Abstract
Iron chelation therapy can be used for the selective removal of Fe3+ ions from spiked human plasma by ion imprinting. N-Methacryloyl-(L)-glutamic acid (MAGA) was chosen as the chelating monomer. In the first step, MAGA was complexed with the Fe3+ ions to prepare the precomplex, and then the ion-imprinted poly(hydroxyethyl methacrylate-N-methacryloyl-(L)-glutamic acid) [PHEMAGA-Fe3+] cryogel column was prepared by cryo-polymerization under a semi-frozen temperature of − 12°C for 24 h. Subsequently, the template, of Fe3+ ions was removed from the matrix by using 0.1 M EDTA solution. The values for the specific surface area of the imprinted PHEMAGA-Fe3+ and non-imprinted PHEMAGA cryogel were 45.74 and 7.52 m2/g respectively, with a pore size in the range of 50–200 μm in diameter. The maximum Fe3+ adsorption capacity was 19.8 μmol Fe3+/g cryogel from aqueous solutions and 12.28 μmol Fe3+/g cryogel from spiked human plasma. The relative selectivity coefficients of ion-imprinted cryogel for Fe3+/Ni2+ and Fe3+/Cd2+ were 1.6 and 4.2-fold greater than the non-imprinted matrix, respectively. It means that the PHEMAGA-Fe3+ cryogel possesses high selectivity to Fe3+ ions, and could be used many times without significantly decreasing the adsorption capacity.
Introduction
Iron is one of the most important essential trace elements for almost all organisms, required for a broad spectrum of metabolic functions which include the synthesis of DNA, the basic building block of all cells in the body, electron transfer, transport, storage, and the activation of oxygen and nitrogen fixation, but its main role is in the production of hemoglobin, the protein within red blood cells that binds oxygen for delivery to the various organs. Neurotransmitters, which are used by the brain cells to communicate with each other, use iron as a component. Iron takes part in the process of production of connective tissue, the cells, and fibrous tissue of the body. The body's immune system also uses iron as an important element. Iron accumulation in different parts of the body such as the liver, heart, and pancreas can cause toxicity in humans. Oxidative stress induced by iron molecules results in toxicity of cells. Toxicity depends on the amount of iron already in the body. Cancer development may be associated with the increase in iron storage (CitationLitovitz et al. 1988). Chelation therapy is the only available supportive treatment for transfusional iron overload and acute iron poisoning. Hereditary hemochromatosis (HH) is a very common disease caused by iron overload (CitationBritton et al. 2002, CitationDe Silva et al. 1996). Although some drugs, as desferrioxamine B (DFO) and deferiprone (DFP), are being used to cure the hazardous effects of excess iron, a high dosage of these drugs may cause toxicity in the body (CitationCrisponi and Remelli 2008). The major limitation to the use of DFO is its unsuitability for such applications in human patients when administered orally, because of the short half-life in plasma and its potential toxicity when given intravenously at therapeutic doses. The use of iron chelators accelerates healing in the animal models suffering from hemorrhagic shock and reperfusion injury (CitationJacobs et al. 1991, CitationVoest et al. 1994). Iron chelating resins, when compared to the suitable iron chelators, have been demonstrated to be effective in removing iron from patients. Efficient iron chelating systems have been studied, to overcome the drawbacks of the soluble iron chelators in the therapy of the iron intoxication, and involve the preparation of effective adsorbents with some advantages in terms of stability, reusability, and minimal damage to biomolecules. Poly(EGDMA-HEMA)-based microspheres carrying iron chelators were developed as polymeric materials by Denizli et al., for removal of heavy metal ions from aqueous media (CitationDenizli et al. 1996, CitationSalih et al. 1996). Reactive dye-ligands, Cibacron Blue F3GA, Alkali Blue 6B, and Congo Red were immobilized onto the poly(EGDMA-HEMA) microspheres as iron chelators, and used for the removal of iron from aqueous solutions and human plasma by the same group (CitationDenizli et al. 1998). CitationYavuz et al. (2001) prepared ferritin-immobilized PHEMA membranes as chelating ligands, which have several advantages such as high porosity, large internal surface area, and high chemical, biological, and mechanical stability for the removal of iron poisoning. The need to develop an efficient iron chelating system has driven the evolution of different types of chromatographic support materials to excrete the iron in the body (CitationLozinsky et al. 2003). Cryogels with many beneficial features such as high porosity, short diffusion path, low pressure drop, and very short residence time for both adsorption and elution studies, are very good alternative adsorbents for bioseparation (CitationPlieva et al. 2007). CitationErgün et al. (2012) used Fe3+ ion-imprinted poly(GMA-MAC) bead-embedded PHEMA cryogels for the selective removal of Fe3+ ions from the plasma of β-thalassemia patients. Molecularly imprinted polymers (MIPs) using molecular recognition-based separation techniques have gained much attention as one of the most promising adsorbents in various fields, because of their high selectivity for target molecules such as drugs, small molecules, peptides, and proteins (CitationAndac et al. 2006, CitationBaydemir et al. 2007). MIPs developed for the removal of iron from human plasma have been reported by a few groups (CitationYavuz et al. 2005, Citation2006, CitationAslıyüce et al. 2010). A strong affinity to the template molecule, compared to other molecules which are eliminated to leave cavities complementary to the template in size, shape, and molecular interactions, is the basic driving force for molecular imprinting. MIPs having higher physical strength, robustness, resistance to elevated pressure and temperature, and inertness against various chemicals (organic solvents, acids, bases, and metal ions), have been employed increasingly in a wide scope of bioanalytical areas such as chromatography, sample pretreatment, purification, catalysts, sensors, and drug delivery (CitationWon et al. 2013). Furthermore, production costs are low, and their usage can be for as long as several years at room temperature (CitationVasapollo et al. 2011). Monolithic materials are regarded as the new generation matrix materials, and have several points of advantage in separation science due to their easy methods of preparation, their good permeability, fast mass transfer, high stability and ease of modification, and the high performance they exhibit in comparison to the conventional beads used in the separation of biomolecules. The Fe3+-imprinted cysteine-based PHEMAC monolithic column was prepared and used for selective removal of Fe3+ ions from an aqueous solution, by CitationÖzkara et al (2011). Slow diffusional mass transfer and the large void volume between the beads are the limitations of conventional packed-bed columns (CitationMcCoy et al. 1996). Recently, porous cryogel materials are being considered as a new kind of stationary phase in the separation technology, because of their easy preparation, excellent flow properties, short diffusion path, low pressure drop, and high performances when compared to other conventional separation technologies (CitationLozinsky et al. 2001, CitationBereli et al. 2008).
