Abstract
The synthesis of silver nanoparticles (AgNPs) by microorganisms is an area attracting growing interest in nanobiotechnology, due to the applications of these nanoparticles in various products including cosmetics and biosensors, and in the biomedical, clinical, and bioimaging fields as well. Various microorganisms have been found to be able to synthesize AgNPs when silver salts are supplied in the reaction system. The main objectives of this study were to evaluate the efficiency of synthesis of AgNPs by the strain Bacillus methylotrophicus DC3, isolated from the soil of Korean ginseng, a traditionally known oriental medicinal plant in Korea. The AgNPs showed maximum absorbance at 416 nm, when assayed by ultraviolet-visible spectroscopy (UV-vis). The field emission transmission electron micrograph (FE-TEM) results showed that the particles were spherical and 10–30 nm in size. In addition, the product was also characterized by energy dispersive X-ray spectroscopy (EDX), which displayed a 3 keV peak corresponding to the silver nanocrystal. Elemental mapping results also confirmed the presence of silver elements in the electron micrograph region. Furthermore, the AgNPs demonstrated antimicrobial activity against various pathogenic microorganisms such as Candida albicans, Salmonella enterica, Escherichia coli, and Vibrio parahaemolyticus, with enhanced antimicrobial activity being exhibited against C. albicans. Therefore, the current study describes the simple, efficient, and green method of synthesis of AgNPs by B. methylotrophicus DC3.
Introduction
Metal nanoparticles have great importance due to their unique electronic, optical, and physiochemical properties, which differ significantly from those of bulk materials (CitationMazur 2004). These special properties could be attributed to their small size, large specific surface area, and high fraction of surface atoms. For these reasons, metal nanoparticles have broad applications in many fields, including biomedicine (CitationBiswas et al. 2004, CitationGovindaraju et al. 2009), and as substances with antimicrobial activity (CitationDuran et al. 2005).
A variety of physical and chemical routes of synthesis have been reported for the preparation of metallic nanoparticles (CitationRaut et al. 2009). However, these methods have several disadvantages, including the use of toxic chemicals and the production of hazardous waste products. There is a growing need to develop an environmentally-friendly process of nanoparticle synthesis (CitationWhitesides 2003). Microorganisms possess the capability to reduce metals into the metal ions, and play a major role in bioremediation (CitationFortin and Beveridge 2000). In addition, these small nanofactories are capable of synthesizing nanoparticles in an eco-friendly way. Numerous silver, gold, and cadmium metal nanoparticles have been synthesized by various microorganisms. Bacteria have been the most commonly used microorganisms, because they are eco-friendly and relatively easy to handle (CitationParikh et al. 2008).
From a medical perspective, silver nanoparticles (AgNPs) present a range of anti-microbial, anti-fungal, and anti-viral applications against a broad range of microorganisms (CitationKim et al. 2008). In addition, AgNPs can be used as optical receptors (CitationSchultz et al. 2000), as catalysts in chemical reactions, as spectrally selective coatings for solar energy absorption, and as intercalation material for electric batteries (CitationKlaus-Joerger et al. 2001), cosmetics (CitationJin and Ye 2007), and therapeutics (CitationProw et al. 2006). Ginseng (Panax ginseng Meyer), is a traditionally known medicinal plant in Korea, China, and Japan. Ginseng and its crude extract have been used to cure various diseases such as cancer, diabetes, Alzheimer's, inflammation, etc. (CitationChoi 2008, CitationMathiyalagan et al. 2014). The soil from the ginseng field has been used to isolate various bacteria, some of which are further utilized for biotransformation of pharmacologically active minor ginsenosides from major ginsenosides (CitationCheng et al. 2007), and other bacteria which promote plant growth (CitationDeepa et al. 2010).
Due to the increasing applications and demand for AgNPs, there is a need for their green synthesis by various microorganisms. Extracellular biosynthesis of AgNPs has been conducted in Escherichia coli, Plectonema boryanum, Pseudomonas stutzeri, and Morganella sp. (CitationGurunathan et al. 2009). In the present study, we have described the biological synthesis and characterizations of AgNPs produced using Bacillus methylotrophicus DC3, which was isolated from ginseng field soil, and explored their antimicrobial efficacy against different pathogenic microorganisms.
