Abstract
Objective: When preliminary tests have confirmed a nano-hydroxyapatite (Nano-HA) content of 20% of the polylactic acid (PLA) composite material of Nano-HA interface fixation material for biomechanical requirements, there is a need for further observation of its biocompatibility and clinical applications, to provide reference data. Methods: Preparation of Nano-HA content of 20% PLA composite Nano-HA bone substitute material and extract. The establishment of the negative control group (containing 10% fetal bovine serum in DMEM complete medium), experimental group (extract), the positive control group (mass concentration of 0.64% phenol), and a co-culture of rabbit bone marrow mesenchymal stem cells (rBMSC) and materials extraction liquid. Observation of the morphological changes in rBMSC in culture at time points of 3, 5, and 7 days, the use of the MTT assay, and determination of the relative growth in the above set of rBMSC in cell culture at 3, 5, and 7 days, to judge the material's cytotoxicity. Results: With time, the absorbance value of the three groups of cells were significantly increased (P < 0.01). The relative growth of the rBMSCs in experimental group in the first 3, 5, and 7 days was 95.3%, 96.8% and 97.6%; the cytotoxicity was according to the national standards I; the difference was not significant (P > 0.05) between the the experimental group and the negative control group; there was a significant difference between the positive control group and the other 2 groups (P < 0.05). Cells in the experimental group were seen having normal morphology, and spindle-shaped adherent growth. Conclusion: PLA composite artificial bone materials and Nano-HA show good cell compatibility, and the values for cytotoxicity, with reference to GB/T16886.5.2003 (China) standards, are in the safe range.
An interface screw is a commonly used tendon fixator in anterior cruciate ligament reconstruction under arthroscopy. However, it has weak mechanical strength and causes foreign body reaction. Its degradation rate does not match with the bone tunnel recovery, and therefore it has no osteoinductive activity. Due to these shortcomings, the healing of the biological interface between tendon and bone, and material and bone, is poor. This becomes an important reason for the postoperative bone tunnel enlargement and ligament relaxation (CitationSultana and Wang 2012). Polylactic acid (PLA) is easily degradable, causing an inflammatory response by increasing the local acidity and possibly altering the dynamic strength of new bones. Therefore, the combination of PLLA, which has good processability, biocompatibility, degradability and absorbability with bioactive nano-hydroxyapatite (Nano-HA) to form the Nano-HA/PLLA composite material can effectively improve the acidic environment caused by the degradation products of PLLA. It is more favorable for the growth of new bone tissue, promoting the healing of the biological interface between tendon and bone (CitationLie et al. 2009, CitationNeuendorf et al. 2008). In order to investigate its cytocompatibility, this study analyzed the cytotoxicity of the composite material using the established bone marrow mesenchymal stem cells with reference to Biological Evaluation Standards of Medical Devices of China (GB/T16886.5.2003) (CitationZhu et al. 2008).
Materials and methods
Materials
Nano-HA/PLLA was developed jointly by the Tissue Engineering Laboratory of the Second People's Hospital of Shenzhen and the Powder Metallurgy Research Institute of Central South University. First, the low-pressure filtration method was used to prepare lactide, and PLLA was obtained by in situ polymerization. Schistose PLLA was prepared by solution spinning. Next, the sol-flocculation method was used to prepare Nano-HA powders. Finally, PLLA and Nano-HA powders were mixed by melt blending to obtain Nano-HA/PLLA composites for interface fixation. Nano-HA/PLLA was processed into cylinder shape with length of 10 mm, diameter of 5 mm and height of 5 mm and disinfected by ethylene oxide (CitationZhu et al. 2013, Citation2014, CitationHuang et al. 2013).
