Abstract
Bcl-2, an antiapoptotic protein, is considered as a potential target in cancer treatment since its oncogenic potential has been proven and is well documented. Antisense technology and RNA interference (RNAi) have been used to reduce the expression of the Bcl-2 gene in many types of cancer cells and are effective as adjuvant therapy along with the chemotherapeutic agents. The lack of appropriate delivery systems is considered to be the main hurdle associated with the RNAi. In this review, we discuss the antiapoptotic Bcl-2 protein, its oncogenic potential, and various approaches utilized to target Bcl-2 including suitable delivery systems employed for successful delivery of siRNA.
Introduction
Though etiology and molecular pathways involved in certain diseases like cancer have been understood over the last three decades, treating cancer is still a challenging task for physicians. Identifying novel targets responsible for tumor induction and growth would greatly help scientists to discover new anticancer molecules. The components of molecular pathways involved in cancer are well identified and studied. These components can be the potential targets for developing effective therapies against cancer. Researchers are focusing on the development of molecules, either synthetic or derived from natural sources, which directly target such components in cancerous cells without affecting the normal cells.
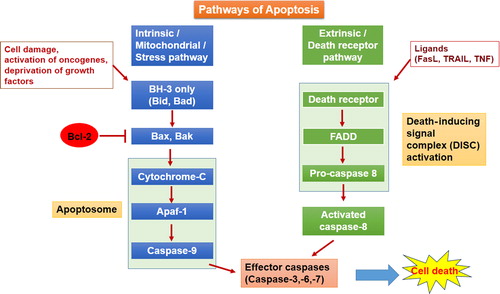
Apoptosis, a naturally occurring phenomenon and often considered programmed cell death, plays a very crucial role in physiological processes such as development, maintaining tissue homeostasis, and defence mechanisms in diseases that occur in higher organisms, from fetal development to the adult lifetime, (CitationHetts 1998). The disruption or dysregulation of apoptotic pathways contribute to the development of certain diseases like neurodegenerative disorders (CitationMattson 2000, CitationThompson 1995), tumor development (CitationReed 1999), and also in some of the autoimmune disorders (CitationLorenz et al. 2000). Apoptosis can be elicited by either of two pathways, namely the intrinsic pathway and the extrinsic pathway (). The intrinsic pathway, also called the mitochondrial or stress pathway, is the most utilized pathway for triggering apoptosis in cells. The intrinsic pathway is initiated upon cell damage, activation of oncogene, and as a response to some stress or deprivation of nutrients. This review discusses the role of the antiapoptotic Bcl-2 (B cell CLL/lymphoma 2) protein in cancer progression, the technical aspects of targeting the Bcl-2 protein, and delivery systems available for utilizing these approaches for targeting Bcl-2.
Antiapoptotic Bcl-2 protein and Bcl-2 protein family
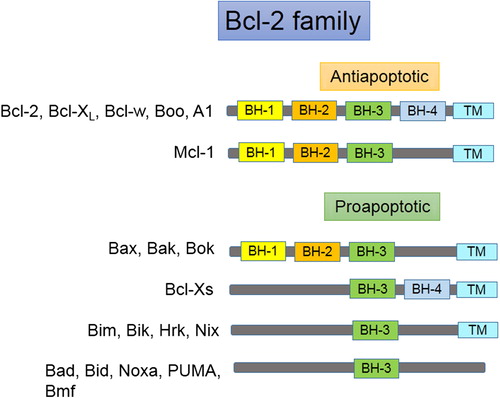
Bcl-2, the antiapoptotic protein from the Bcl-2 family of proteins, can be considered as a regulator of the intrinsic pathway of apoptosis. Initially, the Bcl-2 gene was identified and described as a proto-oncogene in 1984 in human follicular B-cell lymphoma cells carrying translocated Bcl-2 gene to the immunoglobulin heavy chain locus t(14;18)(q32;q21) (CitationMarzo and Naval 2008, CitationHockenbery et al. 1990). The Bcl-2 gene is located at the long (q) arm of chromosome 18 at position 21.3 (CitationMedicine, UNLO). The family of Bcl-2 proteins contains two classes of proteins, proapoptotic and antiapoptotic. Death or survival of the cell depends on the balance between proapoptotic and antiapoptotic proteins (CitationMarzo and Naval 2008). These groups of proteins share one of the four homologous regions known as Bcl homology (BH) domains (BH1–BH4). Antiapoptotic members of the Bcl-2 family include all the four BH domains except Mcl-1, which includes BH-1, 2, and 3. The proapoptotic proteins Bax and Bak contain three domains (BH-1, 2, and 3), and Bad, Bim, Bik, PUMA, NOXA, Bmf, and Hrk contain only the BH3 domain (the BH3-only subfamily) (CitationHeiser et al. 2004). Some of the Bcl-2 family members contain a carboxy terminal transmembrane (TM) region () (CitationKroemer 1997). The Bcl-2 protein (molecular weight of 26 kDa) is found on mitochondrial outer membrane, endoplasmic reticulum and nuclear envelope. It binds with Bax and prevents the release of cytochrome c from mitochondria and the formation of Apaf-1 and apoptosome, thereby preventing the activation of proteolytic demolition in cells (CitationAdams and Cory 1998, CitationElmore 2007). Knock down of Bcl-2 gene in mice displayed early death after birth because of extended apoptosis in cells of the thymus and spleen (CitationVeis et al. 1993). Cell survival depends on the level of Bcl-2 protein in cells.
Oncological Potential of Bcl-2
It was observed that expression of the Bcl-2 protein is enhanced significantly in many types of cancers. Bcl-2 stimulates cancer cell survival rather than inducing proliferation. This concept led the researchers to understand that a disrupted or impaired apoptosis pathway may play a crucial role in tumor development. Disruption of the apoptotic pathway due to mutation is often observed in many cancer types (CitationLowe and Lin 2000). Overexpression of the Bcl-2 gene protects cells from undergoing apoptosis and enhances the survival of the cells in prostate (CitationRaffo et al. 1995), breast (CitationJoensuu et al. 1994), and bladder (CitationMiyake et al. 1999) cancer, acute and chronic leukemia (CitationCampos et al. 1993), follicular lymphoma (CitationReed et al. 1988), B-cell lymphoma, renal (CitationGobé et al. 2002), and neuroblastoma (CitationDole et al. 1994). Induction of apoptosis in cancerous cells can be a link between cancer genetics and treatment (CitationPan et al. 1997, CitationJohnstone et al. 2002). Many chemotherapeutic agents used in the treatment of cancer induce apoptosis in response to drug-induced cell damage (CitationBrown and Attardi 2005, CitationFesik 2005). Defects in the apoptotic pathway also contribute to the drug resistance among cancer cells. The role of the Bcl-2 protein in developing resistance to drug has been proven in certain classes of cancers (CitationDavis et al. 2003, CitationSchmitt et al. 2000, CitationSartorius and Krammer 2002).