Materials and methods
Media and chemicals
All media were purchased from Difco, MB cell (Seoul, Republic of Korea), including analytical grade silver nitrate (AgNO3). Cycloheximide was purchased from Sigma-Aldrich Chemicals, USA. Vancomycin (VA30) 30 μg/disk, oleandomycin (OL15) 15 μg/disk, and penicillin G (P10) 10 μg/disk were purchased from Oxoid Ltd., England.
The pathogenic bacterial strains Vibrio parahaemolyticus [ATCC 33844], Salmonella enterica [ATCC 13076], E. coli [ATCC 10798], and Candida albicans [KACC 30062] were cultured on Tryptic soy agar (TSA) media at 30°C and preserved at − 70°C in glycerol for further study. Candida albicans was cultured on Sabouraud dextrose agar (SDA) at 28°C and preserved at − 70°C in glucose yeast peptone broth (GYP) glycerol stocks.
Isolation and molecular identification of bacteria
Soil samples were collected from the ginseng field at Kyung Hee University, Republic of Korea. The soil samples were serially diluted in sterile 0.8% NaCl and then plated onto TSA media. The colonies were further subcultured on TSA supplemented with 1 mM of filter-sterilized AgNO3 for 48 h at room temperature, and observed for colony growth. Isolated colonies were subcultured to obtain pure cultures.
Molecular identification of the isolated strains was carried out by 16S rRNA sequencing. The genomic DNA of the isolated strain was extracted and purified with the Genomic DNA Isolation Kit (Core Bio System, Republic of Korea). The 16S rRNA gene was amplified with the universal bacterial primer pairs 27F, 518F, 800R, and 1512R (CitationWeisburg et al. 1991). The purified PCR products were sequenced by Genotech (Daejeon, Republic of Korea). The sequences of the 16S rRNA gene were compiled with SeqMan software (version 4.1, DNASTAR, Inc., USA). The 16S rRNA gene sequences of related taxa were obtained from the GenBank database and EzTaxon-e server (CitationKim et al. 2012).
Extracellular biosynthesis of AgNPs
For biological synthesis of AgNPs, the selected bacterial isolate was inoculated into a 250 ml Erlenmeyer flask containing 100 ml of sterile Tryptic soy broth (TSB). The flasks were incubated in a shaking incubator at 28°C for 24 h at 120 rpm. After incubation, the culture was centrifuged at 10,000 rpm for 10 min and the bacterial pellet was removed. The supernatant was mixed with filter- sterilized AgNO3 solution at a 1 mM final concentration, and incubated in an orbital shaker at 200 rpm and 28°C. The extracellular synthesis of AgNPs was monitored by visual observation of the color change in the culture medium. After the completion of incubation, the mixture was first centrifuged at 2000 rpm for 5 min to remove remaining medium components, and then the AgNPs were collected by high-speed centrifugation at 20,000 rpm for 10 min. The product was then washed several times by centrifugation and redispersed in water to remove the unconverted silver ions. Finally, the AgNPs were collected in a pellet and used for characterization.
Characterization of synthesized AgNPs
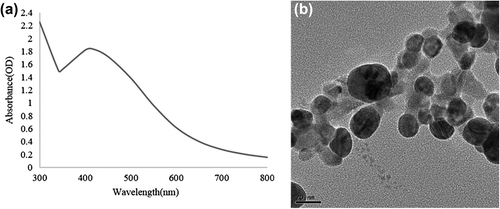
To verify the reduction of silver ions, the solution was scanned at 300–800 nm in a UV-vis spectrophotometer (Ultrospec 2100 pro, Amersham Biosciences Corp., USA).
