat was placed in the blood pressure instrument, with the tail protruding outside. To measure blood pressure, a tubular inflatable cuff was placed around the base of the tail, and a piezoelectric pulse detector was positioned distal to the cuff (CitationAhad et al. 2012, CitationShams et al. 2010).
Induction of hypertension in normotensive rats
After recording the initial blood pressure of rats, the animals were divided into 3 groups (Groups A, B, and C) of 5 animals each. Group A was taken as control. Hypertension was induced in the animals of groups B and C by subcutaneous injection of MPA (20 mg/kg/week) for 2 weeks, according to the reported method (CitationAhad et al. 2012, CitationAqil et al. 2006a). The blood pressure of these rats was measured, and the rats (n = 5 in each group) with a minimum mean blood pressure of 150 mm Hg were selected for further study.
Study of antihypertensive efficacy of valsartan ethosomal formulation on experimental hypertensive rat model
After the induction of hypertension, the blood pressure of hypertensive rats from Groups B and C was measured before the application of the valsartan ethosomal transdermal gel system, on the day of the actual experiment. Group B served as hypertensive control and received no further treatment. Group C received the valsartan ethosomal formulation. The formulation was applied to the rats’ skin on the previously shaven abdominal area, with the entire release surface in intimate contact with the stratum corneum. Treatments given to each group and blood pressure recorded at time intervals are shown in (CitationUbaidulla et al. 2007).
Table I. The groups of rats under investigation, assigned group name and the time points of blood pressure measurement.
Skin irritation study
The skin irritation study was performed according to the method reported by Draize et al., using Wistar rats as an animal model (CitationDraize et al. 1944). The rats were divided into 2 groups (n = 5). The aqueous solution of formalin (0.8% v/v) as a standard irritant, and the valsartan ethosomal formulation, were applied to Groups 1 and 2 respectively. Hair from the abdominal region of rats was shaved by using an electric clipper, approximately 24 h before the application of test formulation. Care was taken to avoid abrading the skin.
Fresh formulation and formalin solution (0.5 g) were applied on the hair-free abdominal side of rats daily for 7 days. After removal of the test formulation, the animals were examined for signs of erythema and edema, and the responses scored within 30–60 min at 24, 48, and 72 h, and on 7th day. An adjacent area of untreated skin of each animal was used as a control for the test. Dermal irritation was scored and recorded according to the visual scoring scale presented in .
Table II. Draize evaluation of skin reactions.
Statistical analysis
Data were analyzed using GraphPad Prism version 3.00 (San Diego, CA, USA). Data were expressed as mean ± standard deviation (S.D.). The comparison between various groups was assessed by Dunnett's multiple comparison test. A value of P < 0.05 was considered to show a significant difference for all comparisons made.
Results and discussion
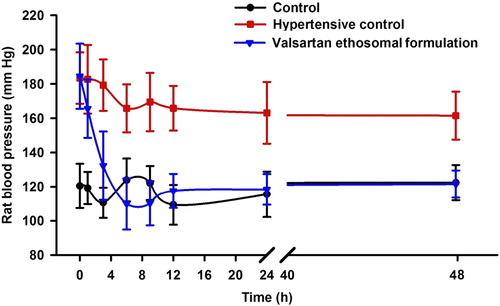
The management of hypertension has been indicated as an international health priority (CitationKearney et al. 2005, CitationRalston et al. 2012). The World Health Report 2002 identified hypertension as the third-ranked factor for disability-adjusted life years. Hypertension affects approximately 78 million individuals in the United States, and approximately 1 billion worldwide (CitationBasile 2003, CitationBerkow and Barnard 2005, CitationGo et al. 2014). Based on a pooled analysis of available national and regional data, it has been predicted that the burden of hypertension will increase by 60%, to approximately 1.56 billion in the year 2025 (CitationChockalingam et al. 2006, CitationKearney et al. 2004). Patients with hypertension need long-term treatment, and life-long therapy is sometimes advised. As transdermal drug delivery offers a better quality of life, it is more accepted than the oral dosage forms (CitationLake and Pinnock 2000, CitationSaroha et al. 2011), and thus drug deliveries via the transdermal route are ideally suited for ailments like hypertension that need chronic treatment (CitationAhad et al. 2011, CitationBairwa et al. 2013, CitationHarries and Armstrong 2012). In addition, a good number of antihypertensive drugs undergo extensive first-pass metabolism, which too can be avoided by transdermal therapy (CitationJain et al. 