Abstract
Background: Umbilical cord blood expresses cluster of differentiation (CD) 26, a fraction of CD34 + cells, negatively regulating in vivo homing and engraftment of hematopoietic stem cells. CD26 is highly expressed in various cells such as HSCs, immune cells, fibroblasts, and epithelial cells. It has been shown that inhibition of the CD26 on CD34 + cells improve the efficiency of transplantation of hematopoietic stem and progenitor cells. This study aimed to investigate the effect of key immune cell cytokines on CD26 expansion. Material and methods: Cord blood mononuclear cells were cultured for 21 days using the stem cell factor, fetal liver tyrosine kinase 3 (Flt3) ligand (FL), interleukin (IL) 2, IL7, and IL15. Harvested cells were analyzed by flow cytometry at distinct time points. Results: Our results showed that utilization of IL7 significantly improved the expression of total CD26 + cells (8.6-fold higher). When either IL2 or IL15 were added to the culture, the expression also improved 2.5-fold. The IL2 and IL7 showed significant effect on the expansion of both the CD26 + and CD26 fractions of the CD34 + cells. However, the effects of IL15 on CD26 + and CD26 −expansion were similar. Conclusion: Taken together, our data suggested that using IL7 causes higher proliferation of CD26 cells in comparison to that seen under other culture conditions.
Introduction
Umbilical cord blood (UCB) has been used as an alternative source of hematopoietic stem and progenitor cells (HSC/HPCs) for transplantation (CitationChristopherson et al. 2007) in hematologic malignancies and non-malignant disorders (CitationDelaney et al. 2010, CitationChristopherson et al. 2007). UCB has low histocompatibility and low risk of graft-versus-host disease (GVHD) (CitationWagner et al. 1995, Citation2002). In UCB transplantation, the CD26 + subset that is a fraction of CD34 + cells, negatively regulates in vivo homing and engraftment of HSCs (CitationChristopherson et al. 2004). CD26 is highly expressed in various cells such as hematopoietic stem cells, immune cells including T, B, and NK cells, fibroblasts, and epithelial cells (CitationDrucker 2007, CitationVanham et al. 1993, CitationKahne et al. 1999). CD26 is a dipeptidase that cleaves cytokines from the N-terminus of peptides (CitationChristopherson et al. 2002). The CD26 marker plays an important role in cell–cell interactions, cell adhesion, apoptosis, immune cell regulation, and cancer cell migration (CitationYu et al. 2006, CitationChristopherson et al. 2012). It also regulates the trafficking of CD34 + HSCs, pre-B lymphocytes, and T lymphocytes from bone marrow (CitationDelaney et al. 2010). Studies have reported that inhibition of the CD26 activity on CD34 + cells can improve HSC/HPC transplantation efficiency (CitationChristopherson et al. 2003, Citation2007, Citation2012, CitationCampbell et al. 2007, CitationEric-Nikolic et al. 2011).
Due to the limited number of cord blood cells collected, many laboratories carry out ex vivo stem cell expansion to make UCB more suitable for transplantation (CitationRao et al. 2012, CitationXu and Reems 2001). However, for clinical application, the expansion of cord blood HSCs is possible by using some extrinsic regulatory factors including hematopoietic cytokines (a group of glycoproteins). Cytokines are produced by many types of hematopoietic and non-hematopoietic cells (CitationMayani et al. 1993). For example, stem cell factor (SCF) and Flt3 ligand (FL), have been used as key cytokines for HSC/HPC proliferation (CitationUeda et al. 2000). It has been shown that SCF improves homing and proliferation of UCB cells in preclinical models (CitationUeda et al. 2000, CitationBoelens et al. 2013). The FL is involved in short-term expansion and also plays a role in the proliferation and differentiation of HSC and HPC (CitationHofmeister et al. 2007). Previous studies have shown that IL7 is crucial for B cell development and the induction of NK cell differentiation (CitationParrish et al. 2009, CitationCavazzana-Calvo et al. 1996). IL15 has a mutual role in the regulation of NK cell differentiation and activation (CitationCavazzana-Calvo et al. 1996, CitationVitale et al. 1996, CitationCheng et al. 2009). Although IL2 is a T cell growth factor, it mediates the activated B cell proliferation and NK cell differentiation (CitationWaldmann 2006, CitationMeazza et al. 2011, CitationCarayol et al. 1998).