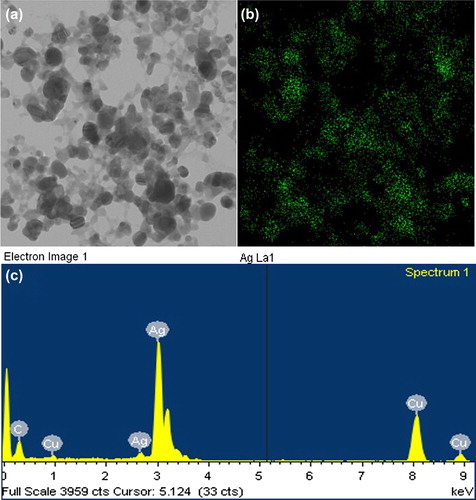
The size, shape, morphology, and distribution of the nanoparticles were analyzed by field emission transmission electron microscopy (FE-TEM), energy dispersive X-ray spectroscopy (EDX), and elemental mapping with a JEM-2100F (JEOL) instrument, operated at 200 kV. The sample was prepared by placing a drop of AgNPs on a carbon-coated copper grid, and subsequently drying it in an oven at 60°C before transferring it to the microscope.
Analysis of antimicrobial activity of AgNPs
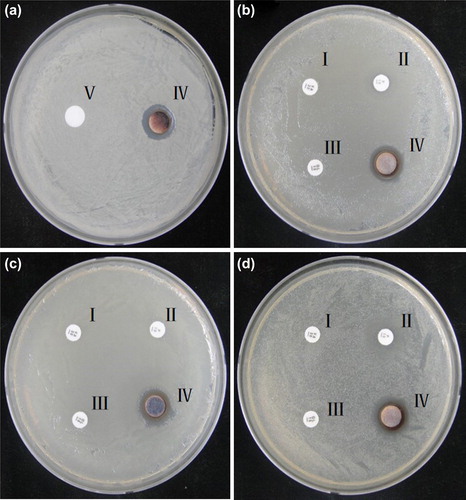
The antimicrobial activity of the biologically synthesized AgNPs against pathogenic organisms such as V. parahaemolyticus, S. enterica, E. coli, and C. albicans was measured on Mueller-Hinton agar (MHA) plates using the disk diffusion method. A 100 μL aliquot of an overnight log culture of V. parahaemolyticus, S. enterica and E. coli was spread evenly on a MHA plate with a glass spreader. The AgNPs (3 μg) were added to a plain disk on the plates. The antibiotic discs VA30, P10, and OL15 were also kept on the same plate, to act as a control, and the plate was incubated at 30°C for 24 h. Similarly, for C. albicans, an overnight log culture was spread evenly on a SDA plate and the sterile paper discs were loaded with 3 μg of cycloheximide and AgNPs, and further kept for 24-h of incubation. After incubation, the zones of inhibition were measured in all the plates.
Results and discussion
Identification of bacteria
On the basis of molecular characterization of the bacterial isolate, the isolated strain showed 99.51% similarity with B. methylotrophicus CBMB205T, thus named as B. methylotrophicus DC3. Moreover, the 16S rRNA sequence of B. methylotrophicus DC3 has been deposited to NCBI, with the KP412492 accession number.
Synthesis and characterization of AgNPs
In the present study, AgNPs were successfully synthesized in the culture supernatant of B. methylotrophicus DC3. The formation of AgNPs by the reduction of AgNO3 in the supernatant of B. methylotrophicus DC3 was indicated by a color change in the reaction mixture (). The color changed gradually from yellow to brown, within 48 h. The brown coloration was due to the formation of AgNPs in the reaction mixture, resulting in the excitation of surface plasmon vibrations (CitationAnil Kumar et al. 2007).