2008). Hence, antihypertensive agents of both therapeutic and prophylactic usage have been subjected to transdermal investigation. Several studies have been investigated to develop a transdermal system of delivery of antihypertensive drugs. CitationAhad et al. (2014a) and CitationAqil et al. (2006b), reviewed in detail the studies focused on the transdermal delivery of various β-blockers (CitationAhad et al. 2014a, CitationAqil et al. 2006b). CitationGungor and Ozsoy (2012), and Selvam et al. have done a brief overview on the different antihypertensive drugs delivered via skin in the literature (CitationGungor and Ozsoy 2012, CitationSelvam et al. 2010). Recently, Ahad et al. reviewed and detailed the transdermal research specifically on angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, and on calcium channel blockers, for the management of hypertension (CitationAhad et al. 2013a, Citation2014b). Transdermal drug delivery has many potential advantages over other routes of administration (CitationAhad et al. 2014d). Various approaches have been utilized to improve transdermal delivery. These include the use of chemical penetration enhancers, iontophoresis, electroporation, sonophoresis, microemulsions, and vesicular drug delivery systems such as liposomes, niosomes, transfersomes and ethosomes, that provide an alternative for improved drug delivery to and through the skin (CitationAlexander et al. 2012). Liposomal carriers for topical drug delivery have been studied since the 1980s, and have evoked a considerable interest. Many researchers have evaluated liposomes with respect to skin delivery, with the majority of them recording localized effects, and relatively few studies showing transdermal delivery effects (CitationEl Maghraby and Williams 2009). Subsequently, transfersomes and ethosomes were developed, with claims about their ability to deliver their payload into and through the skin with efficiencies similar to subcutaneous administration. Since these vesicles are highly flexible, they were thought to penetrate intact skin deep enough to reach the systemic circulation (CitationAkhtar 2014, CitationAmin et al. 2013). Ethosomes have also been developed for delivering the drugs having low penetrative power through the skin. Ethosomes are soft lipid vesicles of sizes ranging from tens of nanometers to microns, containing phospholipids, alcohol (ethanol and isopropyl alcohol) at a relatively high concentration, and water (CitationGoindi et al. 2014). Ethanol acts as a penetration enhancer and fluidizes the ethosomal lipids and the stratum corneum bilayer, thus allowing the soft, malleable vesicles to penetrate the disorganized lipid bilayer. The highest concentration of ethanol (20–50%) is the main reason for better skin permeation ability. Ethanol confers a surface negative net charge to ethosomes, due to which the size of vesicles decreases. Hence, the size of ethosomal vesicles increases with a decrease in the concentration of ethanol. So far, ethosomes have shown the enhancement of pharmacologic efficacy in drug-targeting to transdermal and dermal sites for the treatment of various skin diseases (CitationMbah et al. 2014). In the present study, we have investigated the anti-hypertensive efficacy and skin irritation potential of valsartan ethosomal formulations in Wistar albino rats. The in vivo pharmacodynamic activity of the valsartan ethosomal formulation was assessed using a rat blood pressure measuring instrument based on non-invasive tail-cuff technique. It was observed that hypertension was successfully induced in the normotensive rats by subcutaneous injection of MPA for a period of 2 weeks, and the rats remained hypertensive for 72 h after stopping the MPA injection, as high substantial difference was found in the pre- and post-treatment values. Thus, post treatment with the valsartan ethosomal formulation, blood pressure studies could be performed for up to 48 h. We have observed significant difference (P < 0.001) in blood pressure values of the control (Group A) and hypertensive control (Group B), corroborating the reports that excessive production or administration of glucocorticoid is associated with systemic hypertension (CitationAqil et al. 2004a, CitationRagan 1953). The application of the valsartan ethosomal system resulted in a gradual decrease of blood pressure, with the maximum effect of 109.85 ± 6.48 mm Hg, observed at 6 h on treatment of the experimental hypertensive rats (P < 0.05). It was observed that the valsartan ethosomal formulation presented a 40.43% reduction in blood pressure at a 6 h time point.
