Abstract
We have demonstrated a permeation-enhancing effect of deoxycholic acid (DCA), the bile acid, in diabetic rats. In this study, we designed DCA-based microcapsules for the oral delivery of the antilipidemic drug probucol (PB), which has potential antidiabetic effects. We aimed to further characterize these microcapsules and examine their pH-dependent release properties, as well as the effects of DCA on their stability and mechanical strength at various pH and temperature values. Using the polymer sodium alginate (SA), we prepared PB-SA (control) and PB-DCA-SA (test) microcapsules. The microcapsules were examined for drug content, size, surface composition, release, Micro-CT cross-sectional imaging, stability, Zeta potential, mechanical strength, and swelling characteristics at different pH and temperature values. The microencapsulation efficiency and production yield were also examined. The addition of DCA resulted in microcapsules with a greater density and with reduced swelling at a pH of 7.8 and at temperatures of 25°C and 37°C (p < 0.01). The size, surface composition, production yield, and microencapsulation efficiency of the microcapsules remained similar after DCA addition. PB-SA microcapsules produced multiphasic PB release, while PB-DCA-SA microcapsules produced monophasic PB release, suggesting more controlled PB release in the presence of DCA. The PB-DCA-SA microcapsules showed good stability and a pH-sensitive uniphasic release pattern, which may suggest potential applications in the oral delivery of PB in diabetes.
Introduction
Bile acids are naturally produced in humans and have shown health benefits through their endrocrinological, metabolic, microfloral,and other known and unknown effects (CitationAl-Salami et al. 2007, CitationCalasan et al. 2012, CitationNegrulj et al. 2013). Studies in our laboratory have demonstrated beneficial antidiabetic effects of bile acids, alone or combined with other agents, in vivo (CitationMikov et al. 2006, Citation2007, Citation2008, Citation2012, CitationAl-Salami et al. 2008a, Citation2012) and ex vivo (CitationAl-Salami et al. 2008b, Citation2008c, Citation2008d, Citation2008e, Citation2008f, Citation2009, CitationCalasan et al. 2012), or as membrane-stabilizing molecules in vitro (CitationMooranian et al. 2014a, Citation2014b, Citation2014c, Citation2014d, Citation2014e, Citation2014f). In a recent study, we showed (in vivo) a permeation-enhancing effect of the bile acid deoxycholic acid (DCA), when combined with the antidiabetic drug gliclazide, and administered to diabetic rats (CitationLalic-Popovic et al. 2013). In a follow-up study, we designed a bile acid-based microcapsule platform for the targeted oral delivery of lipophilic drugs in diabetic animals (CitationMooranian et al. 2014a, Citation2014b, Citation2014c, Citation2014d, Citation2014c, Citation2014f). One of the applications of this microcapsule platform is in improving the oral delivery of lipophilic drugs with potential antidiabetic effects (CitationNegrulj et al. 2013). A good approach is to optimize the oral delivery of lipophilic drugs that have already shown desirable antidiabetic effects such as lowering of blood cholesterol and reducing the formation of atherosclerotic plaques (CitationNegrulj et al. 2013). A good example is the microencapsulation of the anti-hyperlipidemic drug, probucol (PB) (CitationMooranian et al. 2014a, Citation2014d).
PB is a highly lipophilic drug with beneficial antidiabetic effects (CitationMooranian et al. 2014a, Citation2014d, CitationNegrulj et al. 2013). These effects include reduction of cholesterol, protection of β-cells of the pancreas, and antiradical and antioxidant effects (CitationWu et al. 2013, CitationYamashita and Matsuzawa 2009). Accordingly, this study aimed to study the suitability of newly designed PB-DCA microcapsules for the oral delivery of PB, in vitro. Sodium alginate (SA) was used as a biodegradable, biocompatible polymer (CitationMooranian et al. 2014a, Citation2014d, CitationNegrulj et al. 2013). The study examined the morphology, shape, size, drug content, surface composition, Micro-CT cross-sectional imaging, swelling properties, release kinetics, mechanical strength, Zeta potential, and the density of the microcapsules. The production yield and the encapsulation efficiency were also examined. In order to investigate the effect of DCA on the PB microcapsules, the study covered both types of microcapsules, PB-SA (as control) and PB-DCA-SA (as test).