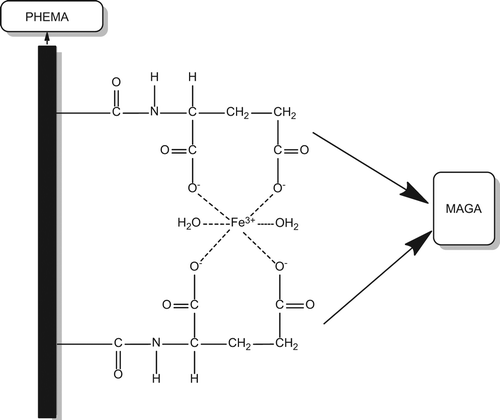
In this study, the Fe3+-imprinted PHEMAGA cryogel column was prepared and used for the selective removal of Fe3+ ions, both from aqueous solution and human plasma. In the first part, MAGA was complexed with Fe3+ ions to prepare MAGA-Fe3+ as the precomplex, and then, the ion-imprinted poly(hydroxyethyl methacrylate-N-methacryloyl-(L)-glutamic acid methyl ester) [PHEMAGA-Fe3+] cryogel column was prepared by cryopolymerization under a semi-frozen temperature. MAGA was selected as the comonomer, because it exhibits a mixture of the chelating bidentate and the bridging bidentate-binding modes with Fe3+ ions (CitationAydoğan et al. 2012). Selective separation abilities of Fe3+ imprinted and non-imprinted cryogels were tested in the presence of competitive ions in a set of adsorption experiments.
Experimental
Materials
Hydroxyethyl methacrylate (HEMA), N,N,N’,N’-tetramethylene diamine (TEMED) and N,N’-methylene bis (acrylamide) (MBAAm) were obtained from Fluka A.G. (Buchs, Switzerland), distilled under reduced pressure in the presence of hydroquinone inhibitor and stored at 4°C until use. L-Glutamic acid hydrochloride, methacryloyl chloride, and ammonium persulfate (APS) were supplied from Sigma (St. Louis, USA). All other chemicals were of reagent grade and purchased from Merck AG (Darmstadt, Germany). All water used in the adsorption experiments was purified using a Barnstead (Dubuque, IA) ROpure LP/reverse osmosis unit with a high-flow cellulose acetate membrane (Barnstead D2731), followed by a Barnstead D3804 NANOpure/organic/colloid removal and ion exchange packed-bed system.
Synthesis of N-methacryloyl-(L)-glutamic acid
Preparation and characterization details of the N-methacryloyl-(L)-glutamic acid (MAGA) have been reported elsewhere (CitationDenizli et al. 2004). The following procedure for synthesis was applied for the MAGA monomer: 5.0 g of L-glutamic acid hydrochloride and 0.2 g of hydroquinone were dissolved in 100 mL of dichloromethane. This solution was cooled down to 0°C. Then, 13.0 g of triethylamine was added to the solution, and 4.0 mL of methacryloyl chloride was poured slowly into this solution under nitrogen atmosphere. This solution was stirred magnetically at room temperature for 2 h. Extraction of unreacted methacryloyl chloride was performed with 10% NaOH solution at the end of the chemical reaction period. After evaporation of the aqueous phase in a rotary evaporator, the residue (i.e., MAGA) was dissolved in ethanol.
Preparation of the Fe3+-MAGA complex
To prepare the MAGA-Fe3+ complex, solid MAGA (2.0 mmol) was added gradually into 15 mL of ethanol/water mixture (50/50 v/v), and then processed with iron nitrate (Fe(NO3)3.9H2O) (1.0 mmol) at room temperature, with continuous stirring for 3 h. Then, the formed metal-monomer complex was filtered, washed with 99% ethanol (250 mL), and dried in a vacuum oven at 50°C for 24 h (pressure: 100 mmHg).
