?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The covalent immobilization of Trichoderma reesei cellulase onto modified ReliZyme HA403 and Sepabeads EC-EP supports were carried out. The optimal immobilization conditions were determined using response surface methodology. The hydrolysis of cellulose using the free and immobilized cellulase preparations in ionic liquids (IL) using cosolvents was investigated. The hydrolytic activities in buffer medium containing 25% (v/v) of 1-butyl-3-methylimidazolium hexafluorophosphate were around 2.6-, 1.6-, and 5.5-fold higher than the activities in buffer medium. The retained initial activities were 32% and 57%, respectively for cellulase preparations immobilized onto Sepabeads EC-EP support and onto modified ReliZyme HA403 support after 5 reuses.
Introduction
The resources of fossil fuel are limited, and their rapid consumption, together with the negative effects in the atmosphere caused by burning fossil fuel have forced researchers to seek renewable and sustainable sources (CitationFarrell et al. 2006). Lignocelluloses are mainly comprised of cellulose, hemicellulose, and lignin structures, and they are not used for food production (CitationEbner et al. 2014). Among them, cellulose is the most abundant natural polymer, and is converted to many industrially important products such as biofuels and value-added chemicals (CitationPandey et al. 2000). Cellulases catalyze the hydrolysis of β-(1–4) linkages in cellulose. They generally consist of a multicomponent enzyme system of endoglucanase, cellobiohydrolase, and cellobiase (CitationOgeda et al. 2012, CitationQin et al. 2008), and act synergistically on cellulose to produce glucose, which can be further fermented to valuable products (CitationHsu et al. 2011). Cellulases have been used in many industries, such as bioenergy (CitationAlftrén and Hobley 2014), food (CitationNadeem et al. 2009), animal feed (CitationJacobs and McAllan 1991), textile (CitationAnish et al. 2007), and paper and pulping (Citationvan Wyk and Mohulatsi 2003). However, it was reported that the ease of inactivation could be due to their multimeric subunits, which may tend to dissociate (CitationBayramoglu et al. 2013). The enzyme immobilization techniques offer a solution to resolve the problem of cellulase inactivation. For instance, CitationDinçer and Telefoncu (2007) immobilized cellulase onto chitosan beads coated with polyanion-modified polyvinyl alcohol, and the immobilized cellulase retained 52% of its initial activity after being reused eight times. CitationWang et al. (2013) reported that cellulase from Trichoderma viride immobilized onto polyamidoamine dendrimer-grafted silica by adsorption and crosslinking methods exhibited better stability with respect to pH and temperature, compared with free enzyme. CitationKhoshnevisan et al. (2011) immobilized cellulase from T. viride on superparamagnetic nanoparticles, and they reported that the immobilized cellulase showed its activity in broader pH and temperature ranges compared to the free enzyme.
ReliZyme and Sepabeads EC are composed of a rigid methacrylic polymer matrix. Sepabeads EC-EP is an epoxy-activated support and can react with different nucleophilic groups at the protein surface, generating a strong multipoint covalent attachment (CitationCerdobbel et al. 2010, CitationGhazi et al. 2005, CitationMateo et al. 2007). ReliZyme HA403 has a hexamethylamino functional group. These primary amino groups on the ReliZyme HA403 surface can be easily activated with a bifunctional coupling agent, such as glutaraldehyde, for enzyme immobilization (CitationBayraktar et al. 2011). In addition, the immobilization of enzyme via glutaraldehyde coupling may prevent dissociation of multimeric enzymes due to the multipoint covalent attachment (CitationFernandez-Lafuente 2009).
Due to its rigid structure, it is difficult to dissolve cellulose in buffers. This creates a bottleneck for accessing cellulose molecules to the enzyme's active site during hydrolysis. Recently, the use of ionic liquids (ILs) as reaction media in biocatalysis have drawn considerable attention since their properties of melting point, viscosity, density and hydrophobicity can be easily altered by changing to the structure of the ions (CitationPlechkova and Seddon 2008, CitationTurner et al. 2003, Citationvan Rantwijk and Sheldon 2007).
Response surface methodology (RSM) has been applied in a variety of biotechnological applications for optimization studies, since it is a simple technique that explains relationships between factors and response, and combines mathematical, statistical, and experimental techniques (CitationTükel et al. 2013, CitationYildirim and Tukel 2013). The Box-Behnken design (BBD) has been widely used to evaluate quadratic models compared to full factorial designs (FFDs) and central composite design (CCD). Besides, BBDs require fewer experiments than FFD and CCD techniques, with the same number of factors.