Reagents and equipments
Fetal calf serum (FCS) (Hangzhou Sijiqing Bioengineering Material Co., Ltd.); High-glucose DMEM culture medium, (GIbco Co.), containing FCS of 0.1 volume fraction, 100 kU/L penicillin, 100 kU/L streptomycin, 10 g/L glutamine, pH adjusted to 7.2. After formulation, it was filtered by millipore filter of 0.22 um for sterilization and preserved. Trypsin (Sigma Inc.); Dimethyl sulfoxide (DMSO) (Sigma Inc.); MTT solution (Sigma Inc.); CO2 incubator with HEPA Class 100 filter (Thermo Electron Corporation); BCM-1000 ultra clean bench (manufactured by Antai Co., Sujing Group); DuR series 600 UV spectrometer (Shanghai Tianpu Analyzer Apparatus Co., Ltd.); Axioshop inverted phase photomicrographic system (ZEISS, German, The Second People's Hospital of Shenzhen); Adjustable micro pipette (Gilson, France); JY92-II ultrasonic cell disruptor (Ningbo Xinzhi Biotechnology Co., Ltd.); Desk centrifuge (TGL-16B, Shanghai Anting Scientific Instrument Factory); 96-well plates (COSTAR, USA); 4°C refrigerator (Qingdao Haier Group); Enzyme- linked immunoassay analyzer (BIO-RAD, USA).
Methods
In vitro isolation and culture of rabbit bone marrow mesenchymal stem cells
Three fetal rabbits younger than 10-day old (Animal Experimental Center of Guangzhou Medical University) were killed by intravenous injection of excess anaesthetics (3% pentobarbital sodium) from the edge of the ear. After cleaning, the rabbits were soaked in 75% ethanol for 5 min for sterilization. The sterilized rabbit was placed on the clean bench, and the residual ethanol was removed. Under aseptic condition, the skin was sliced and the skull (parietal bone and frontal bone) was harvested with scissors. During operation, all operations are subject to review and supervision of the hospital ethics committee, in the course of the experiment the animals to minimize the degree of pain and fear, death process in accordance with humanitarian principles euthanized, in accordance with animal ethics and animal ethics requirements and all the study followed principles in the Declaration of Helsinki. The density gradient centrifugation combined with adherence screening method was used to isolate and culture rabbit bone marrow mesenchymal stem cells (rBMSCs). After 10 − 12 days, the cells were mostly fused and then digested by 0.25% trypsin (containing 0.02% EDTA), to obtain the suspension of primary generation of rBMSCs. The cell count after 3 days was approximately 4 × 104 L− 1. The suspension was preserved for further use.
Preparation of composite extract
The Nano-HA/PLLA sample (10 × 5 × 5 mm) was extracted and disinfected by ethylene oxide. The surface area of the experiment material was calculated at the ratio of 1 cm2/10 mL. The Nano-HA/PLLA sample was put into the complete DMEM medium containing 10% FCS and placed in 37°C incubator for 48 h to obtain the extract. After complete oscillation, 0.22 um millipore filter was used for filter and disinfection. The sample then was preserved in 4°C refrigerator for further use. For positive control group, complete DMEM media containing 0.64% phenol was added; complete DMEM medium containing 10% FCS was added for the negative control group.
Cell inoculation
The rBMSCs in vigorous growth after 48–72 h of passage were prepared into 1 × 107 L− 1 concentration and added into three 6-well plates, 200 μL for each well. The plates were placed in an incubator containing CO2 with volume fraction of 0.05 at 37°C for 24 h for cell adherence. After 24 h, the original culture media was discarded. The culture medium, normal saline, extract and phenol were added to the wells in accordance with the requirements of every group. The groups were set as follows: (1) Experimental group: 100 μL complete DMEM media containing the extract. (2) Negative control group: 100 μL complete DMEM medium containing 10% FCS. (3) Positive control group: 100 μL complete DMEM medium containing phenol of 0.64% mass concentration. The culture plates were placed into an incubator containing CO2 with volume fraction of 0.05 at 37°C. On 3, 5 and 7 days, the samples of all the groups were observed under an inverted microscope.
Detection of relative growth rate
On day 3, 5 and 7, one plate was taken, respectively. The extract and the culture medium in the wells were discarded.
MTT fluid was added for further culture at 20 μL/well for 4 h. The resulting solution was drawn and 200 μL/well DMSO was added. Then the oscillation continued for 10 min. The absorbance at 490 nm was measured on the ELISA reader. The MTT method was used to measure the relative growth rate by the formula: relative growth rate = average absorbance of experiment group/average absorbance of negative control group × 100%. Then, the cytotoxicity of the Nano-HA/PLLA composites was determined according to the relative growth rate. The grading criteria are shown in .
Table I. Reaction grading criteria.
Cell morphology
After the inoculation, the inverted microscope was used to observe the cell morphology and growth in the experimental group, negative control group and positive control group on day 3, 5 and 7. The toxicity grading criteria and cell morphologies are shown in .