Preparation of Fe3+-imprinted PHEMAGA cryogel
The following experimental procedure was applied for the synthesis of PHEMAGA-Fe3+ cryogel. Monomers (1.3 mL of HEMA, 10 mL of N,N’-methylene-bis(acrylamide) (MBAAm), 1mL of MAGA-Fe3+) were dissolved in deionized water, and to remove soluble oxygen, the mixture was degassed under vacuum for about 5 min. Total monomer concentration was 6% (w/v). The cryogel was produced by free radical polymerization initiated by N,N,N’,N’-tetramethylene diamine (TEMED, 25 μL) and ammonium persulfate (APS, 20 mg). After adding APS (1% (w/v) of the total monomers), the solution was cooled in an ice bath for 2–3 min. After adding TEMED (1% (w/v) of the total monomers) to the reaction mixture, and the final solution was poured into a plastic syringe (5 mL, i.d. 0.8 cm) with a closed outlet at the bottom. The prepared polymer solution in the syringe was frozen at − 12°C for 24 h and then thawed at room temperature. The trapped template Fe3+ ions in the [PHEMAGA-Fe3+] cryogel were removed by pumping 0.1 M EDTA solution through the column. The cryogel was then washed with acidic thiourea solution for 48 h at room temperature. Finally, the Fe3+ free column of was rinsed with 0.1 M HNO3 solution. Non-imprinted PHEMAGA cryogel was also prepared in the same manner, in the absence of template Fe3+ ions. Prepared cryogels were stored at 4°C in a buffer solution containing 0.02% sodium azide until use.
Characterization studies
The multipoint Brunauer-Emmett-Teller (BET) apparatus (Quantachrome, Nova 2200E, USA) was used to determine the specific surface area of the PHEMAGA-Fe3+ cryogel in dry state. First, 0.5 g of cryogel sample was placed in the sample holder of BET and degassed by passing through N2 gas at 150°C for 1 h. The adsorption of the N2 gas was performed at − 210°C, while desorption was performed at room temperature. The specific surface area of the cryogel was calculated by experimental values obtained from the desorption step. The BJH (Barrett, Joyner, Halenda) model was used to calculate pore volumes, and an average pore diameter of the cryogel synthesized. For the determination of the swelling degree (S) of the cryogel, the sample was washed on a porous filter until washing was clear. Then, it was sucked dry and then transferred to a pre-weighed vial and weighed (mwet gel). After drying to the constant mass in the oven at 60°C, dried sample mass was measured (mdry gel). The swelling degree was calculated as follows:
The total volume of macropores in the swollen cryogel was roughly estimated by weighing the sample (msqueezed) after squeezing the free water from swollen gel matrix, and then the porosity was calculated as follows:
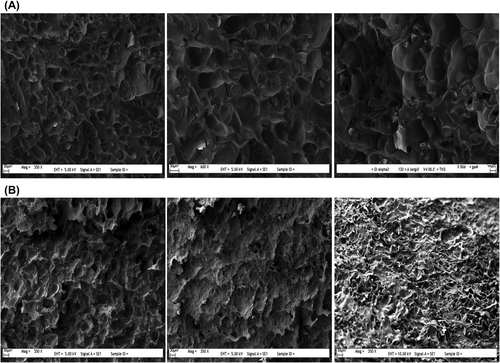
Surface morphologies of Fe3+-imprinted PHEMAGA and PHEMAGA cryogels were examined using scanning electron microscopy (SEM). First, the cryogels were dried in air at room temperature. A piece from the cryogels was mounted on an SEM sample set up and was sputter-coated with gold for 2 min. The samples were then mounted in SEM equipment JEOL JSM 5600 (Tokyo, Japan). The Fourier transform infrared spectrophotometer (FTIR, 8000 Series, Shimadzu, Tokyo, Japan) was used for the estimation of characteristic functional groups of PHEMAGA and PHEMAGA-Fe3+ cryogels. The samples were prepared by mixing 2 mg of dried/crushed cryogels and 98 mg of powdered KBr (IR Grade, Merck, Germany), and pressed into a pellet form. The FTIR spectrum was then recorded in the wavenumber range of 400–4000 cm− 1
Blood compatibility studies
Fibrinogen time (FT)
PHEMAGA and PHEMAGA-Fe3+ cryogels were incubated with human plasma at room temperature for 2 h. Total protein reagent (Ciba Corning Diagnostics Ltd, Halstead, Essex, England; Catalog Ref. No: 712076) was used for measurement of total protein concentration at 540 nm, which is based on the Biuret reaction. The Clauss method was used for fibrinogen determination, by using the Fibrinogen-Kit (Ref. Nos.: 68452 and 68582, bioMérieux Laboratory Reagents and Instruments, Marcy-l’Etoile, France).
Activated partial thromboplastin time
PHEMAGA and PHEMAGA-Fe3+ cryogels were incubated in a phosphate buffer solution (0.1 M pH 7.4) at room temperature for 24 h, and then filtered through a glass filter. Next, 0.5 M NaCl solution, and then distilled water, were used to wash the PHEMAGA and PHEMAGA-Fe3+ cryogels respectively. Next, 0.1 mL of freshly frozen human plasma was preheated to 37°C for 2 min, after thawing at room temperature. Then, 0.3 mL of partial thromboplastin (bioMérieux, Marcy-1’Etoile, France) which was preheated to 37°C for 2 min was mixed with the preheated human plasma. The plasma-thromboplastin mixture was transferred into the PHEMAGA and PHEMAGA-Fe3+ cryogels to measure activated partial thromboplastin time (APTT) time. After 30 s, 0.1 mL of 0.025 M CaCl2 was added to the plasma mixture, and then APTT was determined by the fibrometer method (CitationHarlow and Lane 1998).