It was reported that the immobilization conditions should be optimized to obtain full performance from an immobilized enzyme preparation (CitationYildirim et al. 2014). In the present study, it was aimed to optimize the conditions of immobilization of Trichoderma reesei cellulase onto modified ReliZyme HA403 and Sepabeads EC-EP supports using RSM. For this purpose, a 3-factor and 3-level BBD was applied. The free and immobilized cellulase preparations were characterized in terms of optimum pH and temperature. The hydrolysis of cellulose using the free and immobilized cellulase preparations in ILs, using cosolvents such as 1-butyl-3-methylimidazolium acetate ([Bmim][OAc]), 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([Bmim][TfO]), 1-butyl-3-methylimidazolium tetra fluoroborate ([Bmim][BF4]), and 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) were investigated. Thermal stability studies were conducted at 50°C and 70°C for all the cellulase preparations. The reusability studies were performed in a batch type reactor.
Experimental
Materials
Cellulase from T. reesei ATCC26921 (former name T. viride, lyophilized powder, ≥ 45 U/mg solid) was supplied by Worthington Biochemical Corp. (New Jersey, USA). ReliZyme HA403 (medium, particle size 200–500 μm) and Sepabeads EC-EP (medium, particle size 200–500 μm) supports were kindly provided by Resindion S.r.l. (Rome, Italy). (3-aminopropyl)triethoxysilane (3-APTES), carboxymethyl cellulose (CMC), glutaraldehyde solution (50%, w/w) and 3,5-dinitrosalicylic acid (98%) were obtained from Sigma-Aldrich (St. Louis, MO). [Bmim][OAc], [Bmim][BF4], [Bmim][TfO], and [Bmim][PF6] were purchased from Merck. All the other chemicals used in this study were of analytical grade.
Methods
Modification of ReliZyme HA403 support
ReliZyme HA403 support was activated with glutaraldehyde before the cellulase immobilization, while Sepabeads EC-EP support was used without any modification. The glutaraldehyde activation of ReliZyme HA403 support was performed according to the method detailed by CitationAlptekin et al. (2009).
RSM analysis
The 3-factor and 3-level BBD was applied to optimize the immobilization of T. reesei cellulase onto glutaraldehyde-activated ReliZyme HA403 and Sepabeads EC-EP supports in this study. The parameters were chosen depending on the results of preliminary studies performed, as immobilization pH (X1), immobilization time (X2), and initial protein concentration (X3). The response was the specific activity of each immobilized cellulase preparation. The lowest, middle, and highest levels of parameters are given in . BBD and its analysis were achieved using Design-Expert statistical software (version 8.0.7). A quadratic equation (Equation 1) was used to explain the relationship between the immobilization conditions and specific activity.
Where Y is the predicted response (specific activity of each immobilized cellulase), N is the number of variables, Xi is the independent variable, and β0, βi, βii, and βij are regression coefficients.
Table I. The variables and their lowest, middle, and highest values.
Cellulase immobilization
The immobilizations of cellulase onto modified ReliZyme HA403 support and Sepabeads EC-EP support were carried out at the conditions given in . A total of 0.5 g of modified ReliZyme HA403 support or 0.1 g of Sepabeads EC-EP support was treated with 1 mL of the cellulase solution of various concentrations (0.2, 1.0, or 1.8 mg/mL) prepared to different pH levels (50 mM acetate buffer of pH 5.0, 50 mM phosphate buffer of pH 6.5, or 50 mM phosphate buffer of pH 8.0). The mixtures were gently stirred at various immobilization times (6, 15, or 24 h for Sepabeads EC-EP support and 2, 5, or 8 h for modified ReliZyme HA403 support) at 25°C (). The immobilized cellulase preparations were washed with their immobilization buffers and then stored at 5°C. The protein contents of the filtrates were determined using the Lowry protein assay (CitationLowry et al. 1951). The immobilization yield was calculated according to formula given below:
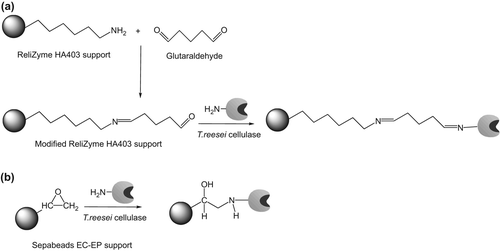
Table II. The results of using 3-factor and 3-level BBD for the optimal conditions of cellulase immobilization.