reveals that the blood pressure of hypertensive rats was controlled for up to 48 h; however, at the 48-h time point, blood pressure was comparable to that of the rats in the control group (P > 0.05). The decrease in blood pressure by the valsartan ethosomal formulation was gradual, because of controlled release of valsartan from the formulation. The valsartan ethosomal formulation was effective, and could control the blood pressure for up to 48 h (). The comparison of blood pressure between the valsartan ethosomal formulation-treated group and the control group, at an interval of 6 to 48 h, showed an insignificant difference in data analyzed by statistical analysis (P > 0.05). The ethosomal formulation of valsartan was successful in reverting the rat blood pressure to normal values in experimental hypertensive rats. The above results suggest that the valsartan ethosomal formulation holds promise for the management of hypertension. Since the administration of valsartan through the ethosomal formulation resulted in sustained and continued drug action for 48 h, the formulation was able to control the hypertension throughout the period. Clearly, the prepared transdermal ethosomal formulation was capable of surmounting the shortcomings of oral administration of valsartan, such as short half-life, and low bioavailability.
In the skin irritation study, the test formulation (valsartan ethosomes) was evaluated for primary skin irritation, according to the method reported by Draize and coworkers (CitationDraize et al. 1944). The purpose of this test was to ascertain the dermal irritation potential of the test formulation on the intact rat skin. After the applications of test formulation and standard irritant (positive control), the application sites were examined for dermal reactions, according to the Draize scoring criteria (). The primary irritation score was calculated following the completion of the study. The results of the skin irritation test for valsartan formulation after applications are reported in . According to Draize et al., compounds producing scores of 2 or less are considered negative, that is they cause no skin irritation (CitationDraize et al. 1944).
Table III. Average response of skin irritation scores following application of formalin (standard irritant) and valsartan ethosomes formulation on Wistar rat skin for period of 7 days (n = 5).
The mean irritation scores of erythema and edema on application of valsartan ethosomal formulation were found to be 0.80 and 0.20 respectively (). The irritation scores revealed that no erythema and edema were observed in the irritation studies at the site of the rat's skin after application of the valsartan formulation. Irritation scores after application of valsartan ethosomes were significantly lower (P < 0.001) in comparison to the standard irritant, formalin, which produced scores of 2.60 and 2.40 for erythema and edema respectively. In light of the above observations, it was concluded that the ethosomal formulation was a safe, less irritating, and well-tolerated formulation for transdermal delivery.
Conclusions
The present study aimed to evaluate valsartan ethosomal formulation in vivo by monitoring the effect of the ethosomal formulation on blood pressure of MPA-induced hypertensive rats. The blood pressure of rats was measured using a noninvasive rat blood pressure instrument based on the tail-cuff technique. A substantial fall in rat blood pressure was observed in the treatment of hypertensive rats with valsartan ethosomal formulation, which was maintained for 48 h. It was observed that valsartan ethosomal formulation presented a 34.11% reduction in blood pressure (184.42 ± 10.75 vs 121.52 ± 10.77 mm Hg). It was concluded that a single application of valsartan ethosomal formulation can effectively control hypertension in rats for 48 h. A study of skin irritation revealed that the valsartan ethosomal formulation was safe, less irritant, and well-tolerated for transdermal delivery. The valsartan ethosomal formulations have great utility and are a viable option for the effective and controlled management of hypertension.
Acknowledgements
Abdul Ahad thanks the Council for Scientific and Industrial Research (CSIR), India (File #09/591 (0084)/2009-EMR-I), for providing financial assistance in the form of a senior research fellowship.
Declaration of interest
All authors have approved the final manuscript, and the authors declare that they have no conflicts of interest to disclose.
References
- Ahad A, Al-Jenoobi FI, Al-Mohizea AM, Akhtar N, Raish M, Aqil M. 2014a. Systemic delivery of beta-blockers via transdermal route for hypertension. Saudi Pharm J. published online 3 January 2014, doi: 10.1016/j.jsps.2013.12.019.
- Ahad A, Al-Jenoobi FI, Al-Mohizea AM, Aqil M, Kohli K. 2013a. Transdermal delivery of calcium channel blockers for hypertension. Expert Opin Drug Deliv. 10:1137–1153.
- Ahad A, Al-Mohizea AM, Al-Jenoobi FI, Aqil M. 2014b. Transdermal delivery of angiotensin II receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEIs) and others for management of hypertension. Drug Deliv. Posted online on July 28, 1–12.
- Ahad A, Aqil M, Ali A. 2014c. Investigation of antihypertensive activity of carbopol valsartan transdermal gel containing 1,8-cineole. Int J Biol Macromol. 64:144–149.