Prothrombin time
Prothrombin time (PT) estimation was performed by using a single step prothrombin method, as follows: After incubation in a phosphate buffer solution (0.1 M, pH 7.4) at room temperature for 24 h, PHEMAGA and PHEMAGA-Fe3+ cryogels were filtered out through a glass filter and washed with a 0.5 M NaCl solution and distilled water. Next, 0.1 mL of freshly frozen human plasma was preheated to 37°C for 2 min, after thawing at room temperature. Then, 0.3 mL of partial thromboplastin (bioMérieux, Marcy-1’Etoile, France) which was preheated to 37°C for 2 min, was mixed with the preheated human plasma. The plasma-thromboplastin mixture were transferred into PHEMAGA and PHEMAGA-Fe3+ cryogels. After 30 s, 0.1 mL of a 0.025 M CaCl2 solution was added to the plasma mixture, and then PT was measured by the fibrometer method (CitationHarlow and Lane 1998).
Adsorption of Fe3+ ions from aqueous solution and human plasma
The adsorption of Fe3+ ions from aqueous solution and human plasma were studied for PHEMAGA-Fe3+ and PHEMAGA cryogels. The adsorption of Fe3+ ions from aqueous solution were studied following the optimization of several parameters affecting the adsorption capacity, such as pH, flow rate, and Fe3+ ion concentration. Fresh human plasma obtained from a healthy donor was applied for all experiments. First, 10 mL of plasma sample, centrifuged at 500 g for 30 min, was treated with different amounts of Fe3+ solutions to obtain different iron concentrations (5, 10, 20, 30, 40, and 50 ppm). The source of Fe3+ ions was nitrate salt (Fe(NO3)3.9H2O). Then, the iron-spiked human plasma was incubated with the cryogels at room temperature for 3 h. After the particular time, the concentration of the Fe3+ ions in the aqueous phase was measured using a graphite furnace atomic absorption spectrophotometer (GFAAS, Analyst 800/Perkin Elmer, USA). Fe3+ solutions with known concentrations were used to check the instrument response periodically. The difference in Fe3+ ion concentration of the pre- and post-adsorption solutions, divided by the weight of dry cryogel, was used for the calculation of adsorption values (mg/g). The studied experiments were repeated three times.
Selectivity experiments
Competitive adsorption studies in the presence of Fe3+-Cd2+ and Fe3+-Ni2+ cation pairs were performed to evaluate the specificity of the Fe3+ ions for the PHEMAGA-Fe3+ cryogel. For this purpose, 2 mL of Cd2+ solution (50 mg/L concentration) and 2 mL of Ni2+ solution (50 mg/L concentration) were mixed with 10 mL of fresh human plasma sample separately. The concentrations of Fe3+, Cd2+, and Ni2+ in the plasma mixtures were detected by GFAAS after the competitive adsorption had reached equilibrium.
Distribution coefficients (Kd) for Cd2+ and Ni2+ with respect to Fe3+ were calculated by Eq. (3) (CitationWon et al. 2013).
Where Kd (mL/g) is a distribution coefficient for the competing metal cation. Ci and Cf are the initial and the final concentrations of metal ions (μg/mL), respectively. V is the volume of the plasma mixture (mL), and m is the mass of cryogel (g).
The generic term in Eq. (4) was adopted to determine a selectivity coefficient (k) for the binding of Fe3+ onto the PHEMAGA-Fe3+ cryogel, in the presence of a competing species such as Cd2+ and Ni2+, in which Kd (competing metal cation) is the distribution coefficient of the competing metal cation.
A ratio of the selectivity coefficients (k’) in Eq. (5), in which kimprinted represents the PHEMAGA-Fe3+ in the presence of the Fe3+-Cd2+ or the Fe3+-Ni2+ cation pairs, and kcontrol represents the PHEMAGA in the presence of the Fe3+-Cd2+ or the Fe3+-Ni2+ cation pairs, was used as an approximation to assess the effect of imprinting on cation selectivity.
Desorption and repeated use
EDTA solution was used as a desorption agent (0.1 M). PHEMAGA-Fe3+ cryogels were placed in this desorption medium and stirred continuously for 1 h at room temperature. The final concentration of Fe3+ ions in desorption medium was measured by GFAAS. The desorption ratio was calculated from the amount of Fe3+ ions adsorbed on PHEMAGA-Fe3+ cryogel and the final Fe3+ ion concentration in the desorption medium. The same imprinted cryogel was used to test the reusability of the Fe3+-imprinted PHEMAGA cryogels, by repeating the Fe3+ ion adsorption–desorption cycle five times. To regenerate and sterilize, the imprinted cryogels were washed with 50 mM NaOH solution after each desorption procedure.