Table II. Cell morphologies and cytotoxicity levels.
Principal observation parameters
(1) Cell relative growth rate and cytotoxicity grading (2) Cell morphology analysis Statistical analysis: SPSS 13.0 was used to analyze the experimental data. The data are expressed as x ± S. The t-test was conducted. P < 0.05 was regarded as statistically significant.
Results
Measurement of cell relative growth rate. See
indicates that with the prolongation of time, the absorbance in the three groups increased significantly. The difference between day 3, 5 and 7 was extremely significant (P < 0.01). The cell relative growth rate of rBMSCs of the experimental group on day 3, 5, and 7 was 95.3%, 96.8% and 97.6%, respectively. In reference to the state standard, the cytotoxicity of Nano-HA/PLLA composites for interface fixation should be of Grade 1. The difference between the experimental group and the negative control group was not significant (P > 0.05). When compared with the positive control group, the difference in the two groups above was significant (P < 0.05), indicating that the extract of the Nano-HA/PLLA composites had no apparent influence on the proliferation of rBMSCs.
Table III. The absorbance values at different time points, the relative growth rate and toxicity grade (n = 6, wells).
Cell morphologies and cell growth
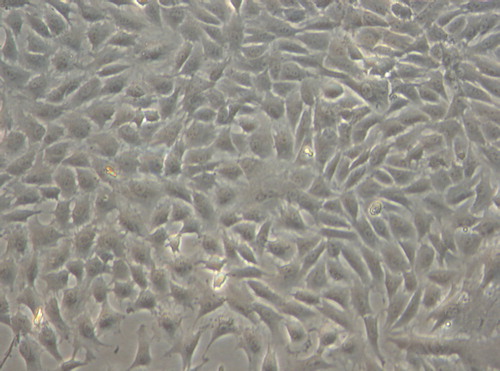
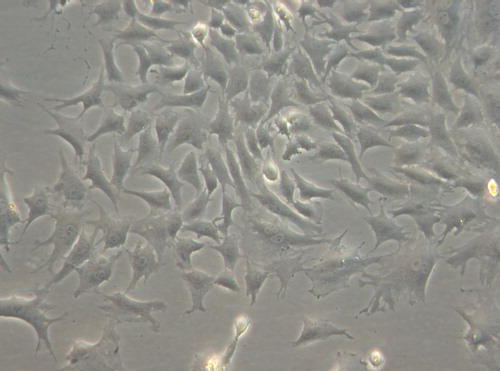
Under inverted phase contrast microscope: the cells in the experimental group adhered nearly completely on day 3 after inoculation. The cells were mostly in spindle shape, presenting fibroblast morphology with scattered distribution (). After 5 days of inoculation, the cell number increased significantly and the growth of cells accelerated. When reaching the overgrowth, the cells were arranged in vortex, palisade-like or radial pattern (). On 7 days, 90% of the cells were in long spindle shape. The cell morphology was normal, the arrangement was compact and regular and the growth of cells was vigorous (). There was no difference between the experimental group to the control group (, and ). According to the cell morphology standard, the Nano-HA/PLLA composites had no cytotoxicity and possessed excellent cytocompatibility.
Figure 1. The cells in the experimental group adhered nearly completely on day 3 after inoculation, spindle-shaped (× 200).
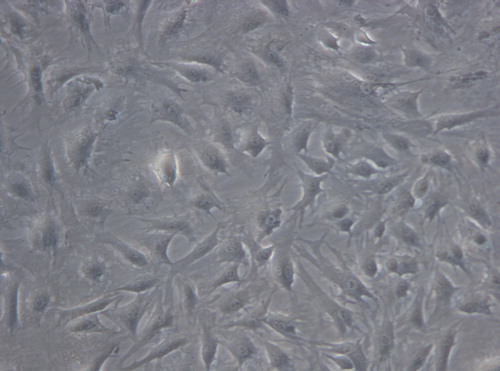
Figure 2. The cells in the experimental group adhered nearly completely on day 5 after inoculation, the cells were palisade-like or radial pattern (× 200).