Measurement of cellulase activity
The activity assay of cellulase was based on the measurement of the amount of reducing sugar released from CMC by the activity of cellulase using 3,5-dinitrosalicylate reagent (DNS). Briefly, 0.5 mL of the free cellulase solution (1 mg/mL) in citrate buffer (100 mM, pH 4.0), and 0.5 mL of CMC solution [1% (w/v)] prepared in the citrate buffer (100 mM, pH 4.0) were incubated at 45°C for 30 min. In case of the immobilized cellulase, 5 mg of the immobilized cellulase preparations, 0.5 mL of citrate buffer (100 mM, pH 4.0), and 0.5 mL of 1% CMC solution were incubated at 45°C for 30 min. After that, the reaction was stopped by the addition of 1 mL of DNS reagent. The resulting mixture was boiled for 15 min and the amount of reducing sugar formed was measured spectrophotometrically at 530 nm. A blank reaction without enzyme was performed under the same conditions. One unit of cellulase specific activity was described as the amount of formed reduced sugar (μmole) per minute per mg of protein.
Characterization of free and immobilized cellulase preparations
The effect of pH on activities of free and immobilized cellulase preparations was assayed in different buffers (50 mM acetate buffers of pH = 3.5 - 5.5, 50 mM citrate buffer of pH = 6.0, 50 mM phosphate buffers of pH = 6.5 and 7.0) at 45°C. The free and immobilized cellulase activities were measured at temperatures ranging from 25 to 60°C, at their optimal pH values and buffer concentrations. The thermal stabilities of all cellulase preparations were tested at 50 and 70°C. The blank reactions without free or immobilized enzyme were performed for each tested condition. The initial activity of each cellulase preparation was assigned as 100%, and the relative activities of the cellulase preparations were measured at different time intervals. The relative activity values (%) in each characterization step were calculated individually for each preparation by dividing the activity of sample at a condition tested by the maximum activity obtained.
Field emission-scanning electron microscopy
Field emission-scanning electron microscopy (FE-SEM) images of the immobilized cellulase preparations were taken using a ZEISS SUPRA 55 field emission scanning electron microscope. Before analysis, the samples were coated with platinum in vacuum using a Quorum Q150R ES sputter coater.
Enzymatic hydrolysis in IL/buffer media
The activities of free and immobilized cellulase preparations were measured in [Bmim][OAc], [Bmim][BF4], [Bmim][TfO], and [Bmim][PF6] as cosolvents under the optimal pH and temperature levels for each preparation. The reaction was started by the addition of 0.5 mL of 1% CMC solution prepared in 25% volume ratio of IL/buffer medium. After 30 min, the reaction was stopped by the addition of 1 mL of DNS reagent and the amount of reducing sugar formed was measured spectrophotometrically at 530 nm. To investigate the effect of volume ratio of the IL/buffer medium, a series of 1% CMC solutions containing different ratios of [Bmim][PF6], such as 2.5, 5, 12.5, 25, 37.5 and 50% (v/v) were prepared and the activities were measured as mentioned above. The blank reactions without free or immobilized enzyme were performed for each condition tested. The activities of free and immobilized cellulase preparations in pure buffer were assigned a value of 100%.
Reusability of immobilized cellulase preparations
The operational stabilities of the immobilized cellulase preparations were tested in a screw-capped glass tube for 1 h with 1% CMC. After each run, the immobilized cellulase preparations were separated by filtration, washed with the buffer before the next cycle, and then reintroduced into another fresh reaction medium.
Results and discussion
Immobilization of cellulase
In this study, the conditions of immobilization of T. reesei cellulase onto the Sepabeads EC-EP support and the glutaraldehyde-activated ReliZyme HA403 support were optimized using a 3-factor and 3-level BBD, and the results have been given in . According to the experimental results, the regression coefficients of Equation 1 were estimated using Design-Expert software, and have been given in Equation 2 and Equation 3, respectively for Sepabeads EC-EP support and the glutaraldehyde-activated ReliZyme HA403 support:
Where Y is the predicted response (specific activity of immobilized cellulase), X1 is the immobilization pH, X2 is the immobilization time (h) and X3 is the initial protein concentration (mg/mL). The values of coefficient of determination (R2) were calculated as 0.85 and 0.96, respectively, for the cellulase immobilized onto the Sepabeads EC-EP support and the modified ReliZyme HA403 support. The model proposed for the immobilization of cellulase onto the Sepabeads EC-EP support was insignificant (p > 0.05), whereas the model proposed for the immobilization of cellulase onto modified ReliZyme HA403 support was significant (p < 0.05) (Supplementary Table I to be found online at http://www.informahealthcare.com/doi/abs/10.3109/21691401.2015.1024842). To carry out further studies, therefore, the optimization studies continued with the cellulase immobilized onto modified ReliZyme HA403 support.