Materials and methods
Materials
Probucol (PB, 99%), sodium alginate (SA, 99%) and deoxycholic acid (DCA, 99%) were purchased from Sigma Chemical Co, USA. Calcium chloride dihydrate (CaCl2.2H2O, 98%) was obtained from Scharlab S.L, Australia. All solvents and reagents were supplied by Merck (Australia), and were of HPLC grade and used without further purification or modification.
Drugs preparations
Stock suspensions of PB (20 mg/ml) and DCA (1 mg/ml) were prepared by adding the powder to 10% Ultra water-soluble gel (CitationMooranian et al. 2014a, Citation2014d). The CaCl2 stock solution (2%) was prepared by adding CaCl2 powder to HPLC water. All preparations were mixed thoroughly at room temperature for 4 h, stored in the refrigerator, and used within 48 h of preparation.
Preparation of microcapsules
Microcapsules of PB-loaded sodium alginate (PB-SA) were prepared using a Büchi-based microencapsulating system (BÜCHI Labortechnik, Switzerland) (CitationMooranian et al. 2014a, Citation2014b, Citation2014c, Citation2014d, Citation2014e). Polymer solutions containing SA and PB with or without DCA were made up to a final concentration (of PB-DCA-SA) in a ratio of 1:3:30 respectively, as described in our previous studies (CitationMooranian et al. 2014a, Citation2014b, Citation2014c, Citation2014d, Citation2014e, Mooranian et al.).
Characterization of loaded microcapsules
Drug content, production yield, microencapsulation efficiency, and stability studies
Drug content, production yield, and microencapsulation efficiency. One gram of microcapsules was carefully weighed, ground, and dissolved in 200 ml of phosphate buffer (pH 7.8), and the suspension was stirred by a magnetic stirrer for 6 h. Aliquots of the dissolution medium (2 ml) were withdrawn at predetermined time points (every 200 s), filtered through a 0.22 μm Millipore filter, and analyzed (CitationMooranian et al. 2014a, Citation2014b, Citation2014c, Citation2014d, Citation2014e, Mooranian et al.).
PB concentrations were calculated from the calibration curve (CitationMooranian et al. 2014a). All analyses were carried out in triplicate (n = 3). The drug contents, production yield, and microencapsulation efficiency were calculated as previously described (CitationMooranian et al. 2014a).
Zeta potential and size analysis. To determine the electrokinetic stability and size uniformity of the microcapsules in the dispersion system, Zeta potential and size distribution for the microencapsulated formulation of PB-SA and PB-DCA-SA were measured by photon correlation spectroscopy using a Nano ZS (Malvern Instruments, Malvern, UK) and by the Mie and Fraunhofer scattering technique using Mastersizer 2000 (Malvern Instruments, Malvern, UK) (CitationMooranian et al. 2014a, Citation2014b, Citation2014c, Citation2014d, Citation2014e, Mooranian et al.). All determinations were performed in triplicate. Results are reported as mean ± SD.
Optical microscopy (OM). Morphological characteristics and particle size analysis were determined utilizing a Nikon YS2-H mounted with a ToupTek photonics FMA050 fixed calibrated microscope adaptor (Japan) (n = 3) (CitationMooranian et al. 2014a, Citation2014b, Citation2014c, Citation2014d, Citation2014e, Mooranian et al.).
Energy Dispersive X-Ray (EDXR) spectroscopy studies. The surface composition of the microcapsules was examined using the EDXR (Oxford Instruments, INCA X-Act, USA) (CitationMooranian et al. 2014c, Citation2014d, Mooranian et al.).
X-ray microtomography (Micro-CT) scanning studies. The morphology and microstructure of the respective microcapsules were examined using a SkyScan 1172A Micro-CT (Kontich, Belgium) machine running Control software (Version 1.5 Build 8) and assembled to a Hamamatsu 10 Mp camera (Hamamatsu, Japan) with a pixel size of 11.48 μm. The scans were acquired under a current of 100 μA and an accelerating voltage of 100 kV using rotation steps of 0.3 degrees for all scans. Analysis was undertaken using a scan exposure time of 1180 milliseconds, and 16 frames were averaged for each exposure in order to minimize background noise/interference. Freshly made microcapsules from the respective formulations were prepared for analysis, and scanning was conducted in triplicate.