In this study, we evaluated the role of IL2, IL7, and IL15 on the CD26 + fraction of cord blood mononuclear cells, and we also assessed the influence of cytokines on the expansion of CD26 + as a subset of CD34 + mononuclear cord blood cells.
Materials and methods
Cell isolation
Cord blood samples were collected from full-term normal deliveries, and diluted at a ratio of 2:1 with phosphate-buffered saline (PBS; SIGMA). Subsequently, mononuclear cells were isolated by centrifugation on Ficoll-Paque (GE healthcare, 1.078 g/ml) at 850 gm for 25 min. The mononuclear cells were collected, washed twice in RPMI1640 (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco), and resuspended in RPMI1640 supplemented with FBS, either for culture or for freezing.
Cell culture and culture condition
A quantity of 105 cord blood mononuclear cells were seeded in 96-well plates in 250 μL of RPMI1640 plus Gluta Max 1-time medium (Gibco) containing 20% FBS (Gibco), 1% penicillin/streptomycin (Gibco), and supplemented with cytokines in final concentrations: SCF (40 ng/ml), FL (40 ng/mL), interleukin-7 (IL7, 40 ng/mL), IL15 (40 ng/mL), and IL2 (40 ng/mL) (all cytokines were purchased from PeproTech). Cells were cultured at 37°C for 21 days, and half of the co-culture medium was replaced weekly. On indicated days, cells were harvested and analyzed by FACS to assess the CD26 and CD34 + cells.
Monoclonal antibodies and flow cytometry
Monoclonal antibodies (conjugated with different fluorochromes) for staining of cell-surface antigens were obtained from Abcam for CD34 (581; Abcam) and from Bioscience for CD26 (M-A261; BD Biosciences). Cultured cells were evaluated by flow cytometric analysis every week. Propidium iodide (1.0 mg/mL; Invitrogen) was used to exclude dead cells from the analysis. Cells were analyzed by BD caliber (BD ebioscience), between 10,000 and 30,000 events were collected, and analyses were performed by using flowing software (Perttu Terho, version 2.5.1.).
Statistical analysis
All data were summarized and expressed as mean (SD). The difference between groups was tested by using the independent T test and one-way Analysis of Variance (ANOVA). P values < 0.05 were considered to be statistically significant.
This study, considering its experimental issues, was approved by Ethical committee of the Tabriz University of Medical Sciences.
Results
Effect of IL7 on CD26 + cell expansion derived from cord blood mononuclear cells
The important role of IL7 during NK cell development (CitationVosshenrich et al. 2005) has been previously shown, along with the complementary role of IL7 during B and T lymphopoiesis, similar to other cytokines of the tyrosine kinase family (CitationSitnicka et al. 2003, Citation2007).
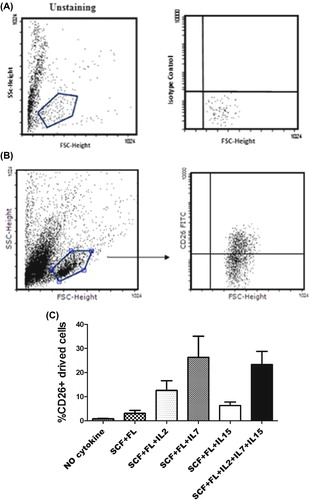
It has been shown that administration of IL7 improves immune reconstitution without GVHD induction after allogeneic bone marrow transplantation (CitationAlpdogan et al. 2001). Moreover, the CD26 + population has a negative effect on the engraftment of the cord blood CD34 + cell transplantation (CitationCampbell et al. 2007). It is unclear whether IL7 has a significant effect on the expansion of CD26 + cell-derived cord blood mononuclear cells. To evaluate the effect of various cytokines with different combinations on CD26 expansion, we cultured 1 × 105 cord blood mononuclear cells for 21 days in the presence of different combinations of SCF, FL, IL2, IL7, and IL15. Harvested cells were evaluated by FACS at distinct time points (). In the presence of SCF and FL, cultured cord blood mononuclear cells expressed 3% of CD26 + cells in comparison to that in the absence of cytokines, on day 14 (). Moreover, their expression did not significantly increase by adding IL15 (6%) and IL2 (12%). On the other hand, adding IL7 significantly increased the expression of CD26 + cells (26%), (8.6-fold, compared with the culture condition without IL7), and it was also 2.5 times more than that when either IL2 or IL15 were added to the culture (). When a combination of IL7, IL2, and IL15 cytokines were used along with SCF and FL, the expression of CD26 + cells was 23% (). The same results have been obtained with a small change on day 21 (data not shown). These data suggest the significant role of IL7 on the expansion of CD26 + cells derived from cord blood mononuclear cells.