Many research groups have proved the oncogenic potential of Bcl-2 in tumor development and metastasis, described elsewhere (CitationHockenbery 1994, CitationMarzo and Naval 2008). CitationReed et al. (1988) demonstrated the oncogenic potential of Bcl-2 by the gene transfer approach in follicular lymphoma, the most common human B cell malignancy (CitationReed et al. 1988). The role of Bcl-2 in B-cell survival and follicular lymphoma was explored in transgenic mice in which Bcl-2 overexpression was targeted to B and T lymphocytes, which caused follicular hyperplasia or T-cell lymphomas (CitationMcDonnell et al. 1989). Bcl-2 contributes to oncogenesis with the synergistic effect of other oncogenes like c-myc and ras (CitationStrasser et al. 1990, CitationReed et al. 1990, CitationCory et al. 1999). To evaluate this synergistic effect of Bcl-2 with c-myc oncogenes, Bcl-2 was transfected through retro virus into the bone marrow of transgenic mice overexpressing the c-myc oncogene. Bcl-2 co-operated with c-Myc to promote the transformation of B cell precursors (CitationVaux et al. 1988). However, Bcl-2 alone did not promote proliferation but inhibited apoptosis induced by cytokine withdrawal (CitationLeskov et al. 2012). CitationSierra A. et al., 1996, reported that Bcl-2 expression was strongly associated with both loss of apoptosis and lymph node metastases (CitationSierra et al. 1996). Donatella CitationDel Bufalo et al. 1997, demonstrated that Bcl-2 overexpression augmented both the tumorigenicity and metastatic potential of the breast cancer cell line MCF7 ADR cells, by injecting MCF7 ADR into nude mice by intravenous or subcutaneous injection after inserting human Bcl-2 gene in the cells (CitationDel Bufalo et al. 1997). CitationMiyake et al., 1999, also proved that the metastatic potential of the human bladder cancer cell line KoTCC-1/BH, transfected with overexpressed Bcl-2 gene in nude mice, was significantly enhanced with improved invasive ability and cell motility (CitationMiyake et al. 1999).
Targeting Bcl-2 for cancer therapy
The role of Bcl-2 in tumor development and metastasis makes it the most promising target for cancer treatment. Induction of apoptosis in cancerous cells by inhibiting the overexpressed Bcl-2 protein can be an attractive and alternate therapy for cancer treatment. Molecules that suppress the expression of the Bcl-2 gene may be considered as a new therapeutic class of drugs in cancer treatment (CitationWang et al. 2003). The Bcl-2 gene is not only involved in the development of cancer but also in the mechanism whereby cancer cells develop resistance to chemotherapeutic agents (CitationLeber et al. 2010). Molecules which suppress the Bcl-2 gene expression and sensitize the cells to conventional chemotherapeutic agents are also gaining more attention as an adjuvant therapy. Several research groups utilizing this approach have synthesized a few molecules that inhibit Bcl-2 proteins. Some of them are already in clinical trials and have shown a promising response () (CitationKang and Reynolds 2009), while some researchers exploited the gene silencing approach, utilizing antisense technology and RNA interference, to inhibit Bcl-2 gene expression.
Table I. List of the molecules targeting Bcl-2 proteins.
Antisense technology was widely used for transcription gene silencing before the discovery of ribozymes and RNAi (CitationKurreck 2003). Antisense oligonucleotides, single-stranded 15–25 base-long DNA molecules complementary to the mRNA of the targeted gene, are used to prevent transcription. When introduced into the cells, antisense oligonucleotides target and bind with the mRNA having a complementary sequence and inhibit the protein synthesis (CitationRe 2000). There are several issues to overcome to enable the success of antisense technology and RNAi as therapeutic tools, including stability, delivery, cost, and toxicity.
A 35S-labeled phosphorothioate oligonucleotide (G3139, Genasense; Genta International Inc., Berkeley Heights, NJ) having 18 bases with sequences complementary to the first six codons of the open reading frame of Bcl-2 (G3139) showed potent antitumor effect against the DoHH2 lymphoma implanted in severe combined immunodeficient female BALB/c mice after single intravenous (i.v.) bolus administration or subcutaneous infusion (s.c) for 1 week (CitationCotter et al. 1996, CitationRaynaud et al. 1997). The results from the preclinical study in mice were encouraging, and researchers conducted this phase I clinical trial of G3139 antisense oligonucleotide in nine patients and later in 21 patients with Bcl-2 positive relapsed non-Hodgkin's lymphoma. Bcl-2 antisense therapy showed good efficacy in patients, with improvement in biochemical and hematological symptoms and downregulation of the Bcl-2 protein in some patients (CitationWebb et al. 1997, CitationWaters et al. 2000). The G3139 antisense oligonucleotide was also evaluated in a phase I study with fludarabine (FL), cytarabine (ARA-C), and granulocyte colony-stimulating factor (G-CSF) (FLAG) salvage chemotherapy in 20 patients with refractory or relapsed acute leukemia. G3139 was administered safely with FLAG chemotherapy and efficiently downregulated Bcl-2 expression in 75% of the patients evaluated (CitationMarcucci et al. 2003). The effect of G3139 was evaluated in prostate cancer in combination with docetaxel (CitationTolcher 2001), in small-cell lung cancer, in combination with carboplatin and etoposide (CitationRudin et al. 2004) and in small-cell lung cancer in combination with paclitaxel in phase I clinical trials (CitationRudin et al. 2002). Oblimersen sodium (Genasense), in combination with dacarbazine, was further evaluated in phase II clinical trials in advanced melanoma (CitationBedikian et al. 2006), in combination with dexamethasone and thalidomide in relapsed multiple myeloma (CitationBadros et al. 2005), in combination with rituximab in recurrent B-cell non-Hodgkin lymphoma (CitationPro et al. 2008), and with carboplatin and etoposide along with or without a combination of oblimersen in small-cell lung cancer (CitationRudin et al. 2008). Phase III clinical trials were completed in combination with fludarabine and cyclophosphamide in recurrent or refractory chronic lymphocytic leukemia, with dexamethasone in recurrent or refractory multiple myeloma, with dacarbazine in metastatic malignant melanoma, and with docetaxel in non-small-cell lung carcinoma. Malignant melanoma did not show a survival benefit from G3139, according to reports of the phase-III clinical trial. Final results from other trials are still awaited (CitationKim et al. 2004).