- Ahad A, Aqil M, Kohli K, Chaudhary H, Sultana Y, Mujeeb M, Talegaonkar S. 2009. Chemical penetration enhancers: a patent review. Expert Opin Ther Pat. 19:969–988.
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M. 2013b. Enhanced transdermal delivery of an anti-hypertensive agent via nanoethosomes: statistical optimization, characterization and pharmacokinetic assessment. Int J Pharm. 443:26–38.
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M. 2014d. Design, formulation and optimization of valsartan transdermal gel containing iso-eucalyptol as novel permeation enhancer: Preclinical assessment of pharmacokinetic in wistar albino rats. Expert Opin Drug Deliv. 11:1149–1162.
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. 2010. Transdermal drug delivery: the inherent challenges and technological advancements. Asian J Pharm Sci. 5:276–288.
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. 2011. Interactions between novel terpenes and main components of rat and human skin: mechanistic view for transdermal delivery of propranolol hydrochloride. Curr Drug Deliv. 8:213–224.
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. 2012. Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan. Nanomedicine. 8:237–249.
- Ahad A, Raish M, Al-Mohizea AM, Al-Jenoobi FI, Alam MA. 2014e. Enhanced anti-inflammatory activity of carbopol loaded meloxicam nanoethosomes gel. Int J Biol Macromol. 67:99–104.
- Akhtar N. 2014. Vesicles: a recently developed novel carrier for enhanced topical drug delivery. Curr Drug Deliv. 11:87–97.
- Alexander A, Dwivedi S, Ajazuddin, Giri TK, Saraf S, Tripathi DK. 2012. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 164:26–40.
- Amin S, Sarfenejad A, Ahmad J, Kohli K, Mir SR. 2013. Nanovesicular transfersomes for enhanced systemic delivery of telmisartan. Adv Sci Eng Med. 5:299–308.
- An K, Sun Y, Wu Y, Yuan H, Cui Z, Xu L. 2013. Preparation and in vitro percutaneous penetration of simvastatin ethosome gel. Artif Cells Nanomed Biotechnol. 41:315–318.
- An K, Sun Y, Xu L, Cui X. 2011. Preparation and in vitro evaluation of simvastatin ethosome. Artif Cells Blood Substit Immobil Biotechnol. 39:347–350.
- Aqil M, Ahad A, Sultana Y, Ali A. 2007. Status of terpenes as skin penetration enhancers. Drug Discov Today. 12:1061–1067.
- Aqil M, Ali A, Sultana Y, Dubey K, Najmi AK, Pillai KK. 2006a. In vivo characterization of monolithic matrix type transdermal drug delivery systems of pinacidil monohydrate: a technical note. AAPS PharmSciTech. 7:E6.
- Aqil M, Ali A, Sultana Y, Parvez N. 2004a. Communication: Matrix type transdermal drug delivery systems of metoprolol tartrate: skin toxicity and in vivo characterization. Ethiop Pharm J. 22:53–60.
- Aqil M, Sultana Y, Ali A. 2006b. Transdermal delivery of beta-blockers. Expert Opin Drug Deliv. 3:405–418.
- Aqil M, Sultana Y, Ali A, Dubey K, Najmi AK, Pillai KK. 2004b. Transdermal drug delivery systems of a beta blocker: design, in vitro, and in vivo characterization. Drug Deliv. 11:27–31.
- Babu RJ, Pandit JK. 2005. Effect of penetration enhancers on the release and skin permeation of bupranolol from reservoir-type transdermal delivery systems. Int J Pharm. 288:325–334.
- Bairwa M, Pilania M, Gupta V, Yadav K. 2013. Hypertension Vaccine may be a boon to millions in developing world. Hum Vaccin Immunother. 10:708–713.
- Basile JN. 2003. Optimizing antihypertensive treatment in clinical practice. Am J Hypertens. 16:13S–17S.
- Berkow SE, Barnard ND. 2005. Blood pressure regulation and vegetarian diets. Nutr Rev. 63:1–8.
- Chockalingam A, Campbell NR, Fodor JG. 2006. Worldwide epidemic of hypertension. Can J Cardiol. 22:553–555.
- Draize JH, Woodard G, Calvery HO. 1944. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 82:377–390.
- Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. 2007a. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 123:148–154.