The synthesis of AgNPs was further confirmed by UV-vis spectral analysis. The product was scanned at 300–800 nm, and the strongest peak was observed at 416 nm, which corresponded to the surface plasmon resonance band of the AgNPs () (CitationBhainsa and D’Souza 2006). Thus, the reaction mixture indicated the formation of AgNPs. FE-TEM images of the AgNPs revealed a spherical shape, with sizes ranging from 10–30 nm (). The EDX analysis of silver nanocrystallites displayed an optical absorption band peak at approximately 3 keV (), which is the typical absorption of metallic silver nanocrystallites (CitationMagudapathy et al. 2001). Elemental mapping analysis of the AgNPs showed maximum distribution of silver (48.36%) (). The distribution of carbon and copper were due to the TEM grid and contamination of chlorine. Overall, we found that the strain B. methylotrophicus DC3 is capable of efficient synthesis of AgNPs. Bacillus has shown activity in biosorption and biodegradation (CitationLi et al. 2014), and B. methylotrophicus has been reported in the biosynthesis of levan by levansucrase (CitationZhang et al. 2014), and biocontrol of rice blast (CitationShan et al. 2013). To our knowledge, this is first time B. methylotrophicus DC3 has been found to be capable of synthesizing spherically shaped AgNPs. The previous studies suggest that the enzyme and proteins secreted by the microorganisms extracellularly in the culture medium play a major role in the reduction of AgNO3 to AgNPs. The study, based on the extracellular synthesis of AgNPs by B. licheniformis, showed that the nitrate reductase secreted by the bacteria in the medium was responsible for the synthesis of AgNPs. Thus, the synthesis of AgNPs occurs extracellularly in the culture medium (CitationKalimuthu et al. 2008), which completely eludes the downstream processing needed for intracellular synthesis.
Antimicrobial activity of AgNPs
In our study, the AgNPs were tested for antimicrobial activity against a range of pathogenic microorganisms, which included C. albicans (), S. enterica (), E. coli (), and V. parahaemolyticus (). After incubation, the mean diameter of the zone of inhibition was determined by measuring the diameter of the zone, and antimicrobial activity was observed against C. albicans, V. parahemolyticus, E. coli, and S. enterica. The results showed that the strains were completely resistant to these antibiotics, but sensitive to AgNPs. The AgNPs exerted more antimicrobial activity as compared to standard antibiotics. The antimicrobial activity of AgNPs has also been reported for a methicillin-resistant Staphylococcus sp. (CitationSaravanan and Nanda 2010). In our study, we showed the synthesis of spherically shaped AgNPs by an eco-friendly method, without the use of any toxic or hazardous chemicals. Furthermore, the particles were found to exert remarkable antimicrobial activity against human pathogenic strain, and hence can be applied on a clinical platform against pathogenic and multidrug-resistant microorganisms. Moreover, the nanoparticles are well known for target specificity. Many studies highlight that apart from target specificity, AgNPs are also capable of antimicrobial activity, biofilm degradation, therapeutic action, inducing hyperthermia, enhancing radiotherapy, silencing genes, and delivering drugs; the anticoagulant effect of AgNPs has also been demonstrated, which further corresponds to their application on the biomedical platform. Thus, the AgNPs were synthesized by B. methylotrophicus DC3 in an eco-friendly, economical, and biocompatible method and showed antimicrobial activity against pathogenic microorganisms.
Conclusions
The study highlights the synthesis of spherically shaped AgNPs by B. methylotrophicus DC3 in an eco-friendly manner, without using any toxic or hazardous chemicals. The spherically shaped particles were well characterized by prevailing instrumentation techniques and applied for antimicrobial activity against human pathogenic microorganisms. The particles were found to exert remarkable antimicrobial activity against human pathogenic strains C. albicans, S. enterica, E. coli, and V. parahemolyticus; hence, they can be applied on a clinical platform against pathogenic and multidrug-resistant microorganisms. Additionally, the extracellular synthesis of nanoparticles could easily be adapted to large-scale operations and downstream processing. Thus, the rapid and eco-friendly synthesis of AgNPs and antimicrobial activity against human pathogenic microorganisms corresponds to their application on the biomedical platform.
Acknowledgments
This research was supported by the Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry & Fisheries (KIPET NO: 313038-03-2-SB010), and also was supported by Business for Cooperative R & D between Industry, Academy, and Research Institute funded KSMB (Grants No.C0214183).
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anil Kumar S, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmad A, Khan MI. 2007. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett. 29:439–445.
- Bhainsa KC, D’Souza SF. 2006. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B Biointerfaces. 47:160–164.
- Biswas A, Aktas O, Schrmann U, Saeed U, Zaporojtchenko V, Faupel F, Strunskus T. 2004. Tunable multiple plasmon resonance wavelengths response from multicomponent polymer-metal nanocomposite systems. Appl Physics Lett. 84:2655–2657.