Swelling Studies. To determine the swelling properties of the microcapsules, 50mg of dry microcapsules were weighed and placed in 20 mL of buffer, under conditions of two pH values (3 and 7.8) and two temperatures (25°C and 37°C) for 6 h (CitationMooranian et al. 2014a, Citation2014b, Citation2014e).
Drug release studies (in-vitro dissolution test). A weighed sample (2 g) of PB and DCA-loaded microcapsules were suspended in 200 ml of phosphate buffer solution at pH values of 1.5, 3, 6, and 7.8 for 6 h, as appropriate (CitationMooranian et al. 2014a, Citation2014e). The dissolution medium was stirred at 200 rpm. All the experiments were carried out at 25°C. All absorbances of the solution were measured every 30 min using our Hewlett Packard-based Time-Controlled UV-Spec mounted with a close-loop flow system (CitationMooranian et al. 2014a, Citation2014e). All analyses were carried out in triplicate (n = 3).
Mechanical resistance testing
In order to test the mechanical stability of the microcapsules, mechanical resistance testing was carried out using a Boeco Multishaker PSU 20 (Boeco Company, Hamburg, Germany) over a 24-h period (CitationMooranian et al. 2014b).
Physical and chemical stability. The stability test was carried out by placing predetermined amounts of freshly prepared microcapsules onto sterile Petri dishes (30 microcapsules in each) and storing them in thermostatically controlled ovens (environmental stability chambers) at 20°C, 5°C, 25°C, and 40°C, with relative humidity set at 35%, for a period of 72 h. The experiment was conducted using a stability chamber (Angelantoni Environmental and Climatic Test Chamber, Italy) (CitationMooranian et al. 2014a, Citation2014b, Citation2014e).
Statistical analysis
Values are expressed as means ± SD. Drug content, production yield, and microencapsulation efficiency were assessed using the Student's t-test. Statistical analysis was performed using a two-way ANOVA with Tukey HSD post-hoc comparison of means only when the associated main effect or interaction was statistically significant. The best fit model was derived using GraphPad Prism software (V6; GraphPad Software, Inc., USA). Statistical significance was set at p < 0.05.
Results and discussion
Drug content, production yield, microencapsulation efficiency, Zeta potential, and microcapsule size
shows the drug content (%), production yield, encapsulation efficiency, Zeta potential, and size of the PB-SA (control) and PB-DCA-SA (test) microcapsules. Data show that the addition of DCA only affected Zeta potential in reducing the surface charge from − 72.9 ± 2 to − 20.6 ± 1 (p < 0.01). This suggests an ionic interaction between the DCA and the polymer SA. Data also suggest that DCA addition did not have any detrimental effect on the microcapsules’ size or drug content, or the efficiency of the microencapsulation process.
Table I. Drug contents, production yield, encapsulation efficiency, Zeta potential, and mean particle size of PB-SA and PB-DCA-SA microcapsules. n = 3, data are averages ± SD. **p < 0.01.
Optical microscopy
The PB-SA () and PB-DCA-SA microcapsules were analyzed for their particle size and morphology (). Both microcapsules showed sphericity and homogeneity, with similar sizes, and the diameter of both microcapsules averaged around 850 μm. This suggests that the addition of DCA did not adversely change the size or morphology of the microcapsules.