- Dubey V, Mishra D, Jain NK. 2007b. Melatonin loaded ethanolic liposomes: physicochemical characterization and enhanced transdermal delivery. Eur J Pharm Biopharm. 67:398–405.
- El Maghraby GM, Williams AC. 2009. Vesicular systems for delivering conventional small organic molecules and larger macromolecules to and through human skin. Expert Opin Drug Deliv. 6:149–163.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. 2006. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 322:60–66.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. 2014. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 129: e28–e292.
- Goindi S, Dhatt B, Kaur A. 2014. Ethosomes-based topical delivery system of antihistaminic drug for treatment of skin allergies. J Microencapsul. 31:716–724.
- Gungor S, Ozsoy Y. 2012. Systemic delivery of antihypertensive drugs via skin. Ther Deliv. 3:1101–1116.
- Harries C, Armstrong I. 2012. A review of the management of pulmonary arterial hypertension associated with congenital heart disease. Eur J Cardiovasc Nurs. 11:239–247.
- Jain R, Aqil M, Ahad A, Ali A, Khar RK. 2008. Basil oil is a promising skin penetration enhancer for transdermal delivery of labetolol hydrochloride. Drug Dev Ind Pharm. 34:384–389.
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. 2005. Global burden of hypertension: analysis of worldwide data. Lancet. 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. 2004. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 22:11–19.
- Lake Y, Pinnock S. 2000. Improved patient acceptability with a transdermal drug-in-adhesive oestradiol patch. Aust N Z J Obstet Gynaecol. 40:313–316.
- Mbah CC, Builders PF, Attama AA. 2014. Nanovesicular carriers as alternative drug delivery systems: ethosomes in focus. Expert Opin Drug Deliv. 11:45–59.
- Mura S, Manconi M, Fadda AM, Sala MC, Perricci J, Pini E, Sinico C. 2013. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil: in vitro evaluation of drug permeation by infrared spectroscopy. Pharm Dev Technol. 18:1339–1345.
- Ragan C. 1953. Corticotropin, cortisone and related steroids in clinical medicine; practical considerations. Bull N Y Acad Med. 29:355–376.
- Ralston RA, Lee JH, Truby H, Palermo CE, Walker KZ. 2012. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J Hum Hypertens. 26:3–13.
- Saroha K, Yadav B, Sharma B. 2011. Transdermal patch: A discrete dosage form. Int J Curr Pharm Res. 3:98–108.
- Selvam RP, Singh AK, Sivakumar T. 2010. Transdermal drug delivery systems for antihypertensive drugs - A review. Int J Pharm Biomed Res. 1:1–8.
- Shams MS, Alam MI, Ali A, Sultana Y, Aqil M. 2010. Pharmacodynamics of a losartan transdermal system for the treatment of hypertension. Drug Dev Ind Pharm. 36:385–392.
- Singh D, Pradhan M, Nag M, Singh MR. 2014. Vesicular system: Versatile carrier for transdermal delivery of bioactives. Artif Cells Nanomed Biotechnol. published online 25 February 2015, doi: 10.3109/21691401.2014.883401.
- Sinha VR, Kaur MP. 2000. Permeation enhancers for transdermal drug delivery. Drug Dev Ind Pharm. 26:1131–1140.
- Song CK, Balakrishnan P, Shim CK, Chung SJ, Chong S, Kim DD. 2012. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surf B Biointerfaces. 92:299–304.
- Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. 2000. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 65:403–418.
- Touitou E, Dayan N, Levi-Schaffer F, Pilipoponsky A. 1998. Novel lipid vesicular system for enhanced delivery. J Lipid Res. 8:113–114.
- Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, Levi-Schaffer F. 2001. Intracellular delivery mediated by an ethosomal carrier. Biomaterials. 22:3053–3059.
- Ubaidulla U, Reddy MV, Ruckmani K, Ahmad FJ, Khar RK. 2007. Transdermal therapeutic system of carvedilol: effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics. AAPS PharmSciTech. 8:2.
- Verma P, Pathak K. 2010. Therapeutic and cosmeceutical potential of ethosomes: an overview. J Adv Pharm Technol Res. 1:274–282.
- Verma P, Pathak K. 2012. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine. 8:489–496.
- Williams AC, Barry BW. 2004. Penetration enhancers. Adv Drug Deliv Rev. 56:603–618.