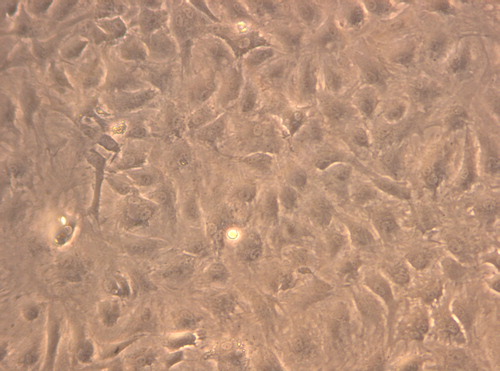
Figure 3. The cells in the experimental group adhered nearly completely on day 7 after inoculation, the cell morphology was normal (× 200).
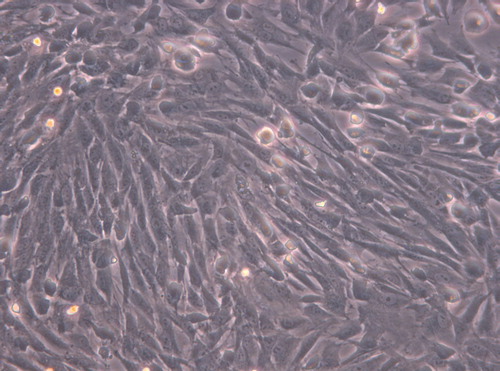
Figure 4. The cells in the control group adhered nearly completely on day 7 after inoculation, cells were spindle-shaped (× 200).
Discussion
The bionic composites for interface fixation can be degraded into products absorbable by human body. During the early and middle stages of tendon-bone healing stages, these composites can provide good mechanical strength and properties to fix the transplanted tendon. With the degradation and absorption of the biomaterials, the load is transferred and the stress shielding effect is reduced. Due to the action of stress, the osteogenesis is enhanced and the healing of the local tendon-bone interface is accelerated. Thus, a series of implications are avoided. The Nano-HA/PLLA composites for interface fixation are currently at the research and development stage. Earlier experiments confirmed that when the Nano-HA content reached 20%, the bending strength of the Nano-HA/PLLA composites was 175 Mpa, which satisfied the requirements of biomechanics for the fixation of the transplanted tendon (CitationHam 1995).
After the entry of medical materials into the human body, they are in direct contact with human tissues and cells. Therefore, medical materials must have good biocompatibility. The Nano-HA/PLLA composite for interface fixation is a new type degradable material for tendon-bone healing. The evaluation of its biosafety is an important step before the clinical application. According to Biological Evaluation Standards of Medical Devices (GB/T16886.5.2003) (CitationZhu et al. 2008), the bone mesenchymal stem cells established as cell strain were used for the cytotoxicity study of the composite material. Dead cells have no such reactions. DMSO can dissolve the formazan accumulated in the cells. The color of the solution is in positive correlation with the formazan amount in the cells. With ELISA reader, the absorbance was measured in this study to represent the number of living cells. This method is more accurate, quicker and easier than the manual cell counting. The MTT method uses the extract to measure the cytotoxicity. It has high sensitivity to the toxicity of the dissolved substance from the test material. This conforms to the toxicity experiment results in animals. Thus, it is a satisfactory method to evaluate the cytotoxicity of biomaterials in vitro (CitationZhu et al. 2012).
The results of this study showed no significant difference between the experimental group and the blank control group in terms of the number and morphology of rBMSCs. On day 3, 5, and 7, the rBMSCs adhered to the composite and were in long spindle or flat polygon shape with long processes. There was no difference from the blank control, indicating that the Nano-HA/PLLA composites had no apparent cytotoxicity and did not influence the normal functions of cells. On day 3, 5, and 7, the cell relative growth rate was higher than 90%. With the prolongation of time, cells proliferated in large quantity and the proliferation rate increased continuously. The proliferation rate on day 3 was 95.3%, on day 5, 96.8%, and on day 7, 97.6%. In accordance to the state standards, the cytotoxicity of the Nano-HA/PLLA composites for interface fixation should be of Grade 0 or Grade 1. The Nano-HA/PLLA composites fabricated in this study met the medical requirements for biomaterials.