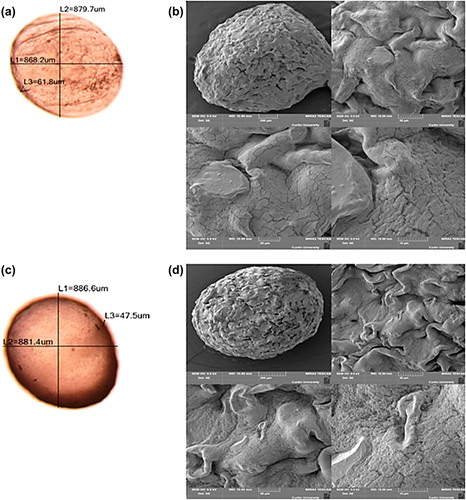
EDXR studies
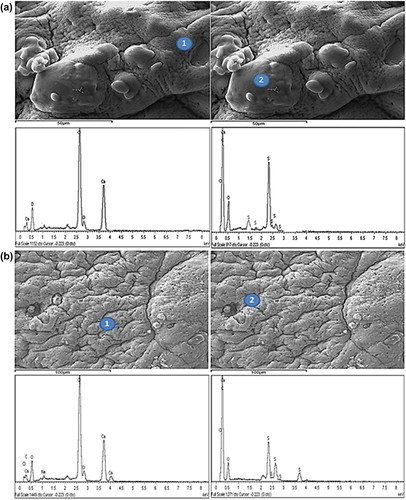
Microcapsule surface analysis was undertaken for both PB-SA and PB-DCA-SA microcapsules using EDXR technology. This analytical technique allows for the determination of the elemental composition of microcapsule surfaces, since each element displays unique energy-dependent X-ray spectroscopic patterns (CitationTam et al. 2005). shows an example of both PB-SA and PB-DCA-SA microcapsule surfaces with the corresponding surface sites where EDXR analysis was conducted. Surface analysis of both microcapsules showed similar elemental contents. This suggests that the bile acid, DCA, is distributed within the microcapsules and not on the surface, which means the pH-targeted delivery of the PB and DCA will take place only after the microcapsule membrane dissociates and its contents are released.
X-ray microtomography (Micro-CT) scanning studies
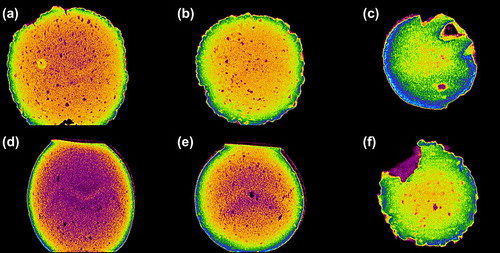
Computer-assisted X-ray Micro-CT scanning is often used to determine the microstructure, internal complexity, and morphology of alginate-based microencapsulated systems (CitationArifin et al. 2011, Citation2012). depicts images of a PB-SA microcapsule, and depicts images of a PB-DCA-SA microcapsule at various cross-sectional scan exposure times. Overall, microcapsule shape and morphology were similar across the scanned time points for both microcapsules, but there were notable differences in their internal complexity. Specifically, there was a greater encapsulated density of material in the PB-DCA-SA microcapsules owing to bile acid partitioning within the microcapsule layers. In addition, the PB-DCA-SA microcapsules presented well-defined membrane borders as opposed to the PB-SA microcapsules, and this may be explained by the membrane-stabilizing and structural support properties of the bile acid, which is in line with our previous findings (CitationMooranian et al. 2014a, Citation2014b, Citation2014c, Citation2014d, Citation2014e, Mooranian et al., CitationNegrulj et al. 2013).
Swelling studies
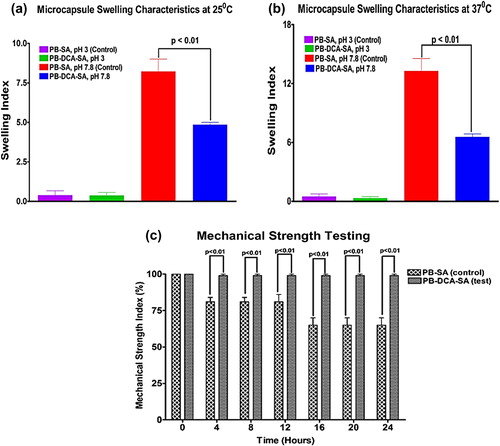
and show that at both 25°C and 37°C, the presence of DCA reduced the swelling properties of the microcapsules only at a high pH (7.8), which suggests that DCA exerts a membrane-stabilizing effect, particularly at higher pH values, thereby exerting a more controlled dissolution pattern. This effect is desirable, as the targeted region of PB release is the lower gastrointestinal tract (CitationMooranian et al. 2014a, CitationNegrulj et al. 2013).