shows the response surface plot of the effect of immobilization pH and immobilization time on the specific activity of immobilized cellulase preparation at the fixed initial cellulase concentration of 1 mg/mL. The specific activity of immobilized cellulase decreased from 25.8 (run number 9) to 21.6 U mg protein− 1 (run number 4). Although the immobilization pH was increased to 8.0 and the other parameters were maintained at the same level, the specific activity of immobilized cellulase was constant (run number 7). This situation may be attributed to the cellulase retaining its active conformation against the change of pH.
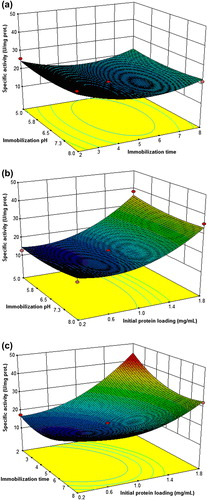
shows the response surface plot of the effect of immobilization pH and initial protein concentration on the specific activity of the immobilized cellulase at the fixed immobilization time of 5 h. The specific activity of immobilized cellulase was determined as 12.6 U/mg protein when the immobilization pH was 5.0 and initial protein concentration was 0.2 mg/mL (run number 6). Increasing the initial protein concentration from 0.2 to 1.8 mg/mL caused an increase in the specific activity of immobilized cellulase, and the specific activity was measured as 38.1 U/mg protein when the immobilization pH was 5.0 and initial protein concentration was 1.8 mg/mL (run number 14). The specific activity of immobilized cellulase decreased to 35.3 U/mg protein (run number 2) when the immobilization pH was 8.0 and initial protein concentration was 1.8 mg/mL.
When the immobilization pH was 5.0 and the immobilization time was 2 h, the specific activity of immobilized cellulase was determined as 25.8 U/mg protein (run number 9) for the initial protein concentration of 1.0 mg/mL (). When the immobilization pH was increased to 8.0 and the other immobilization conditions were kept constant, the specific activity of immobilized cellulase was measured as 27.1 U/mg protein (run number 12). Increasing the immobilization time from 2 to 8 h, the specific activity of immobilized cellulase was 21.6 U/mg protein (run number 7).
The optimal conditions for immobilization of cellulase onto modified ReliZyme HA403 support were determined as a pH of 6.5, a time of 2 h, and an initial protein concentration of 1.8 mg/mL, after the desirability analysis. The further characterization studies were continued with the cellulase preparation immobilized onto modified ReliZyme HA403 support, prepared at the aforementioned conditions. The cellulase preparation immobilized onto Sepabeads EC-EP support was also used in the characterization studies. The optimal conditions for cellulase immobilization onto Sepabeads EC-EP support were taken as a pH of 6.5, a time of 6 h, and an initial protein concentration of 1.8 mg/mL, based on the specific activity and immobilization yield.
Characterization of cellulase preparations
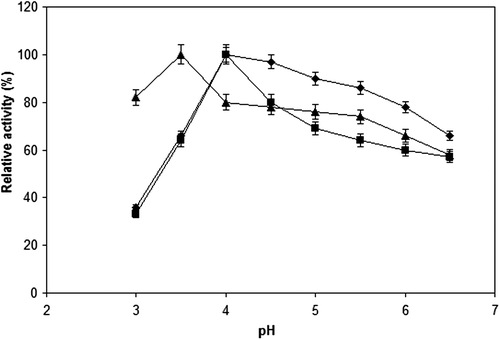
The activities of free and immobilized cellulase preparations were measured in the pH range of 3.0–6.5. At pH 3.0, the residual activities of the free cellulase, cellulase immobilized onto Sepabeads EC-EP support, and cellulase immobilized onto modified ReliZyme HA403 support were observed as 36, 33 and 82%, respectively. As shown in , the maximum activity was observed at pH 3.5 for cellulase immobilized onto modified ReliZyme HA403 support, whereas the maximum activities of both free cellulase and cellulase immobilized onto Sepabeads EC-EP support were observed at pH 4.0. CitationYildirim et al. (2011) reported that the silanization of the support with 3-APTES may result in positively charged free amino groups on the support, depending on the degree of silanization. Therefore, more OH− ions are attracted by the immobilized enzyme, resulting in shift of the optimal pH of the immobilized enzyme. The relative activities of all the cellulase preparations decreased at the pH values above their optimal pH values, and the relative activities were measured as 66, 57, and 58% respectively, at pH 6.5.