Results and discussion
Characterization studies
In , the specific surface area, macroporosity, and water uptake of PHEMAGA and PHEMAGA-Fe3+ cryogels have been presented. The values for specific surface area of PHEMAGA and PHEMAGA-Fe3+ cryogels were determined by a multipoint BET apparatus, and found to be 7.5 and 45.7 m2/g polymer, respectively. The equilibrium swelling degree and porosity of the PHEMAGA and PHEMAGA-Fe3+ cryogels were 8.3 g water/g cryogel and 80.4%, 10.6 g water/g cryogel and 69.4%, respectively.
Table I. Surface area measurements of PHEMAGA and PHEMAGA-Fe3+ cryogels.
The average pore sizes of the pores on PHEMAGA and PHEMAGA-Fe3+ cryogels were determined by SEM as 150 μm in diameter, in which the pore diameters range from 50 to 200 μm, suggesting the presence of supermacropores of the PHEMAGA-Fe3+ cryogel. The scanning electron micrographs of the internal structures of PHEMAGA and PHEMAGA-Fe3+ cryogels are shown in . Large continuous interconnected pores (10–100 μm diameter, supermacroporous) that provide channels for the mobile phase of the PHEMAGA-Fe3+ cryogel to flow through have been produced in such a way that they have porous and thin polymer walls.
MAGA was chosen as the comonomer and used for the selective separation of Fe3+ ions from human plasma. After synthesizing MAGA from glutamic acid hydrochloride and methacryloyl chloride, complexation of Fe3+ ions with MAGA was performed. The HEMA monomer was introduced to the MAGA-Fe3+ precomplex. The HEMAGA-Fe3+ complex is shown in .
The FTIR spectrum of MAGA has the characteristic stretching vibration of carboxyl–carbonyl and amide–carbonyl absorption bands at 1732 and 1655 cm− 1. The N–H bending peak appearing at 1540 cm− 1 is associated with the amide vibration of MAGA. To determine the characteristics of the complex, due to the formation of the linear coordinate covalent complex, the characteristic strong carbonyl stretching vibration band at 1655 cm− 1 shifts to the higher frequency field at 1690 cm− 1, as a result of decreasing the electron density of carbonyl group of the MAGA monomer. The FTIR spectrum of Fe3+-imprinted PHEMAGA cryogel has the characteristic stretching vibration band of hydrogen-bonded alcohol, O–H, at around 3550 cm− 1, carbonyl at 1650 cm− 1, and amide II absorption bands at 1520 cm− 1, respectively.
The nephelometric measurements, and evaluation of prothrombin time (PT), APTT, and fibrinogen level were performed using the coagulation instrument Diagnostica Stago. Briefly, healthy human blood plasma (anticoagulated blood with 1/10 (v/v) of 3.8% tri-sodium citrate solution) was collected. 10 mg of imprinted and non-imprinted cryogel in 500 μL of plasma was incubated for 1 h at 37°C. After the incubation, coagulation times were measured. Each sample was tested twice. The results of the coagulation tests, including APTT, PT, and fibrinogen levels, did not change significantly. However, in the imprinted and non-imprinted polymers, fibrinogen concentrations were increased by 0.1–0.2 ± 0.12 g/L; PT values were increased by 0.1–0.3 ± 0.14 min, and APPT values were increased by 2–4 ± 0.42 min. As seen in , when compared to the control plasma, the clotting times in the presence of iron-imprinted cryogels were increased (CitationAslıyüce et al. 2010), which is in the tolerable range by the body (CitationDenizli 2002). Therefore, it is concluded that the blood compatibility of PHEMAGA-Fe3+ and the clotting times determined are consistent with the relevant literature (CitationKim and Jacops 1996).
Table II. Coagulation times in human plasma (reported in seconds).a
Adsorption of Fe3+ from human plasma
Effect of Fe3+ ion concentration
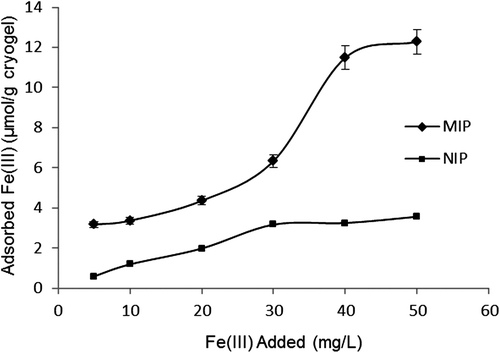
The effect of different concentrations of Fe3+ ions on the adsorption capacity of the PHEMAGA and PHEMAGA-Fe3+ cryogels has been shown in . The adsorption values were increased with increasing concentration of Fe3+ ions. A saturation value, representing the saturation of the active binding cavities on the PHEMAGA-Fe3+ cryogel, was achieved at the 50 mg/L concentration of Fe3+ ions. Maximum adsorption capacity was 12.28 μmol/g for PHEMAGA-Fe3+ and 3.56 μmol/g for the PHEMAGA cryogel.
Adsorption time
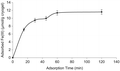
The change in the adsorption amount of Fe3+ ions with time has been shown in . As shown in the figure, the adsorption capacity is increased within 60 min. It was found that the geometric shape memory between the Fe3+ ions and their cavities having similar size, shape and chemical similarity to the template molecule, created by removing them from the polymeric structure, affects the adsorption equilibrium rate, which was achieved rapidly. The maximum adsorption capacity for Fe3+ ions was 11.6 μmol per gram weight of the PHEMAGA-Fe3+ cryogel.