The main mechanisms responsible for water penetration and matrix erosion at higher pH are likely to be from alginate water uptake and subsequent matrix porosity and dissolution (CitationTakka and Cali, 2012, CitationGoh et al. 2012). Clearly, swelling was reduced by the DCA reinforcement. This could be due to the hydrophobic nature of the DCA, repelling water molecules that would otherwise penetrate and rupture the membrane, causing bursts of drug release. On the other hand, at acidic pH 3, alginate does not absorb water, and thus maintains the shape of the microcapsules (CitationGoh et al. 2012, CitationGeorge and Abraham 2006). In order to test the resistance of the alginate matrix to pressure, both with and without DCA, mechanical strength testing was performed.
Mechanical resistance testing
The results of the mechanical strength testing () were in line with swelling data and showed stronger and more intact microcapsules in the presence of DCA compared with control. By the end of a 24-h period, almost all PB-DCA-SA microcapsules remained intact while a significant percentage of PB-SA microcapsules had lost their contents. This may also suggest that PB-DCA-SA microcapsules will be able to withstand pressure as they move through the gastrointestinal tract and until they reach the lower intestine, where PB is maximally absorbed. The desired outcome of the swelling and mechanical resistance testing is that the PB-DCA-SA microcapsules will withstand gut pressure until reaching the lower intestine, where the pH exceeds 6 and the release of PB and DCA commences (CitationMooranian et al. 2014a, CitationNegrulj et al. 2013).
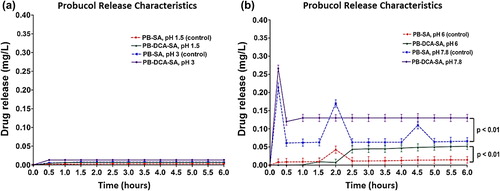
Drug release studies
and show PB release from the microcapsules at four different pH values: 1.5, 3, 6, and 7.8. Data show no release at the lower pH values () while there was little release at pH 6. There is a clear difference in the PB release pattern at pH 6 from both microcapsules, with PB starting to be released at 1.5 h (control) compared with 2.0 h (test). At pH 7.8, PB exhibited a multiphasic release pattern and the addition of DCA caused a more controlled, monophasic release pattern suggesting optimized delivery. This is beneficial as in vivo studies showed that PB has huge inter-individual variation in absorption and this has been associated with its severe side effects and limited clinical applications (CitationNegrulj et al. 2013).
Accelerated stability studies (environmental chamber)
Accelerated stability studies can provide useful information on how the microcapsules react to change in temperature in terms of their physical characteristics (CitationMooranian et al. 2014a, Citation2014b, Citation2014e). The stability testing at various temperatures revealed key differences in the microcapsules’ morphology, size, and texture. While there was no change in morphology, size, or texture of microcapsules at − 20°C and 5°C, there were significant changes at higher temperatures, 25°C and 40°C. At 25°C, the morphology did not change, but the color changed from creamy white to pale yellow. The texture also became more brittle and stiff. At 40°C, the microcapsules changed color to dark yellow, and became very brittle and stiff while the size became smaller and the microcapsules shrunk significantly. The changes at higher temperature can be explained by the loss of moisture contents resulting in a reduction in size. There was also an increase in surface hardness and color change, suggesting significant dehydration of the alginate matrix, and subsequent smaller, harder, and darker microcapsules. The presence of DCA made no difference to the shape, texture, color, or size of the microcapsules, suggesting that DCA did not change or compromise the reaction of microcapsules to heat, especially at higher temperatures.
Conclusion
The study used a host of methods to examine newly designed bile acid-based microcapsules for the orally targeted delivery of a highly lipophilic drug with potential applications in diabetes. The bile acid used was DCA, which has previously shown permeation-enhancing effects, while the lipophilic drug used was PB, which has previously shown protective effects of pancreatic β-cells. In order to examine the morphological and drug-distribution properties, optical as well as SEM, EDXR, and Micro-CT imaging were used, while, in order to examine the stability and physical properties of the formulations, Zeta potential, swelling, and mechanical strength were tested. In order to examine the orally targeted delivery, release studies were performed at various pH and temperature values. Overall, the data show that DCA incorporation into PB microcapsules did not affect drug contents or the size of the microcapsules, but brought about a more dense core, with DCA accumulating mainly in the inside of the microcapsules. Based upon in vitro examination of the microcapsules’ swelling properties, as well as mechanical strength and PB release, our findings suggest that it would be possible to achieve targeted delivery of PB and DCA into the lower intestine, where both are meant to have best absorption. A future study may examine either the impact of other bile acids on PB release or examine the in vivo effects of the microcapsules, using an animal model of diabetes.