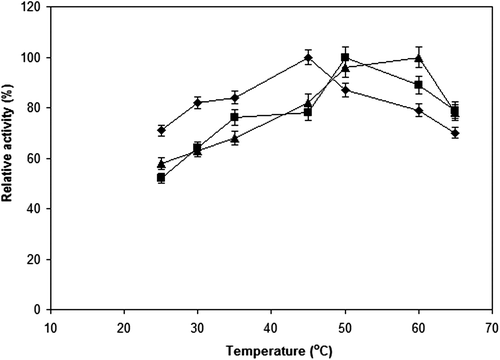
The free cellulase had a maximum activity at 45°C, whereas the maximum activities of cellulase preparations immobilized onto Sepabeads EC-EP support and onto modified ReliZyme HA403 support were observed at 50 and 60°C, respectively. The relative activity of free cellulase decreased above 45°C, and 70% of its maximum activity was measured at 65°C. In case of the immobilized cellulases, 79 and 78% of their maximum activities were retained at the same temperature for the cellulase preparations immobilized onto Sepabeads EC-EP support and onto modified ReliZyme HA403 support, respectively (). CitationInce et al. (2012) reported that the optimum temperature values of both free T. viride cellulase and its form immobilized onto polyaniline-coated poly(styrene-divinylbenzene) were 40°C. CitationKhoshnevisan et al. (2011) reported that the optimum temperature values of both free T. viride cellulase and the corresponding preparation immobilized onto supermagnetic nanoparticles were 60°C.
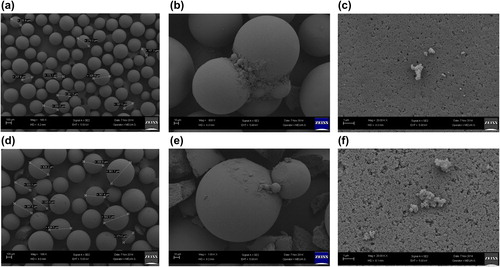
(a–f) shows the FE-SEM images of Sepabeads EC-EP and modified ReliZyme HA403 supports before and after cellulase immobilization. Before cellulase immobilization, both the supports remained spherical in structure. After the cellulase immobilization, both the support surfaces were filled with protein aggregates.
Activity of cellulase preparations in ILs/buffer media
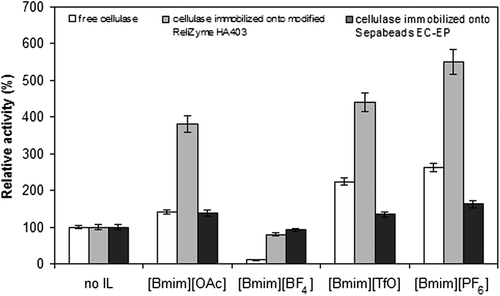
The cellulose solubility and also the activity of cellulase are highly affected by the composition and concentration of ILs (CitationSwatloski et al. 2002, CitationTurner et al. 2003). To investigate the effect of ILs on the activities of free and immobilized cellulase preparations, the reaction was tested in ILs/buffer media (25%, v/v). As shown in , the hydrolytic activities were significantly dropped in [Bmim][BF4]/buffer mixture for all the cellulase preparations, especially for the free cellulase. Only 10% of relative activity was observed for the free cellulase when [Bmim][BF4] was used as cosolvent; however, the observed activities were 92% and 80%, respectively for cellulase immobilized onto Sepabeads EC-EP and onto modified ReliZyme HA403 supports in the same medium. When [Bmim][OAc], [Bmim][TfO] and [Bmim][PF6] were used as cosolvents, the cellulase activities increased depending on the buffer for all the preparations, and the highest increase was observed when [Bmim][PF6] was used as the cosolvent. The activities measured in [Bmim][PF6]/buffer medium were around 2.6-, 1.6-, and 5.5-fold higher than those of the activities in buffer medium. The glutaraldehyde spacer may confer flexibility on the cellulase, and therefore, the cellulase immobilized onto modified ReliZyme HA403 support can show higher activity than the cellulase immobilized onto Sepabeads EC-EP support. CitationPaljevac et al. (2006) reported that cellulase from Humicola insolens (Celluzyme 0.7T) showed better activity in [Bmim][PF6], when compared to that in [Bmim][BF4].