- Cheng LQ, Na JR, Kim MK, Bang MH, Yang DC. 2007. Microbial conversion of ginsenoside Rb1 to minor ginsenoside F2 and gypenoside XVII by Intrasporangium sp. GS603 isolated from soil.J Microbiol Biotechnol. 17:1937–1943.
- Choi KT. 2008. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 29:1109–1118.
- Deepa CK, Dastager S, Pandey A. 2010. Isolation and characterization of plant growth promoting bacteria from non-rhizospheric soil and their effect on cowpea (Vigna unguiculata (L.) Walp.) seedling growth. World J Microbiol Biotechnol. 26:1233–1240.
- Duran N, Marcato PD, Alves OL, Souza GI, Esposito E. 2005. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol. 3:8.
- Fortin D, Beveridge TJ. 2000. Biomineralization: From biology to biotechnology and medical application. In: Baeuerlein E, (Ed.). Germany: Wiley-VCH, Weinheim.
- Govindaraju K, Kiruthiga V, Kumar VG, Singaravlu G. 2009. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J Nanosci Nanotechnol. 9:5497–5501.
- Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, Muniyandi J, et al. 2009. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces. 74:328–335.
- Jin S, Ye K. 2007. Nanoparticle-mediated drug delivery and gene therapy. Biotechnol Prog. 23:32–41.
- Kalimuthu K, Suresh Babu R, Venkataraman D, Bilal M, Gurunathan S. 2008. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 65:150–153.
- Kim KJ, Sung WS, Moon SK, Choi JS, Kim JG, Lee DG. 2008. Antifungal effect of silver nanoparticles on dermatophytes. J Microbiol Biotechnol. 18:1482–1484.
- Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, et al. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 62:716–721.
- Klaus-Joerger T, Joerger R, Olsson E, Granqvist C. 2001. Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol. 19:15–20.
- Li X, Ding C, Liao J, Lan T, Li F, Zhang D, et al. 2014. Biosorption of uranium on Bacillus sp. dwc-2: preliminary investigation on mechanism. J Environ Radioact. 135:6–12.
- Magudapathy P, Gangopadhyay P, Panigrahi BK, Nair KGM, Dhara S. 2001. Electrical transport studies of Ag nanoclusters embedded in glass matrix. Physica B Condensed Matter. 299: 142–146.
- Mathiyalagan R, Subramaniyam S, Kim YJ, Kim YC, Yang DC. 2014. Ginsenoside compound K-bearing glycol chitosan conjugates: synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr Polym. 112:359–366.
- Mazur M. 2004. Electrochemically prepared silver nanoflakes and nanowires. Electrochem Commun. 6:400–403.
- Parikh RY, Singh S, Prasad BL, Patole MS, Sastry M, Shouche YS. 2008. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: towards understanding biochemical synthesis mechanism. Chembiochem. 9:1415–1422.
- Prow T, Greebe R, Merges C, Smith JN, Mcleod DS, Leary JF, Lutty GA. 2006. Nanoparticle tethered antioxidant response element as a biosensor for oxygen induced toxicity in retinal endothelial cells. Mol Vis. 12:616–625.
- Raut R, Lakkakula J, Kolekar N, Mendhulkar V, Kashid S. 2009. Phytosynthesis of silver nanoparticle using Gliricidia sepium (Jacq.). Curr Nanosci. 5:117–122.
- Saravanan M, Nanda A. 2010. Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf B Biointerfaces. 77:214–218.
- Schultz S, Smith DR, Mock JJ, Schultz DA. 2000. Single-target molecule detection with nonbleaching multicolor optical immunolabels. Proc Natl Acad Sci U S A. 97:996–1001.
- Shan H, Zhao M, Chen D, Cheng J, LI J, Feng Z, et al. 2013. Biocontrol of rice blast by the phenaminomethylacetic acid producer of Bacillus methylotrophicus strain BC79. Crop Protect. 44:29–37.
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173:697–703.
- Whitesides GM. 2003. The ‘right’ size in nanobiotechnology. Nat Biotech. 21:1161–1165.
- Zhang T, Li R, Qian H, Mu W, Miao M, Jiang B. 2014. Biosynthesis of levan by levansucrase from Bacillus methylotrophicus SK 21.002. Carbohydr Polym. 101:975–981.