Hydroxyapatite (HA) is the most common type of bioactive materials. It is one of the inorganic compositions of human bone tissue and has good biocompatibility and bone conductibility (CitationTang et al. 2012). In recent years, nanohydroxyapatite material began to bewidely used in the field of tissue engineering. PLLA is currently the most used scafold in tissue engineering, as it has good biocompatibility and controllable biodegradability. Moreover, the degraded products can be metabolized (CitationZhu et al. 2010). The nHA/PLLA material of this research is diferent from any other single material. It is composed of Nano-HA and PLLA via computer-aided rapid prototyping technology and freeze-drying compound. Thus, it has the characteristics of the two kinds of materials. (1) It is more advantageous for extracellular matrix protein adhesion and interaction due to the nano structure of material. It is able to guide tissue cells to grow, and the biodegradability increases as the result of the increase of surface area. (2) PLLA degradation products can be neutralized to some extent by nHA, thus preventing the aseptic inflammation effectively. It can also accelerate the degradation of the material. (3) Its composition, shape, microstructure, and mechanical properties can be predicted in advance and it can be made to meet the clinical needs in the process of material production.
This mixed culture of composite extract and the rBMSCs was employed to measure the cytotoxicity of the Nano-HA/PLLA composites for interface fixation. The results indicated that the Nano-HA/PLLA composite extract showed no toxicity in a thermostat incubator containing CO2 with volume fraction of 0.05 at 37°C, which was used to simulate the internal environment of the human body. This indicated that the Nano-HA/PLLA composites for interface fixation had good cytocompatibility and the cytotoxicity was classified as Grade I, which met the medical standards for biomaterials. It is a promising degradable biomaterial for tendon-bone healing.
Acknowledgment
This study received financial support from Guangdong Science and Technology Project (project number 2013B021800100) Shenzhen Science and Technology Project (project number CXZZ20130321152713220 and JCYJ20140414170821164).
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ham MF. 1995. In vitro study of biodegradation of a Co-Cr alloy using a human cell culture model. J Biomater Sci Polym Ed. 6:809–814.
- Huang J, Xiong J, Liu J, Zhu W, Wang D. 2013. Investigation of the in vitro degradation of a novel polylactide/Nanohydroxyapatite composite for artificial bone. J Nanomat.2013:1–11.
- Lie Shen,Hui Yang,Jia Ying, Qiao F, Peng M. 2009. Preparation and mechanical properties of carbon fiber reinforced hydroxyapatite/polylactide biocomposites. J Mater Sci Mater Med. 20:2259–2265.
- Neuendorf RE, Saiz E, Tomsia AP, Ritchie RO. 2008. Adhesion between biodegradable polymers and hydroxyaptite: relevance to synthetic bone-1ike materials and tissue engineering scaffolds. Acta Biomater. 4:1288–1296.
- Sultana N, Wang M. 2012. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: surface modification and in vitro biological evaluation. Biofabrication. 4:15–24.
- Tang Z, Cao J, Wen N, Wang HB, Zhang ZW, Liu ZQ, et al. 2012. Posterolateral spinal fusion with nano- hydroxyapatite- collagen/PLA composite and autologous adipose-derived mesenchymal stem cells in a rabbit model. J Tissue Eng Regen Med. 6:325–336.
- Zhu W, Chen K, Lu W, Sun Q, Peng L, Fen W, et al. 2014. In vitro study of nano-HA/PLLA composite scaffold for rabbit BMSC differentiation under TGF-β1 induction. In Vitro Cell Dev Biol Anim. 50:214–220.
- Zhu W, Huang J, Lu W, Sun Q, Peng L, Fen W, et al. 2013. Performance test of Nano-HA/PLLA composites for interface fixation. Artif Cells Nanomed Biotechnol. 42:331–335.
- Zhu W, Lu W, Zhang X, Cai Z, Liu H, Peng L, et al. 2012. Nano- hydroxyapatite/fibrin glue/recombinant human osteogenic protein-1 artificial bone for repair of bone defect in an animal model. Micro & Nano Letters. 7:467–471.
- Zhu W, Xiao J, Wang D, Liu J, Xiong J, Liu L, et al. 2008. Experimental study of nano-HA artificial bone with different pore sizes for repairing the radial defect. Int Orthop. 33:567–571.
- Zhu W, Zhang X, Wang D, Lu W, Ou Y, Han Y, et al. 2010. Experimental study on the conduction function f nano-hydroxyapatite artificial bone. Micro & Nano Letters. 1:19–27.