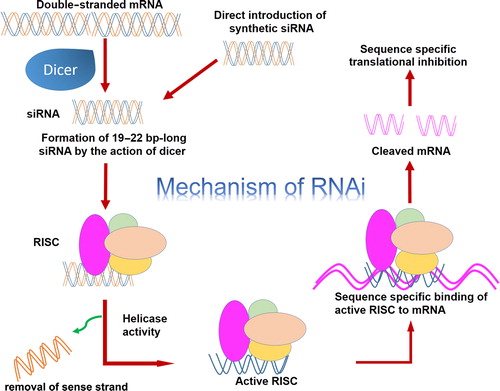
RNA interference (RNAi) is a natural biological mechanism in which double-stranded RNA (dsRNA) intrudes with the gene expression having a homologous sequence with the dsRNA, when introduced. RNAi is widely used to regulate gene expression in many studies, like basic research, functional genomics, drug discovery, drug validation, and transgenic designing. RNAi-induced gene silencing can be achieved by utilizing small interfering RNA (siRNA) of 21–23 nucleotide length that is inherently produced from long dsRNA by the enzyme Dicer, which has RNase-III-like activity. These siRNA are integrated into a multi-protein RNA-inducing silencing complex (RISC). The duplex siRNA is unwound by helicase of RISC, causing the formation of sense and antisense strands. The antisense strand and RISC directed to its complementary mRNA and by the action of the exonuclease and endonuclease domain of RISC, targeted mRNA is cleaved by the action of the exonuclease and endonuclease domain of RISC () (CitationElbashir et al. 2001).
Many research groups have selected the Bcl-2 gene as the target, utilizing RNAi technology in developing treatment for many cancer types. siRNA specific for the Bcl-2 gene can be designed utilizing many available bioinformatics tools. In 2002, Takashi Futami et al. used the siRNA expression system-based vector-targeting Bcl-2 gene in HeLa cells to induce apoptosis. The siRNA vectors made HeLa cells more sensitive to doxorubicin treatment (CitationFutami et al. 2002). Silencing of Bcl-2 in melanoma cells by specific siRNA led to an increase in apoptotic cell death and inhibition of cell growth. siRNA-targeting Bcl-2 in combination with cisplatin showed a massive increase in apoptotic cell death (CitationWacheck et al. 2003). Thereafter, the siRNA-targeting Bcl-2 gene was intensively used in pancreatic (CitationOcker et al. 2005), liver (CitationFeng et al. 2006, CitationWarmann et al. 2008), breast (CitationAkar et al. 2008), ovarian (CitationSaad et al. 2008), gastric (CitationHao et al. 2007), and bladder (CitationKunze et al. 2008) cancer, and in human glioblastoma (CitationGeorge et al. 2009a, Citation2009b) and neuroblastoma (CitationShen et al. 2012), in vitro and in vivo.
Various studies show that siRNA and antisense nucleotide-based nucleic acid drugs are very effective in controlling the expression of genes such as Bcl-2. These nucleic acid-based drugs can be effective only if they can reach their target present within the cell. However, a number of factors hinder the in vivo applications of these drugs (CitationYin et al. 2013, CitationDraz et al. 2014).
Charge: Nucleic acid based drugs are negatively charged. Therefore, these drugs cannot cross the cell membrane easily.
Stability: Upon entry into the systemic circulation, siRNA is recognized by the immune system and is rapidly cleared. siRNA can pass through the glomerular filtration barrier and therefore undergoes rapid renal clearance. These drugs are degraded by the highly acidic endosomes and serum nucleases.
Off-target effect: Binding siRNA with other mRNA rather than the targeted mRNA could result in prevention of translation of genes coding for essential proteins. These negatively charged nucleotides can also bind with serum proteins, resulting in decreased bioavailability.
Toxicity: siRNA is capable of activating Toll-like receptors, leading to increase in the cytokine levels. Nuclease-cleaved RNA can activate innate immunity.
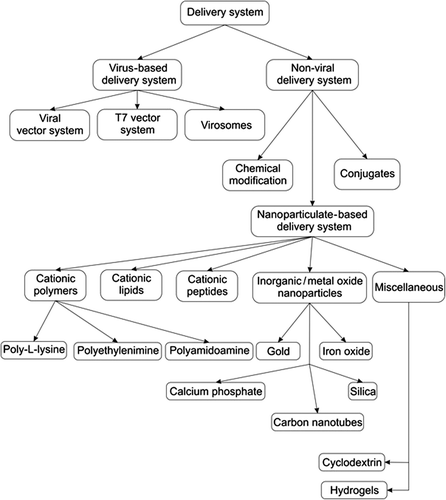
Therefore, it is necessary to develop and adopt proper techniques so that these nucleic acid-based drugs are able to reach their targets. The following section discusses some of the currently tested techniques, with a focus on nanoparticulate-based delivery systems useful in assisting these drugs reach their targets ().
Virus-based delivery system
Viruses are “naturally evolved vehicles” that have the highest ability to easily transfer their genetic material into the hosts. Due to this, a number of viral vector systems have been screened for possible use as drug carriers to deliver genes of interest. The viruses are selected based on the safety, ability to carry foreign genes, transfection efficiency, and gene expression. Among the various virus groups, retroviruses have been the virus of choice for delivery systems. Nearly 40% of the clinical trials involved in studies using viral vector systems utilize recombinant retroviruses as the carrier. Other viral vector systems include the adenovirus, adeno-associated virus, herpes simplex virus, and lentivirus. These viral vectors are well characterized and easy to manipulate (CitationWalther and Stein 2000).