Adsorption of Fe3 + from aqueous solutions
Fe3+ adsorption onto the PHEMAGA-Fe3+ cryogel from aqueous solutions was studied in a continuous system. The effect of flow rate effect on Fe3+ adsorption onto cryogel was evaluated in the range of 0.5–2.0 mL/min. To determine the effect of pH of the solution on the adsorption capacity, the pH of the solution was varied between pH levels of 4.0 and 8.0. The effect of the equilibrium concentration on Fe3+ ion adsorption was studied in the range of 5–50 mg/L.
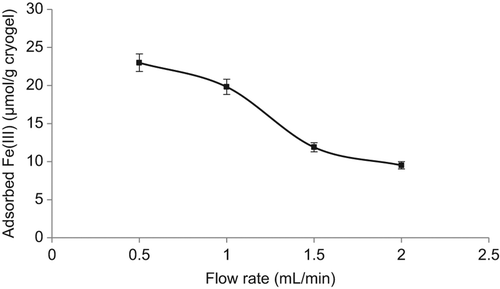
Flow rate
The study on the effect of flow rate on Fe3+ adsorption was implemented at different flow rates, given in . According to the results of the study, the adsorption capacity decreased significantly with the increase in the flow rate. The main reason for this is the reduction of the contact time between Fe3+ ions and the Fe3+-imprinted PHEMAGA cryogel at high flow rates. When the flow rate decreases, the contact time in the column is longer. Therefore, the diffusion into the pores becomes more effective. Thus, there will be more time for the diffusion of iron ions through the macroporous cryogel column, and a better adsorption capacity is obtained.
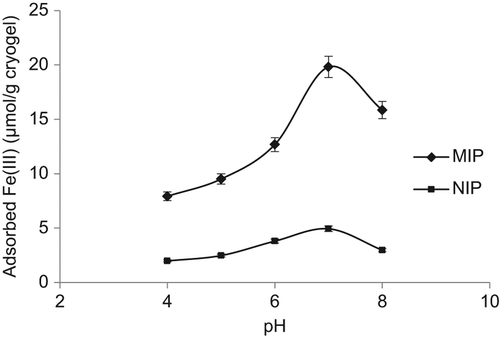
Effect of pH
The pH value of the solution is an important controlling parameter in the metal ion adsorption process, which influences both the metal binding sites on the adsorbent surface and the metal chemistry in water. The pH range was varied between the levels of 4.0 and 8.0. The effect of pH on the Fe3+ binding of the PHEMAGA-Fe3+ cryogel has been shown in . As seen here, the binding of Fe3+ ions increased with increasing pH, and then decreased at a pH level of around 8.0. The increasing pH of the solution favored complex formation between the carboxyl groups of the MAGA comonomer and Fe3+ ions. The specific adsorption of Fe3+ ions via the MAGA groups was pH-dependent. Fe3+-binding at a pH of around 7.0 was high, may be because of deprotonation of the functional groups on the MAGA structure. Because of the formation of Fe(OH)3 at increased pH values, less interaction is expected between the MAGA monomer and Fe3+ ions.
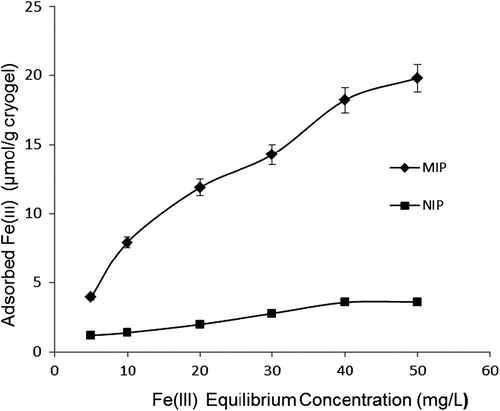
Effect of Fe3+ ion concentration
The results of adsorption were based on the binding of Fe3+ ions onto PHEMAGA and PHEMAGA-Fe3+ cryogels, from the experiments performed in the media containing the different Fe3+ concentrations, as shown in . The adsorption of Fe3+ ions was observed to increase linearly per unit mass of the imprinted cryogel, as the amount of initial concentration of Fe3+ ions increased gradually from 5 to 50 mg/L. The amount of Fe3+ ions adsorbed on the cryogel was 19.81 μmol/g for the MIP column. At the same conditions, the amount of Fe3+ ion adsorbed onto the NIP column was estimated as 3.61 μmol/g. A plateau value was achieved at a concentration of 50 mg/L for the MIP column and 30 mg/L for the NIP column.
Two important physico-chemical aspects for evaluating an adsorption process as a unit operation rate are the kinetics and the equilibrium of the adsorption process. Equilibrium data are usually modeled using the Langmuir or Freundlich isotherms (CitationAydoğan et al. 2012). Equations (6) and (7) give the Langmuir and Freundlich isotherms, respectively.