shows the influence of volume ratio of [Bmim][PF6]/buffer medium on the hydrolysis of CMC. When the volume ratio of [Bmim][PF6] was increased from 2.5 to 25%, the CMC hydrolysis catalyzed by the free and immobilized cellulase preparations increased and reached a maximum in [Bmim][PF6]/buffer medium containing 25% of [Bmim][PF6] (v/v). The further increase in [Bmim][PF6] resulted in a decrease in the activity of all the cellulase preparations. Similar results have also been reported in the literature. CitationKamiya et al. (2008) reported that the cellulose hydrolysis yield catalyzed by T. reesei cellulase was increased in a ¼(v/v) mixture of 1-ethyl-3-methylimidazolium diethyl phosphate and water. CitationJones and Vasudevan (2010) demonstrated that a 2.7-fold higher initial reaction rate was observed by the addition of 2% (v/v) 1-ethyl-3-methylimidazolium diethyl phosphate during hydrolysis of cellulose, as compared to none using ionic liquid medium.
![Figure 7. The hydrolysis of CMC in different volume ratios of [Bmim][PF6]/buffer media. The pH/temperature values were 4.0/45°C, 4.0/50°C and 3.5/60°C, respectively, for the free cellulase, cellulase immobilized onto Sepabeads EC-EP, and onto modified ReliZyme. Values for 100% activities are 170, 66, and 60 U/mg protein, respectively, for the free cellulase, cellulase immobilized onto Sepabeads EC-EP, and onto ReliZyme HA403.](/cms/asset/0a9dd0cd-889b-4224-aec4-02004fdd06d7/ianb_a_1024842_f0007_b.gif)
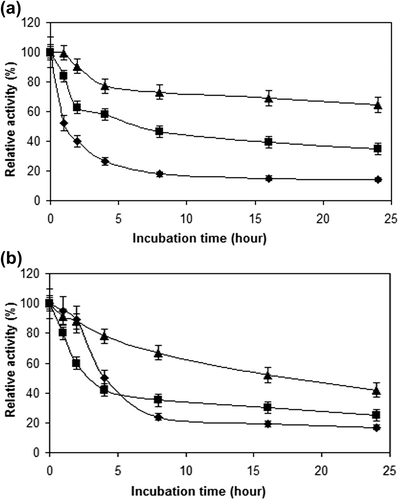
shows the thermal stabilities of all the cellulase preparations at 50 and 70°C. The free cellulase retained around 14% of its initial activity at the end of the 24-h incubation time at 50°C, whereas cellulases immobilized onto Sepabeads EC-EP and onto modified ReliZyme HA403 supports retained 35% and 65% of their initial activities, respectively, at the same incubation time and temperature. At 70°C, the retained activities were 12, 25 and 42%, respectively, for the free cellulase, cellulase immobilized onto Sepabeads EC-EP, and cellulase immobilized onto ReliZyme HA403. CitationWang et al. (2013) reported that the free cellulase lost more than 50% of its initial activity at the end of the 2-h incubation time at 70°C. However, the cellulases immobilized onto PAMAM-grafted silica by adsorption and cross-linking methods retained 53% and 66% of their initial activities after a 3-h incubation time at the same temperature.
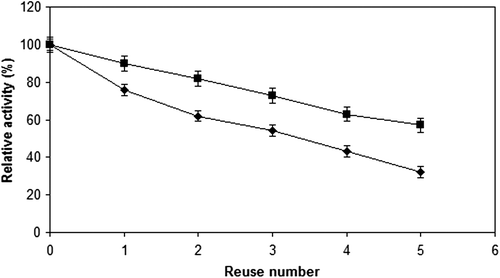
As shown in , the retained activities of immobilized cellulase preparations were gradually decreased depending on the reuse number. The initial activities retained were 32% and 57%, respectively for cellulase preparations immobilized onto Sepabeads EC-EP support and onto modified ReliZyme HA403 support, after 5 reuses. CitationDinçer and Telefoncu (2007) reported that A. niger cellulase immobilized onto modified polyvinyl alcohol-coated chitosan beads retained 52% of its initial activity after eight reuses. CitationHung et al. (2011) showed that the retained activity of cellulase immobilized onto electrospun polyacrylonitrile nanofibrous membranes reduced gradually and was around 55% at the fifth cycle.