T7 vector-based delivery systems
T7 vector-based delivery systems are useful to obtain a sufficient amount of mRNA within the target cells. These vectors can be made to specifically target Bcl-2 by preparing the “T7 promoter-driven siRNA expression vector system (Bcl-2/T7)”. These kinds of vectors are utilized in reducing the level of Bcl-2 mRNA (CitationHolle et al. 2004).
Virosomes
Virosomes are prepared by incorporating proteins derived from the envelope of the virus. This delivery system is useful for the cytosolic release of drugs (CitationFelnerova et al. 2004). Detailed descriptions of the virus as a drug carrier for delivery of nucleic acid-based drugs have been well documented (CitationWalther and Stein 2000, CitationEl-Aneed 2004).
Chemical modifications
One way of successfully using siRNA in vivo is through suitable chemical modification. siRNA can be modified in a number of ways, to increase their stability and efficacy. Replacing oxygen atoms (which are not involved in bridging two ribonucleotides) present in the phosphate backbone, with atoms of sulfur, helps in preparing siRNA with ‘phosphorothioate’ linkages. This technique increases the resistance against serum nucleases. Similarly, if the replacing atom is ‘boron’, then siRNA will contain ‘boranophosphate’ linkages. This siRNA is believed to produce a better gene-silencing effect even at a lower concentration, in addition to the increased stability in serum. Bridging 2’ ribose carbon with 4’ carbon results in methylene linkages which produce nucleotides with higher affinity binding and better efficacy. This kind of siRNA is often referred to as “Locked nucleic acid (LNA)” nucleotides (CitationCorey 2007). Readers can refer the article “Chemical modification: the key to clinical application of RNA interference?” by David R. Corey, for complete explanation on various approaches available (CitationCorey 2007).
Conjugates
The stability of genetic material such as siRNA can also be improved by preparing them in the form of conjugates which can improve pharmacokinetic properties, particularly the half-life and volume of distribution. Cholesterol-siRNA conjugates are found to be absorbed better by the tissues (CitationWolfrum et al. 2007, CitationShim and Kwon 2010). Through suitable modification and by using Bcl-2-specific siRNA, this approach could be adopted to target various cancer types.
Cationic polymers
Poly(L-lysine) (PLL)
PLL is a biodegradable lysine-containing linear polypeptide. The main drawback of a PLL-based delivery system is the rapid clearance from the plasma (CitationEl-Aneed 2004). However, PLL can be easily grafted to make it suitable for specific applications (CitationLollo et al. 2002). PLL can be even grafted using materials such as silica, to make it suitable for oral delivery of genes (CitationBadros et al. 2005). Modified PLL is useful in delivering antisense oligonucleotides specific for Bcl-2 (CitationRead et al. 2000). Modified PLL is also useful as a delivery system for co-administration of siRNA and anticancer agents. This combination drug strategy is effective in downregulating the expression of the Bcl-2 gene and enhancing the effect of the chemotherapeutic agent (CitationZheng et al. 2013).
Poly(ethylenimine) (PEI)
Among the cationic polymers, PEI is the most widely used. This is mainly due to its ability to buffer a wide pH range. PEI is highly positively charged due to the presence of nitrogen atoms. Molecules such as DNA and RNA are negatively charged. Therefore, these molecules are easily complexed by the highly positively charged PEI. Although there is no clear proof on the mechanism by which PEI is able to resist highly acidic conditions, it is believed that these PEI complexes are able to escape lysosomal degradation due to the “proton sponge” effect (CitationBenjaminsen et al. 2013). Cytotoxicity, activation of immune cells, slow release of siRNA, and the tendency to form aggregates are some of the limitations associated with PEI-mediated genetic delivery systems (CitationWen and Meng 2014). Among the various factors, viz., particle size, molecular weight, degree of branching, etc., it is believed that the high positive charge on the PEI contributes to its toxicity (CitationEl-Aneed 2004, CitationShen et al. 2013). Therefore, approaches such as PEGylation help in reducing the toxicity. PEGylation further improves the stability and biocompatibility of the complexes (CitationShen et al. 2013).
Polyamidoamine
The ease with which the charge density and molecular weight can be altered has made polyamidoamine one of the preferred carrier systems for the delivery of siRNA. The amines present at the surface of the polyamidoamine aid in binding with the siRNA. The tertiary amine present in the interior of the polyamidoamine helps in the escape from endosomal degradation (CitationWen and Meng 2014).
Cationic (amphipathic) peptides (CP)
CP are amphiphilic in nature and therefore have the ability to undergo conformational change when exposed to acidic conditions. This helps them to escape lysosomal degradation and deliver their cargoes. The positively charged amino acids present in the cationic peptides help them to condense the negatively charged materials such as DNA and siRNA and deliver inside the cell (CitationEl-Aneed 2004). CP are particularly useful when used along with other carriers such as cationic polymers (CitationKichler et al. 2006). The properties that CP should possess for the delivery of nucleic acid-based drugs include solubility in aqueous solutions, presence of positive charge (preferably amphipathic in nature), cell membrane destabilizing ability, and short size (preferably less than 30 amino acids) (CitationKichler et al. 2006, CitationVerdurmen and Brock 2011). If it is decided that CP will be used as a drug carrier, the CP should be carefully selected as CP possess intrinsic biologic properties (CitationVerdurmen and Brock 2011).
Gold nanoparticles (GNP)
Interest in the use of GNP as a delivery system for nucleic acid-based drugs, particularly siRNA, has steadily increased, with the first report published in 2006 (CitationLytton-Jean et al. 2011). siRNA can be conjugated with GNP either through chelation of gold-thiol or through electrostatic interaction. Newly synthesized GNP are negatively charged. These negatively charged GNP are surface-coated with suitable materials/polymers such as PEI, so that they acquire positive charge. They can then bind with siRNA through electrostatic interaction (CitationLytton-Jean et al. 2011, CitationWen and Meng 2014). Scavenger receptors present on the cell surface help in internalization of GNP. The circulation time of GNP improves upon PEGylation or attachment with a suitable ligand (CitationWen and Meng 2014). GNP are able to protect siRNA from nucleases and shield them from the immune system (CitationLytton-Jean et al. 2011).