Above, QL is the Langmuir monolayer adsorption capacity (μmol/g), Ce is the equilibrium Fe3+ concentration (mg/L), b is the constant related to the affinity binding sites, KF is the Freundlich constant, and n is the Freundlich exponent. The adsorption isotherm that best describes Fe3+-binding can be determined by how well the data follow the linear forms of the Langmuir and Freundlich equations, which are given by Equations (8) and (9),
compares the experimental adsorption behavior with the Langmuir and Freundlich adsorption isotherms. Based on the higher correlation coefficient (R2 = 0.993), the data is in more conformity with the Freundlich isotherm. Moreover, the maximum adsorption capacity obtained from the experimental results, that is 19.81 μmol/g, is also very close to the calculated Freundlich adsorption isotherm. It can be concluded that adsorption shows multilayer behavior. The Langmuir and Freundlich adsorption isotherm constants are shown in .
Figure 8. Experimental data of adsorbed Fe3+ ions onto PHEMAGA and PHEMAGA-Fe3+ cryogels compared with Langmuir and Freundlich isotherms.
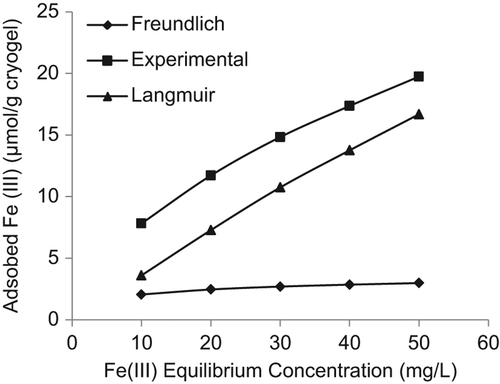
Table III. Langmuir and Freundlich adsorption isotherm constants.
Selectivity experiments
Because the selectivity of binding is a crucial parameter, the competitive adsorption of Ni2+/Fe3+ and Cd2+/Fe3+ from the binary solutions was also studied in a batch system. Ni2+ and Cd2+ were chosen as competitive metal ions. The ionic radii of Ni2+ (70 pm) and Cd2+ (95 pm) are larger than that of Fe3+ ions (65 pm). summarizes the Kd, k, and k’ values of Ni2+ and Cd2+ with respect to Fe3+. A comparison of the Kd values for the Fe3+-imprinted PHEMAGA samples with the control samples shows an increase in Kd for Fe3+, while Kd is decreased for Ni2+ and Cd2+. The relative selectivity coefficient has been introduced as an indicator to evaluate the selectivity properties of imprinted cryogel and an adsorption affinity of recognition sites to the imprinted Fe3+ ions. These results show that the relative selectivity coefficients of the ion-imprinted cryogel for Fe3+/Ni2+ and Fe3+/Cd2+ were 1.6 and 4.2 times greater than the non-imprinted matrix, respectively ().
Table IV. Kd and k values of Cd2 values of Cd2+ and Ni2+ with respect to Fe3+.
Table V. Comparison with the related literature.
Desorption and repeated use
The regeneration of the adsorbent material is a very important issue in improving process economy. Desorption of the Fe3+ ions from the PHEMAGA-Fe3+ cryogel was carried out in the continuous system. The diverse parameters determining the rate of Fe3+ desorption can be the extent of hydration of metal ions, the structure of the polymer, etc. However, the important parameter appears to be the strength of the binding. In this work, the time of desorption was found to be 15 min, with a very high desorption ratio (up to 97%). The adsorption-desorption cycles were repeated ten times by using the same cryogel column, in order to test the reusability. The adsorption capacity of the recycled PHEMAGA-Fe3+ cryogel can still be maintained at a 90%-level at the 10th cycle. It can be concluded that the PHEMAGA-Fe3+ cryogels can be used many times without decreasing their adsorption capacities significantly.
Conclusion
The Fe3+-imprinted PHEMA-based cryogel was successfully synthesized with the assistance of the functional monomer MAGA, chelated with Fe3+ ions, for the recognition of Fe3+ ions from aqueous solutions and spiked human plasma. The shape memory effect is the major factor affecting the imprinting formation and template recognition. The PHEMAGA-Fe3+ cryogel exhibits the properties of high selectivity and recognition for the template Fe3+ in the presence of competitive metal ions. The imprinted cryogel can be used for the separation of Fe3+ ions in chromatography applications.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Andac M, Özyapı E, Senel S, Say R, Denizli A. 2006. Ion-selective imprinted beads for aluminum removal from qqueous solutions. Ind Eng Chem Res. 45:1780–1786.
- Aslıyüce S, Bereli N, Uzun L, Onur MA, Say R, Denizli A. 2010. Ion-imprinted supermacroporous cryogel for in vitro removal of iron out of human plasma with beta thalassemia. Sep Purif Technol. 73:243–249.
- Aydoğan C, Andaç M, Bayram E, Say R, Denizli A. 2012. Molecularly imprinted cryogel for L-glutamic acid separation. Biotechnol Pro. 28:459–466.
- Baydemir G, Andac M, Bereli N, Say R, Denizli A. 2007. Selective removal of bilirubin from human plasma with bilirubin-imprinted particles. Ind Eng Chem Res. 46:2843–2852.
- Bereli N, Andaç M, Baydemir G, Say R, Galaev IY, Denizli A. 2008. Protein recognition via ion-coordinated molecularly imprinted supermacroporous cryogels. J Chromatogr A 1190:18–26.