Figure 1. IL7 Ligand generates increased CD26 + cells from cord blood mononuclear cells. 105 cord blood mononuclear cells cultured on 96 well plate by using different combination of cytokines and CD26 + cells analyzed by FACS in day 7, 14, 21. (A) isotype control sample. (B) Representative FACS profiles from mononuclear cord blood cultured cells. (C) Mean (SD) proportion of CD26 + cells after 14 days; p < 0.001.
Role of cytokines in generation of CD26 + cord blood mononuclear cells
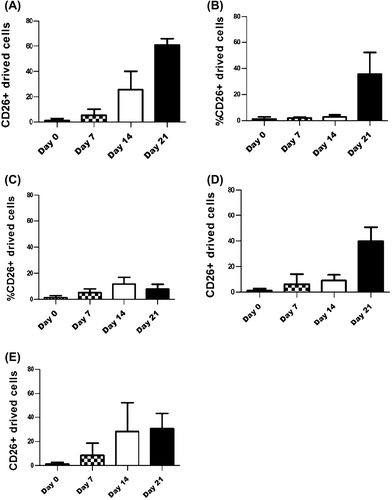
Previous studies have shown the crucial role of IL7 for B cell development and IL2 induction in NK cell differentiation (CitationParrish et al. 2009, CitationCavazzana-Calvo et al. 1996) and T cell proliferation, and also a mediatory role in B cell proliferation (CitationMeazza et al. 2011, CitationCarayol et al. 1998, CitationWaldmann 2006). Furthermore, IL15 has a significant role in the regulation of NK cell differentiation from CD34 + progenitor cells (CitationCavazzana-Calvo et al. 1996, CitationVitale et al. 1996, CitationCheng et al. 2009). Using the combination of cytokines is an alternative option to increase the survival, differentiation, and proliferation of cord blood cells in transplantation (CitationMayani et al. 1993, CitationBordeaux-Rego et al. 2010). Regarding the influence of IL2, IL7, and IL15 on the expansion of the CD26 + subset, cord blood mononuclear cells (1 × 105) were cultured in the presence of IL2, IL7, and IL15 in 96-well plates, and the harvested cells were evaluated by FACS on days 0, 7, 14 and 21. The findings revealed that CD26+-derived cells had increased in the culture condition of SCF and FL, and the results were similar in both cases of addition of IL15, or of a combination of IL2, IL7, and IL15 (30–60%) (). In the presence of cytokines SCF, FL, and IL2, the percentage of CD26 reached 11% on day 14, but it declined to 7.8% on day 21(). The CD26 + cells dramatically increased with a combination of SCF, FL, and IL7, from day 0 to day 14, and remained at the same level on day 21 (30%) (). Taken together, the CD26 + fraction increased with IL7 and IL2, but on day 21, it remained constant for IL7 and reduced for IL2.
Figure 2. Cytokines can efficiently produce CD26 + cells. 105 cord blood mononuclear cells cultured with different combination of cytokines. Cultured cells harvested in identical time points and analyzed by FACS. Mean proportion of CD26 + cells have shown with combination of SCF+ FL+ IL2 + IL7+ IL15; p < 0.0001 (A), SCF+ FL; p < 0.0007 (B), SCF+ FL+ IL2; no significant (C), SCF+ FL+ IL15; p < 0.0001 (D) and SCF+ FL+ IL7; p < 0.01 (E).