Iron oxide-based nanoparticles (INP)
INP are extensively used as diagnostic agents and also in hypothermic-based cancer treatment. Among various types of iron oxides available, magnetite (Fe3O4) and maghemite (γ-Fe2O3) are widely used. This is due to their biocompatibility, stability within biological conditions, and low cost of preparation (CitationFiguerola et al. 2010). Recent studies show that INP could be used as carriers in delivering cargo specifically into the target site. These nanocarriers are even useful to target specific proteins such as Bcl-2. Coupling the oncopeptide NuBCP-9 with INP allows specific targeting of Bcl-2. These INP are particularly useful in delivering levoform proteins and peptides to the targets lying within the cell (CitationKumar et al. 2014). Although the aqueous solubility of INP is the main limitation, this is easily overcome through surface coating of these nanoparticles (CitationFiguerola et al. 2010). Reports even suggest that ultra-small superparamagnetic iron oxide (USPIO) can be easily synthesized through suitable modification in the method of synthesis and subsequent coating with amphiphilic polymers (CitationYang et al. 2013). These USPIO are made Bcl-2-specific by binding them with Bcl-2 monoclonal antibody using a N-hydroxysuccinimide/ethyl(diethylaminopropyl) carbodiimide-based method (CitationYang et al. 2013).
An important application of superparamagnetic iron oxide (SPIO) is the possible use as a non-viral vector. Conjugation of SPIO further allows monitoring of the effectiveness of the therapy. Bcl-2-specific siRNA was found to be effective in controlling the expression of Bcl-2 in neuroblastoma cells when delivered along with a delivery system comprising a single-chain antibody conjugated with the PEG-PEI-SPIO system (CitationShen et al. 2013). Other miscellaneous, inorganic or metal-based particulate delivery systems used in the delivery of nucleic acid-based drugs include cyclodextrin, calcium phosphate, carbon nanotubes, silica, and silicon-based nanoparticles (CitationYin et al. 2013, CitationWen and Meng 2014, CitationDraz et al. 2014).
Hydrogels
Hydrogels have a hydrophilic three-dimensional network capable of absorbing large quantities of water and biological fluids. They could be virtually made up of any hydrophilic polymers and can be modified easily for different shapes and properties (CitationDraz et al. 2014, CitationHoare and Kohane 2008). Among the macro, micro, and nano hydrogels, nano hydrogels are extensively studied for possible application as a drug carrier for delivery of drugs into the cell. Due to their porous nature, hydrogels have high loading efficiency and can be easily employed for targeted delivery. This allows sustained release of drugs such as siRNA (CitationDraz et al. 2014).
Cationic Liposomes
Cationic liposomes are one of the most studied nonviral-based delivery systems for nucleic acids and siRNA. Cationic lipids like DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) and DOTMA (1,2-di-O-octadecenyl-3-trimethylammonium propane) have been extensively studied to deliver siRNA to target sites (CitationMa et al. 2005, CitationZhang et al. 2007). The cationic charge on the surface of particles helps to form stable complexes with negatively charged siRNA due to electrostatic interaction, which improves the stability of the complexes. Cationic liposomes will interact with the negatively charged cell membrane and easily get transfected, leading to enhanced cellular uptake. Activation of the immune response, the interaction with blood components and the removal by the reticuloendothelial system, leading to poor blood circulation, are considered as drawbacks for cationic liposomes. Cationic liposomes can be modified with polymers like polyethylene glycol (PEG) (CitationKim et al. 2010), peptides (CitationYang et al. 2014), galactose (CitationSonoke et al. 2011), transferrin (CitationNakase et al. 2005), and many other small molecules.
For effectively targeting Bcl-2, the potential drug candidates have to be delivered to their target sites. This is particularly challenging due to various factors including the presence of charged cell membranes and various efflux mechanisms present within the cell. Therefore, the small size of nanodelivery systems makes them useful in delivering the drugs into the cell. A viral-based delivery system is useful in achieving higher bioavailability. However, the chances of immune response have led to the search for newer non-viral based carriers. Although chemical modification and the use of conjugates helps in the delivery of drugs across the cell membrane, not all the drugs can be modified without affecting their activities. In such cases, nanoparticulate-based delivery systems are useful. In addition, nanoparticulate delivery systems provide more choice with respect to drug targeting. However, there is no complete information on nanoparticulate delivery systems, particularly their toxicity and degradation. Nevertheless, nanoparticulate delivery systems hold a great promise for targeting sites such as Bcl-2 present within the cell.
Conclusion
Cancer therapies have made a paradigm shift in approaches for proper understanding of cancer biology. The identification of genes having potential roles in the development of cancer has led to the selection of new targets for cancer therapy. One of the potential targets is the antiapoptotic Bcl-2 protein, proved to be a potential target due to its role in the development of cancer. Silencing the overexpressed Bcl-2 gene, preventing its translation, and using well-conceptualized RNAi and antisense technology, are proving to be viable and effective alternatives in cancer therapy. Developing formulations to deliver siRNA and antisense oligonucleotides to targeted sites is an issue. Many nanotechnology-based delivery systems are being developed and evaluated to achieve this goal. Hence, this approach holds a great promise for developing an alternative therapy for cancer or as an adjuvant therapy, thereby enhancing the effectiveness of existing anticancer drugs.
Acknowledgements
The authors would like to acknowledge the authorities of the Council of Scientific and Industrial Research (CSIR), New Delhi, for providing a fellowship to Mr. Hitesh Vitthal Jagani, and Manipal University for providing the necessary research facilities.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adams JM, Cory S. 1998. The Bcl-2 protein family: arbiters of cell survival. Science. 281:1322–1326.
- Akar U, Chaves-Reyez A, Barria M, Tari A, Sanguino A, Kondo Y, et al. 2008. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 4:669.
- Badros AZ, Goloubeva O, Rapoport AP, Ratterree B, Gahres N, Meisenberg B, et al. 2005. Phase II study of G3139, a Bcl-2 antisense oligonucleotide, in combination with dexamethasone and thalidomide in relapsed multiple myeloma patients. J Clin Oncol. 23:4089–4099.
- Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. 2006. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 24:4738–4745.
- Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. 2013. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 21:149–157.
- Brown JM, Attardi LD. 2005. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 5:231–237.
- Campos L, Rouault J-P, Sabido O, Oriol P, Roubi N, Vasselon C, et al. 1993. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 81:3091–3096.
- Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. 2006. A novel Bcl-2/Bcl-XL/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 26: 2374–2380.
- Corey DR. 2007. Chemical modification: the key to clinical application of RNA interference? J clin Invest. 117:3615–3622.
- Cory S, Vaux DL, Strasser A, Harris AW, Adams JM. 1999. Insights from Bcl-2 and Myc: malignancy involves abrogation of apoptosis as well as sustained proliferation. Cancer Res. 59:1685s–1692s.
- Cotter F, Corbo M, Raynaud F, Orr R, Pocock C, Bryan B, et al. 1996. Bcl-2 antisense therapy in lymphoma: in vitro and in vivo mechanisms, efficacy, pharmacokinetic and toxicity studies. Ann Oncol. 7:327.
- Davis JM, Navolanic PM, Weinstein-Oppenheimer CR, Steelman LS, Hu W, et al. 2003. Raf-1 and Bcl-2 induce distinct and common pathways that contribute to breast cancer drug resistance. Clin Cancer Res. 9:1161–1170.
- Del Bufalo D, Biroccio A, Leonetti C, Zupi G. 1997. Bcl-2 overexpression enhances the metastatic potential of a human breast cancer line. FASEB J. 11:947–953.
- Delia D, Aiello A, Formelli F, Fontanella E, Costa A, Miyashita T, et al. 1995. Regulation of apoptosis induced by the retinoid N-(4-hydroxyphenyl) retinamide and effect of deregulated bcl-2. Blood. 85: 359–367.
- Dole M, Nuñez G, Merchant AK, Maybaum J, Rode CK, Bloch CA, Castle VP. 1994. Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res. 54:3253–3259.
- Draz MS, Fang BA, Zhang P, Hu Z, Gu S, Weng KC, et al. 2014. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 4:872–892.
- El-Aneed A. 2004. An overview of current delivery systems in cancer gene therapy. J Control Release. 94:1–14.
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498.
- Elmore S. 2007. Apoptosis: a review of programmed cell death. Toxicol Pathol. 35:495–516.
- Felnerova D, Viret J-Fo, Glück R, Moser C. 2004. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 15:518–529.
- Feng LF, Zhong M, Lei XY, Zhu BY, Tang SS, Liao DF. 2006. Bcl-2 siRNA induced apoptosis and increased sensitivity to 5-fluorouracil and HCPT in HepG2 cells. J Drug Target. 14:21–26.
- Fesik SW. 2005. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
- Figuerola A, Di Corato R, Manna L, Pellegrino T. 2010. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol Res. 62:126–143.
- Futami T, Miyagishi M, Seki M, Taira K. 2002. Induction of apoptosis in HeLa cells with siRNA expression vector targeted against bcl-2. Nucl Acids Symp Ser (Oxford Univ Press). 251–252.
- George J, Banik NL, Ray Sk. 2009a. Bcl-2 siRNA augments taxol mediated apoptotic death in human glioblastoma U138MG and U251MG cells. Neurochem Res. 34:66–78.
- George J, Banik NL, Ray SK. 2009b. Combination of taxol and Bcl‐2 siRNA induces apoptosis in human glioblastoma cells and inhibits invasion, angiogenesis and tumour growth. J Cell Mol Med. 13:4205–4218.
- Gobé G, Rubin M, Williams G, Sawczuk I, Buttyan R. 2002. Apoptosis and expression of Bcl-2, Bcl-XL, and Bax in renal cell carcinomas. Cancer Invest. 20:324–332.
- Hao J-H, Gu Q-L, Liu B-Y, Li J-F, Chen X-H, Ji Y-B, et al. 2007. Inhibition of the proliferation of human gastric cancer cells SGC-7901 in vitro and in vivo using Bcl-2 siRNA. Chin Med J-(Engl). 120:2105.
- Heiser D, Labi V, Erlacher M, Villunger A. 2004. The Bcl-2 protein family and its role in the development of neoplastic disease. Exp Gerontol. 39:1125–1135.
- Hetts SW. 1998. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 279:300–307.
- Hoare TR, Kohane DS. 2008. Hydrogels in drug delivery: progress and challenges. Polymer. 49:1993–2007.
- Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 348:334–6.
- Hockenbery DM. 1994. Bcl-2 in cancer, development and apoptosis. J Cell Sci Suppl. 18:51–55.
- Holle L, Hicks L, Song W, Holle E, Wagner T, Yu X. 2004. Bcl-2 targeting siRNA expressed by a T7 vector system inhibits human tumor cell growth in vitro. Int J Oncol. 24:615–621.
- Joensuu H, Pylkkänen L, Toikkanen S. 1994. Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol. 145:1191.
- Johnstone RW, Ruefli AA, Lowe SW. 2002. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 108:153–164.
- Kang MH, Reynolds CP. 2009. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 15: 1126–1132.
- Khan S, Maududi T, Barton K, Ayers J, Alkan S. 2004. Analysis of histone deacetylase inhibitor, depsipeptide (FR901228), effect on multiple myeloma. Br J Haematol. 125:156–161.
- Kichler A, Mason AJ, Bechinger B. 2006. Cationic amphipathic histidine-rich peptides for gene delivery. Biochim Biophys Acta. 1758:301–307.
- Kim HK, Davaa E, Myung CS, Park JS. 2010. Enhanced siRNA delivery using cationic liposomes with new polyarginine-conjugated PEG-lipid. Int J Pharm. 392:141–147.
- Kim R, Emi M, Tanabe K, Toge T. 2004. Therapeutic potential of antisense Bcl‐2 as a chemosensitizer for cancer therapy. Cancer. 101:2491–2502.
- Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed Jc. 2008. Bcl-2 antagonist apogossypol (NSC736630) displays single-agent activity in Bcl-2–transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048). Blood. 111: 3211–3219.
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo Pp, Kitada S, et al. 2006. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer cell. 10:375–388.