- Britton RS, Leicester KL, Bacon BR. 2002. Iron toxicity and chelation therapy. Int J Hematol. 76:219–228.
- Crisponi G, Remelli M. 2008. Iron chelating agents for the treatment of iron overload. Coord Chem Rev. 252:1225–1240.
- De Silva DM, Askwith CC, Kaplan J. 1996. Induction of the root cell plasma membrane ferric reductase (An exclusive role for Fe and Cu). Phisiol Rev. 76:31.
- Denizli A, Salih B, Pişkin E. 1996. Congo red and Cu(II) carrying poly(ethylene glycol dimethacrylate-hydroxyethyl methacrylate) microbeads as specific sorbents albumin adsorption/desorption. J Chromatogr A. 731:57–63.
- Denizli A, Salih B, Pişkin E. 1998. New chelate-forming polymer microspheres carrying dyes as chelators for iron overload. J Biomater Sci Polym Edn. 9:175–187.
- Denizli A. 2002. Preparation of immuno-affinity membranes for cholesterol removal from human plasma. J Chromatogr B. 772:357–367.
- Denizli A, Sanlı N, Garipcan B, Patır S, Alsancak G. 2004. Methacryloylamidoglutamic acid incorporated porous poly(methyl methacrylate) beads for heavy-metal removal. Ind Eng Chem Res. 43:6095–6101.
- Ergün B, Baydemir G, Andaç M, Yavuz H, Denizli A. 2012. Ion imprinted beads embedded cryogels for in vitro removal of iron from β-thalassemic human plasma. J Appl Polym Sci. 125:254–262.
- Harlow E, Lane D. 1998. Antibodies: A Laboratory Manual New York: Cold Spring Harbor Laboratory.
- Jacobs DM, Julsrud FM, Bubrick MP. 1991. Iron chelation with a deferoxamine conjugate in hemorrhagic shock. J Surg Res. 51:484–490.
- Kim SW, Jacops H. 1996. Design of nonthrombogenic polymer surfaces for blood-contacting medical devices. Blood Purif. 14:357–372.
- Litovitz TL, Schmitz BF, Matyunas N, Martin TG. 1988. 1987 Annual Report of the American Association of Poison Control Centers National Data Collection System. Am J Emerg Med. 6:479–515.
- Lozinsky VI, Plieva FM, Galaev IY, Mattiasson B. 2001. The potential of polymeric cryogels in bioseparation. Bioseparation. 10: 163–188.
- Lozinsky VI, Galaev IY, Plieva FM, Savina IN, Jungvid H, Mattiasson B. 2003. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 21:445–451.
- McCoy M, Kalghatgi K, Regnier FE, Afeyan N. 1996. Perfusion chromatography-characterization of column packings for chromatography of proteins. J Chromatogr A. 743:221–229.
- Özkara S, Say R, Anda C, Denizli A. 2008. An Ion-Imprinted Monolith for in Vitro Removal of Iron out of Human Plasma with Beta Thalassemia. Ind. Eng. Chem. Res. 47:7849–7856.
- Özkara S, Andaç M, Karakoç V, Say R, Denizli A. 2011. Ion-imprinted PHEMA based monolith for the removal of Fe3 + ions from aqueous solutions. J Appl Polym Sci. 120:1829–1836.
- Plieva FM, Galaev IY, Mattiasson B. 2007. Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications. J Sep Sci. 30:1657–1671.
- Saatçılar Ö, Şatıroǧlu N, Say R, Bektaş S, Denizli A. 2006. Binding behavior of Fe3 + ions on ion-imprinted polymeric beads for analytical applications. J Appl Polym Sci. 101:3520–3528.
- Salih B, Denizli A, Engin B, Tuncel A, Piskin E. 1996. Congo red attached poly(EGDMA–HEMA) microspheres as specific sorbents for removal of cadmium ions. J Appl Polym Sci. 60:871–877.
- Utku S, Yılmaz E, Türkmen D, Uzun L, Garipcan B, Say R, Denizli A. 2008. Ion-Imprinted Thermosensitive Polymers for Fe3+ Removal from Human Plasma. Hacettepe J. Biol Chem. 36:291–304.
- Vasapollo G, Sole RD, Mergola L, Lazzoi MR, Scardino A, Scorrano S, Mele G. 2011. Molecularly imprinted polymers: Present and future prospective. Int J Mol Sci. 12:5908–5945.
- Voest EE, Vreugdenhil G, Marx JJM. 1994. Iron-chelating agents in non-iron overload conditions. Ann Intern Med. 120:490–499.
- Won J, Song HY, Ali F. 2013. Molecular imprinted polymers for separation science: a review of reviews. J Sep Sci. 36:609–628.
- Yavuz H, Arıca Y, Denizli A. 2001. Therapeutic affinity adsorption of iron(III) with dye- and ferritin-immobilized pHEMA adsorbent. J Appl Polym Sci. 82:186–194.
- Yavuz H, Say R, Denizli A. 2005. Iron removal from human plasma based on molecular recognition using imprinted beads. Mater Sci Eng C. 25:521–528.
- Yavuz H, Andac M, Uzun L, Say R, Denizli A. 2006. Molecular recognition based removal from human plasma with imprinted membranes. Int J Artif Organs. 29:900–911.