Acknowledgments
The authors acknowledge the CHIRI at Curtin University, and the Curtin-seeding grant for the support provided, and also acknowledge the use of equipment and the scientific and technical assistance of the Curtin University Electron Microscope Facility, which has been partially funded by the University, State, and Commonwealth Governments.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al-Salami H, Butt G, Fawcett JP, Tucker IG, Golocorbin-Kon S, Mikov M. 2008a. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet. 33:101–106.
- Al-Salami H, Butt G, Tucker I, Fawcett PJ, Golocorbin-Kon S, Mikov I, Mikov M. 2009. Gliclazide reduces MKC intestinal transport in healthy but not diabetic rats. Eur J Drug Metab Pharmacokinet. 34:43–50.
- Al-Salami H, Butt G, Tucker I, Golocorbin-Kon S, Mikov M. 2012. Probiotics decreased the bioavailability of the bile acid analog, monoketocholic acid, when coadministered with gliclazide, in healthy but not diabetic rats. Eur J Drug Metab Pharmacokinet. 37:99–108.
- Al-Salami H, Butt G, Tucker I, Mikov M. 2008b. Influence of the semisynthetic bile acid MKC on the ileal permeation of gliclazide in vitro in healthy and diabetic rats treated with probiotics. Methods Find Exp Clin Pharmacol. 30:107–113.
- Al-Salami H, Butt G, Tucker I, Mikov M. 2008c. Influence of the semisynthetic bile acid MKC on the ileal permeation of gliclazide in vitro in healthy and diabetic rats treated with probiotics. Methods Find Exp Clin Pharmacol. 30:107–113.
- Al-Salami H, Butt G, Tucker I, Skrbic R, Golocorbin-Kon S, Mikov M. 2008d. Probiotic pre-treatment reduces gliclazide permeation (ex vivo) in healthy rats but increases it in diabetic rats to the level seen in untreated healthy rats. Arch Drug Inf. 1:35–41.
- Al-Salami H, Butt G, Tucker IG, Mikov M. 2008e. The influence of pre-treatment with probiotics on the in vitro ileal permeation of the antidiabetic drug gliclazide, in healthy and diabetic rats. Drug Metabolism Reviews. 40:81–82.
- Al-Salami H, Grant B, Ian T, Mikov M. 2008f. The influence of probiotics pre-treatment, on the ileal permeation of gliclazide, in healthy and diabetic rats. The Archives of Drug Information. 1:35–41.
- Al-Salami H, Kansara H, King J, Morar B, Jayathilaka B, Fawcett PJ, Mikov M. 2007. Bile acids: a bitter sweet remedy for diabetes. The New Zealand Pharmacy Journal. 27:17–20.
- Arifin DR, Long CM, Gilad AA, Alric C, Roux S, Tillement O, et al. 2011. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology. 260:790–798.
- Arifin DR, Manek S, Call E, Arepally A, Bulte JW. 2012. Microcapsules with intrinsic barium radiopacity for immunoprotection and X-ray/CT imaging of pancreatic islet cells. Biomaterials. 33:4681–4689.
- Calasan J, Al-Salami H, Mikov M. 2012. Bile acids and probiotics could help treating diabetes. Febs Journal. 279:267–267.
- George M, Abraham TE. 2006. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan–a review. J Control Release. 114:1–14.
- Goh CH, Heng PWS, Chan LW. 2012. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydrate Polymers. 88:1–12.
- Lalic-Popovic M, Vasovic V, Milijasevic B, Golocorbin-Kon S, Al-Salami H, Mikov M. 2013. Deoxycholic acid as a modifier of the permeation of gliclazide through the blood brain barrier of a rat. J Diabet Res. 2013:598603.