- Kroemer G. 1997. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 3:614–620.
- Kumar M, Singh G, Sharma S, Gupta D, Bansal V, Arora V, et al. 2014. Intracellular delivery of peptide cargos using iron oxide based nanoparticles: studies on antitumor efficacy of BCL-2 converting peptide, NuBCP-9. Nanoscale. 1–15.
- Kunze D, Wuttig D, Fuessel S, Kraemer K, Kotzsch M, Meye A, et al. 2008. Multitarget siRNA inhibition of antiapoptotic genes (XIAP, BCL2, BCL-XL) in bladder cancer cells. Anticancer Res. 28: 2259–2263.
- Kurreck J. 2003. Antisense technologies. Eur J Biochem. 270:1628–1644.
- Leber B, Geng F, Kale J, Andrews DW. 2010. Drugs targeting Bcl-2 family members as an emerging strategy in cancer. Exp Rev Mol Med. 12:e28.
- Leskov I, Pallasch C, Drake A, Iliopoulou B, Souza A, Shen C, et al. 2012. Rapid generation of human B-cell lymphomas via combined expression of Myc and Bcl2 and their use as a preclinical model for biological therapies. Oncogene. 32:1066–1072.
- Lollo CP, Banaszczyk MG, Mullen PM, Coffin CC, Wu D, Carlo AT, et al. 2002. Poly-L-Lysine-Based Gene Delivery Systems. Gene therapy protocols. New York: Springer, pp. 1–13.
- Lorenz H, Herrmann M, Winkler T, Gaipl U, Kalden J. 2000. Role of apoptosis in autoimmunity. Apoptosis. 5:443–449.
- Lowe SW, Lin AW. 2000. Apoptosis in cancer. Carcinogenesis. 21: 485–495.
- Lytton-Jean AKR, Langer R, Anderson DG. 2011. Five years of siRNA delivery: spotlight on gold nanoparticles. Small. 7:1932–1937.
- Ma Z, Li J, He F, Wilson A, Pitt B, Li S. 2005. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun. 330:755–759.
- MacVicar G, Kuzel T, Curti B, Poiesz B, Somer B, Greco F, et al. 2008.An open-label, multicenter, phase I/II study of AT-101 in combination with docetaxel (D), prednisone (P) in men with hormone refractory prostate cancer (HRPC). J Clin Oncol (Meeting Abstracts). 16043.
- Manero F, Gautier F, Gallenne T, Cauquil N, Grée D, Cartron P-F, et al. 2006. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 66:2757–2764.
- Marcucci G, Byrd JC, Dai G, Klisovic MI, Kourlas PJ, Young DC, et al. 2003. Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood. 101:425–432.
- Marzo I, Naval J. 2008. Bcl-2 family members as molecular targets in cancer therapy. Biochem Pharmacol. 76:939–946.
- Mattson MP. 2000. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 1:120–130.
- McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, Mckearn JP, Korsmeyer SJ. 1989. Bcl-2-Immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation . Cell. 57:79–88.
- Medicine, UNLO Genetic Home Reference [Online]. 2014. Available: http://ghr.nlm.nih.gov/gene/BCL2 [Accessed 10th October 2014].
- Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S. 1999. Overexpression of Bcl-2 enhances metastatic potential of human bladder cancer cells. Br J Cancer. 79:1651.
- Nakase M, Inui M, Okumura K, Kamei T, Nakamura S, Tagawa T. 2005. p53 gene therapy of human osteosarcoma using a transferrin- modified cationic liposome. Mol Cancer Ther. 4:625–631.
- Ocker M, Neureiter D, Lueders M, Zopf S, Ganslmayer M, Hahn E, et al. 2005. Variants of bcl-2 specific siRNA for silencing antiapoptotic bcl-2 in pancreatic cancer. Gut. 54:1298–1308.
- Pan H, Yin C, VAN Dyke T. 1997. Apoptosis and cancer mechanisms. Cancer Surv. 29:305.
- Pro B, Leber B, Smith M, Fayad L, Romaguera J, Hagemeister F, et al. 2008. Phase II multicenter study of oblimersen sodium, a Bcl‐2 antisense oligonucleotide, in combination with rituximab in patients with recurrent B‐cell non‐Hodgkin lymphoma. Br J Haematol. 143:355–360.
- Raffo AJ, Perlman H, Chen M-W, Day ML, Streitman JS, Buttyan R. 1995. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 55:4438–4445.
- Raynaud FI, Orr RM, Goddard PM, Lacey HA, Lancashire H, Judson IR, et al. 1997. Pharmacokinetics of G3139, a phosphorothioate oligodeoxynucleotide antisense to bcl-2, after intravenous administration or continuous subcutaneous infusion to mice. J Pharmacol Exp Ther. 281:420–427.
- Re R. 2000. The application of antisense technology to medicine. Ochsner J. 2:233–236.
- Read ML, Dash PR, Clark A, Howard KA, Oupicky D, Toncheva V, et al. 2000. Physicochemical and biological characterisation of an antisense oligonucleotide targeted against the bcl-2 mRNA complexed with cationicâhydrophilic copolymers. Eur J Pharm Sci. 10: 169–177.
- Reed JC. 1999. Dysregulation of apoptosis in cancer. J Clin Oncol. 17:2941–2941.
- Reed JC, Cuddy M, Slabiak T, Croce CM, Nowell PC. 1988. Oncogenic potential of bcl.-2 demonstrated by gene transfer. Nature. 336: 259–261.
- Reed JC, Haldar S, Croce C, Cuddy M. 1990. Complementation by BCL2 and C-HA-RAS oncogenes in malignant transformation of rat embryo fibroblasts. Mol Cell Biol. 10:4370–4374.
- Rudin C, Otterson G, Mauer A, Villalona-Calero M, Tomek R, Prange B, et al. 2002. A pilot trial of G3139, a bcl-2 antisense oligonucleotide, and paclitaxel in patients with chemorefractory small-cell lung cancer. Ann Oncol. 13:539–545.
- Rudin CM, Kozloff M, Hoffman PC, Edelman MJ, Karnauskas R, Tomek R, et al. 2004. Phase I study of G3139, a bcl-2 antisense oligonucleotide, combined with carboplatin and etoposide in patients with small-cell lung cancer. J Clin Oncol. 22:1110–1117.