Conclusion
In this study, RSM was conducted to optimize immobilization conditions of T. reesei cellulase onto modified ReliZyme HA403 support and onto Sepabeads EC-EP support. The equation proposed for the immobilization conditions of T. reesei cellulase onto modified ReliZyme HA403 support predicted the responses with a statistically significant accuracy, whereas the equation proposed for the immobilization conditions of T. reesei cellulase onto Sepabeads EC-EP support did not predict the responses with a statistically significant accuracy. The optimal working pH/temperature values were determined as 4.0/45°C, 4.0/50°C and 3.5/60°C, respectively, for the free cellulase, cellulase immobilized onto Sepabeads EC-EP support, and cellulase immobilized onto modified ReliZyme HA403 support. The use of ILs such as [Bmim][OAc], [Bmim][TfO], and [Bmim][PF6] as cosolvents appeared to be an attractive option for CMC hydrolysis. The highest hydrolytic activities were determined in 25% [Bmim][PF6]/buffer (v/v) medium for all the cellulase preparations. The cellulase immobilized onto modified ReliZyme HA403 support showed better thermal performance and reusability than the cellulase immobilized onto Sepabeads EC-EP support.
Supplementary material available online
ianb_a_1024842_sm1139.pdf
Download PDF (96.2 KB)Acknowledgement
The authors are grateful to Resindion S.r.l. (Rome, Italy) for the generous donation of Sepabeads EC-EP and ReliZyme HA403 supports.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by Research Grants of FEF 2010 D 1 from the University of Cukurova.
References
- Alftrén J, Hobley TJ. 2014. Immobilization of cellulase mixtures on magnetic particles for hydrolysis of lignocellulose and ease of recycling. Biomass Bioenergy. 65:72–78.
- Alptekin O, Tükel SS, Yildirim D, Alagöz D. 2009. Characterization and properties of catalase immobilized onto controlled pore glass and its application in batch and plug-flow type reactors. J Mol Catal B: Enzym. 58:124–131.
- Anish R, Rahman MS, Rao M. 2007. Application of cellulases from an alkalothermophilic Thermomonospora sp. in biopolishing of denims. Biotechnol Bioeng. 96:48–56.
- Bayraktar H, Serilmez M, Karkaş T, Çelem EB, Önal S. 2011. Immobilization and stabilization of α-galactosidase on Sepabeads EC-EA and EC-HA. Int J Biol Macromol. 49:855–860.
- Bayramoglu G, Senkal BF, Arica MY. 2013. Preparation of clay–poly(glycidyl methacrylate) composite support for immobilization of cellulase. App Clay Sci. 85:88–95.
- Cerdobbel A, Desmet T, De Winter K, Maertens J, Soetaert W. 2010. Increasing the thermostability of sucrose phosphorylase by multipoint covalent immobilization. J Biotechnol. 150:125–130.
- Dinçer A, Telefoncu A. 2007. Improving the stability of cellulase by immobilization on modified polyvinyl alcohol coated chitosan beads. J Mol Catal B: Enzym. 45:10–14.
- Ebner G, Vejdovszky P, Wahlström R, Suurnäkki A, Schrems M, Kosma P, et al. 2014. The effect of 1-ethyl-3-methylimidazolium acetate on the enzymatic degradation of cellulose. J Mol Catal B: Enzym. 99:121–129.
- Farrell AE, Plevin RJ, Turner BT, Jones AD, O’Hare M, Kammen DM. 2006. Ethanol can contribute to energy and environmental goals. Science. 311:506–508.
- Fernandez-Lafuente R. 2009. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb Technol. 45:405–418.
- Ghazi I, De Segura AG, Fernández-Arrojo L, Alcalde M, Yates M, Rojas-Cervantes ML, et al. 2005. Immobilisation of fructosyltransferase from Aspergillus aculeatus on epoxy-activated Sepabeads EC for the synthesis of fructo-oligosaccharides. J Mol Catal B: Enzym. 35:19–27.
- Hsu C-L, Chang K-S, Lai M-Z, Chang T-C, Chang Y-H, Jang H-D. 2011. Pretreatment and hydrolysis of cellulosic agricultural wastes with a cellulase-producing Streptomyces for bioethanol production. Biomass Bioenergy. 35:1878–1884.
- Hung TC, Fu CC, Su CH, Chen JY, Wu WT, Lin YS. 2011. Immobilization of cellulase onto electrospun polyacrylonitrile (PAN) nanofibrous membranes and its application to the reducing sugar production from microalgae. Enzyme Microb Technol. 49:30–37.