- Mikov M, Al-Salami H, Golocorbin-Kon G. 2012. Potentials and Limitations of Bile Acids and Probiotics in Diabetes Mellitus.
- Mikov M, Al-Salami H, Golocorbin-Kon S, Skrbic R, Raskovic A, Fawcett JP. 2008. The influence of 3alpha,7alpha-dihydroxy-12-keto-5beta-cholanate on gliclazide pharmacokinetics and glucose levels in a rat model of diabetes. Eur J Drug Metab Pharmacokinet. 33:137–142.
- Mikov M, Boni NS, Al-Salami H, Kuhajda K, Kevresan S, Fawcett JP. 2006. Pharmacokinetics and hypoglycaemic effect of 3 alpha, 7 alpha-dihydroxy-12-oxo-5beta-cholanate (MKC) in diabetic rat. Febs Journal. 273:210–210.
- Mikov M, Boni NS, Al-Salami H, Kuhajda K, Kevresan S, Golocorbin-Kon S, Fawcett JP. 2007. Bioavailability and hypoglycemic activity of the semisynthetic bile acid salt, sodium 3alpha,7alpha-dihydroxy-12-oxo-5beta-cholanate, in healthy and diabetic rats. Eur J Drug Metab Pharmacokinet. 32:7–12.
- Mooranian A, Negrulj R, Al-Sallami HS, Fang Z, Mikov M, Golocorbin-Kon S, et al. 2014a. Probucol release from novel multicompartmental microcapsules for the oral targeted delivery in type 2 diabetes. AAPS PharmSciTech. 16:45–52.
- Mooranian A, Negrulj R, Arfuso F, Al-Salami H. 2014b. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic β-cells. Artif Cells Nanomed Biotechnol. 11:1–7.
- Mooranian A, Negrulj R, Chen-Tan N, Al-Sallami HS, Fang Z, Mukkur T, et al. 2014c. Novel artificial cell microencapsulation of a complex gliclazide-deoxycholic bile acid formulation: a characterization study. Drug Des Devel Ther. 8:1003–1012.
- Mooranian A, Negrulj R, Chen-Tan N, Al-Sallami HS, Fang Z, Mukkur T, et al. 2014d. Microencapsulation as a novel delivery method for the potential antidiabetic drug, Probucol. Drug Des Devel Ther. 8:1221–1230.
- Mooranian A, Negrulj R, Mathavan S, Martinez J, Sciarretta J, Chen-Tan N, et al. 2014e. Stability and release kinetics of an advanced gliclazide-cholic acid formulation: the use of artificial-cell microencapsulation in slow release targeted oral delivery of antidiabetics. J Pharm Innov. 9:150–157.
- Mooranian A, Negrulj R, Mathavan S, Martinez J, Sciarretta J, Chen-Tan N, et al. 2014f. An advanced microencapsulated system: a platform for optimized oral delivery of antidiabetic drug-bile acid formulations. Pharm Dev Technol. 0:1–8.
- Negrulj R, Mooranian A, Al-Salami H. 2013. Potentials and limitations of bile acids in type 2 diabetes mellitus: applications of microencapsulation as a novel oral delivery system. J Endocrinol Diabet Mellitus. 1:49–59.
- Takka S, Cali AG. 2012. Bile salt-reinforced alginate-chitosan beads. Pharm Dev Technol. 17:23–29.
- Tam SK, Dusseault J, Polizu S, Ménard M, Hallé J-P, Yahia LH. 2005. Physicochemical model of alginate–poly-L-lysine microcapsules defined at the micrometric/nanometric scale using ATR-FTIR, XPS, and ToF-SIMS. Biomaterials. 26:6950–6961.
- Wu R, Zhang W, Liu B, Gao J, Xiao X-Q, Zhang F, et al. 2013. Probucol ameliorates the development of nonalcoholic steatohepatitis in rats fed high-fat diets. Dig Dis Sci. 58:163–171.
- Yamashita S, Matsuzawa Y. 2009. Where are we with probucol: a new life for an old drug? Atherosclerosis. 207:16–23.