- Rudin CM, Salgia R, Wang X, Hodgson LD, Masters GA, Green M, Vokes EE. 2008. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 26:870–876.
- Saad M, Garbuzenko OB, Minko T. 2008.Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond). 3: 761–776.
- Sartorius UA, Krammer PH. 2002. Upregulation of bcl‐2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 97:584–592.
- Schmitt CA, Rosenthal CT, Lowe SW. 2000. Genetic analysis of chemoresistance in primary murine lymphomas. Nat Med. 6:1029–1035.
- Shen M, Gong F, Pang P, Zhu K, Meng X, Wu C, et al. 2012. An MRI-visible non-viral vector for targeted Bcl-2 siRNA delivery to neuroblastoma. Int J Nanomed. 7:3319.
- Shen M, Gong F, Pang P, Zhu K, Meng X, Wu C, et al. 2013. An MRI-visible non-viral vector for targeted Bcl-2 siRNA delivery to neuroblastoma. Int J Nanomed. 7:3319–3332.
- Shim MS, Kwon YJ. 2010. Efficient and targeted delivery of siRNA in vivo. FEBS J. 277:4814–4827.
- Sierra A, Castellsague X, Tortola S, Escobedo A, Lloveras B, Peinado MA, et al. 1996. Apoptosis loss and bcl-2 expression: key determinants of lymph node metastases in T1 breast cancer. Clin Cancer Res. 2:1887–1894.
- Sinicrope FA, Penington RC, Tang XM. 2004. Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand–Induced Apoptosis Is Inhibited by Bcl-2 but Restored by the Small Molecule Bcl-2 Inhibitor, HA 14-1, in Human Colon Cancer Cells. Clin Cancer Res. 10:8284–8292.
- Song T, Chang X, Zhang Z, Liu Y, Shen X. 2012. S1, a novel pan-BH3 mimetic, induces apoptosis in Mcl-1-overexpressing cells through Bak. J Pharmacol Sci. 119:330–340.
- Sonoke S, Ueda T, Fujiwara K, Kuwabara K, Yano J. 2011. Galactose-modified cationic liposomes as a liver-targeting delivery system for small interfering RNA. Biol Pharm Bull. 34:1338–1342.
- Strasser A, Harris AW, Bath Ml, Cory S. 1990. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 348:331–333.
- Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, et al. 2007. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 67:1176–1183.
- Thompson CB. 1995. Apoptosis in the pathogenesis and treatment of disease. Science. 267:1456–1462.
- Tolcher AW. 2001. Preliminary phase I results of G3139 ( bcl-2 antisense oligonucleotide) therapy in combination with docetaxel in hormone-refractory prostate cancer. Semin Oncol. 28:67–70.
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. 2008. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68:3421–3428.
- Vaux DL, Cory S, Adams JM. 1988. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature (London). 335:440–442.
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. 1993. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 75:229–240.
- Verdurmen WPR, Brock R. 2011. Biological responses towards cationic peptides and drug carriers. Trends Pharmacol Sci. 32:116–124.
- Wacheck V, Losert D, Günsberg P, Vornlocher H-P, Hadwiger P, Geick A, et al. 2003. Small interfering RNA targeting bcl-2 sensitizes malignant melanoma. Oligonucleotides. 13:393–400.
- Walther W , Stein U. 2000. Viral vectors for gene transfer. Drugs. 60: 249–271.
- Wang S, Yang D, Lippman ME. 2003. Targeting Bcl-2, Bcl-XL with nonpeptidic small-molecule antagonists. Seminars Oncol (Elsevier). 30:133–142.
- Warmann SW, Frank H, Heitmann H, Ruck P, Herberts T, Seitz G, Fuchs J. 2008. Bcl-2 gene silencing in pediatric epithelial liver tumors. J Surg Res. 144:43–48.
- Waters JS, Webb A, Cunningham D, Clarke PA, Raynaud F, Di Stefano F, Cotter Fe. 2000. Phase I clinical and pharmacokinetic study of bcl-2 antisense oligonucleotide therapy in patients with non-Hodgkin's lymphoma. J Clin Oncol. 18:1812–1823.
- Webb A, Cunningham D, Cotter F, Clarke P, Di Stefano F, Ross P, et al. 1997. BCL-2 antisense therapy in patients with non-Hodgkin lymphoma . Lancet. 349:1137–1141.
- Wei J, Kitada S, Rega MF, Stebbins JL, Zhai D, Cellitti J, et al. 2009. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 52:4511–4523.
- Wei J, Stebbins JL, Kitada S, Dash R, Zhai D, Placzek WJ, et al. 2011. An optically pure apogossypolone derivative as potent pan-active inhibitor of anti-apoptotic Bcl-2 family proteins. Front Oncol. 1:28.
- Wen Y, Meng Ws. 2014. Recent In Vivo Evidences of Particle-Based Delivery of Small-Interfering RNA (siRNA) into Solid Tumors. J Pharm Innov. 9:158–173.
- Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, et al. 2007. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 25:1149–1157.
- Yang B, Cai H, Qin W, Zhang B, Zhai C, Jiang B, Wu Y. 2013. Bcl-2-functionalized ultrasmall superparamagnetic iron oxide nanoparticles coated with amphiphilic polymer enhance the labeling efficiency of islets for detection by magnetic resonance imaging. Int J Nanomed. 8:3977–3990.
- Yang Z-Z, Li J-Q, Wang Z-Z, Dong D-W, Qi X-R. 2014. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 35:5226–5239.
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. 2013. Non-viral vectors for gene-based therapy. Nat Rev Genet. 15:541–555.
- Zhang S, Zhao B, Jiang H, Wang B, Ma B. 2007. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Rel. 123:1–10.
- Zhang Z, Song T, Zhang T, Gao J, Wu G, An L, Du G. 2011. A novel BH3 mimetic S1 potently induces Bax/Bak‐dependent apoptosis by targeting both Bcl‐2 and Mcl‐1. Int J Cancer. 128:1724–1735.
- Zheng C, Zheng M, Gong P, Deng J, Yi H, Zhang P, et al. 2013. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials. 34:3431–3438.