- Ince A, Bayramoglu G, Karagoz B, Altintas B, Bicak N, Arica MY. 2012. A method for fabrication of polyaniline coated polymer microspheres and its application for cellulase immobilization. Chem Eng J. 189–190:404–412.
- Jacobs JL, McAllan AB. 1991. Enzymes as silage additives. 1. Silage quality, digestion, digestibility and performance in growing cattle. Grass Forage Sci. 46:63–73.
- Jones PO, Vasudevan PT. 2010. Cellulose hydrolysis by immobilized Trichoderma reesei cellulase. Biotechnol Lett. 32:103–106.
- Kamiya N, Matsushita Y, Hanaki M, Nakashima K, Narita M, Goto M, Takahashi H. 2008. Enzymatic in situ saccharification of cellulose in aqueous-ionic liquid media. Biotechnol Lett. 30:1037–1040.
- Khoshnevisan K, Bordbar A-K, Zare D, Davoodi D, Noruzi M, Barkhi M, Tabatabaei M. 2011. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem Eng J. 171:669–673.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Mateo C, Grazú V, Pessela BCC, Montes T, Palomo JM, Torres R, et al. 2007. Advances in the design of new epoxy supports for enzyme immobilization-stabilization. Biochem Soc Trans. 35:1593–1601.
- Nadeem MT, Butt MS, Anjum FM, Asgher M. 2009. Improving bread quality by carboxymethyl cellulase application. Int J Agric Biol. 11:727–730.
- Ogeda TL, Silva IB, Fidale LC, El Seoud OA, Petri DF. 2012. Effect of cellulose physical characteristics, especially the water sorption value, on the efficiency of its hydrolysis catalyzed by free or immobilized cellulase. J Biotechnol. 157:246–252.
- Paljevac M, Habulin M, Knez Z. 2006. Ionic liquids as (co) solvents for enzymatic reactions. Chem Ind Chem Eng Q. 12:181–186.
- Pandey A, Soccol CR, Nigam P, Soccol VT. 2000. Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresour Technol. 74:69–80.
- Plechkova NV, Seddon KR. 2008. Applications of ionic liquids in the chemical industry. Chem Soc Rev. 37:123–150.
- Qin Y, Wei X, Liu X, Wang T, Qu Y. 2008. Purification and characterization of recombinant endoglucanase of Trichoderma reesei expressed in Saccharomyces cerevisiae with higher glycosylation and stability. Protein Expr Purif. 58:162–167.
- Swatloski RP, Spear SK, Holbrey JD, Rogers RD. 2002. Dissolution of cellose with ionic liquids. J Am Chem Soc. 124:4974–4975.
- Tükel S, Sahin PB, Yildirim D. 2013. Optimization of lipase-catalyzed synthesis of fructose stearate using response surface methodology. Artif Cells Nanomed Biotechnol. 41:344–351
- Turner MB, Spear SK, Huddleston JG, Holbrey JD, Rogers RD. 2003. Ionic liquid salt-induced inactivation and unfolding of cellulase from Trichoderma reesei. Green Chem. 5:443 - 447.
- van Rantwijk F, Sheldon RA. 2007. Biocatalysis in ionic liquids. Chem Rev. 107:2757–2785.
- van Wyk JPH, Mohulatsi M. 2003. Biodegradation of wastepaper by cellulase from Trichoderma viride. Bioresour Technol. 86:21–23.
- Wang S, Su P, Ding F, Yang Y. 2013. Immobilization of cellulase on polyamidoamine dendrimer-grafted silica. J Mol Catal B: Enzym. 89:35–40.
- Yildirim D, Tukel SS, Alagoz D, Alptekin O. 2011. Preparative-scale kinetic resolution of racemic styrene oxide by immobilized epoxide hydrolase. Enzyme Microb Technol. 49:555–559.
- Yildirim D, Tukel SS. 2013. Immobilized Pseudomonas sp. lipase: A powerful biocatalyst for asymmetric acylation of (+/−)-2-amino-1-phenylethanols with vinyl acetate. Process Biochem. 48:819–30.
- Yildirim D, Tükel SS, Alptekin Ö, Alagöz D. 2014. Optimization of immobilization conditions of Mucor miehei lipase onto Florisil via polysuccinimide spacer arm using response surface methodology and application of immobilized lipase in asymmetric acylation of 2-amino-1-phenylethanols. J Mol Catal B: Enzym. 100:91–103.