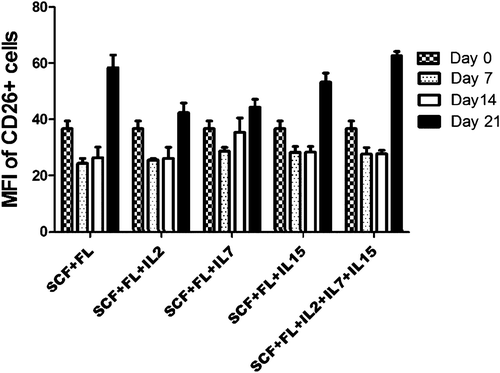
It has been shown that mean fluorescence intensity (MFI) of the CD26 + population in lymphoid cells is low in breast cancer (CitationEric-Nikolic et al. 2011). Moreover, previous studies have reported that the MFI and the percentage of CD26 + cells in the PHA-stimulated PBMC or lymphoblasts increased in the presence of IL2 (CitationCordero et al. 1997, CitationSalgado et al. 2000). Considering the influence of key immune cell cytokines on the CD26 + fraction on the cord blood mononuclear cells, which has not been studied yet, our data showed that the MFI of CD26 + cells increased from day 0 to day 21 in the presence of SCF and FL, and also in the presence of IL2, IL7, and IL15 (). For the SCF and FL groups, the MFI was in the same level. The same results were obtained when IL15 was added and also when three cytokines together (IL2, IL15, IL7) were added. However, on day 21, MFI increased with the use of IL2 and IL7 (43%), which was lower when using IL15 (53%).
Role of the cytokines in the expansion of CD26 + and CD26 − fractions of CD34 + cord blood mononuclear cells
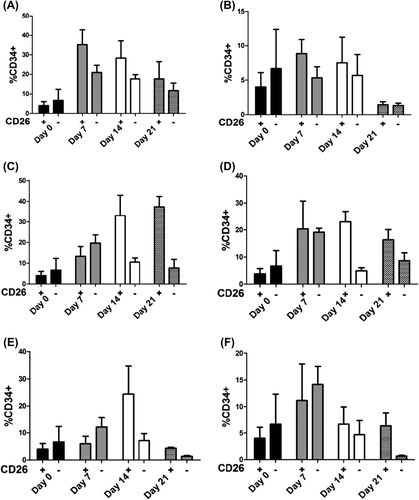
Previous studies have reported that a subset of CD34 + cord blood cells express CD26. Moreover, inhibition of CD26 + cells improves engraftment of CD34 + cells (CitationChristopherson et al. 2004, Citation2007, CitationCampbell et al. 2007). Since it is important to know the role of key immune cytokines on the CD26 + versus the CD26 − fraction of CD34 + mononuclear cord blood cells, we cultured mononuclear cord blood cells in the presence of SCF and FL, by adding IL2, IL7, and IL15 separately and also simultaneously. The flow cytometric analysis of cells harvested on days 0, 7, 14, and 21 () showed that CD26 + and CD26 − fractions expanded on day 7 without the addition of any cytokine (35%, 21% respectively); however, both declined until day 21 (17%, 11%) (). In the presence of all cytokines in culture (SCF, FL, IL2, IL7 and IL15), the expansion of CD26 + and CD26 − did not show any difference, but both declined on day 21 (). The CD26 + fraction expanded from day 7 (13%) to day 21 (37%) with SCF and FL, whereas the CD26 − population declined from 19% to 7.6% (). CD26 + and CD26 − cells increased by the same amount on day 7, with the addition of IL2 (20%), but CD26 + cells slightly declined and CD26 cells sharply reduced to 8.6% till day 21 (). The CD34 + CD26+ fraction increased with SCF, FL, and IL7 on day 14, and reduced on day 21 (from 24% to 4%), although CD34 + and CD26 − population had not significantly changed during the study periods (). The CD26 + and CD26 − fractions of CD34 + expansion were in the same amount at indicated time points (day 7, 14), while CD26 − reduced on day 21 (). These data suggest the significant role of cytokines on the expansion of CD26 fraction of CD34 + cells.
Figure 4. Representative FACS profile of 105 cultured cord blood mononuclear cells in identical time points. CD34+ CD26+ and CD34+CD26− evaluated by gating on lymphoid population in FSC versus SSC.
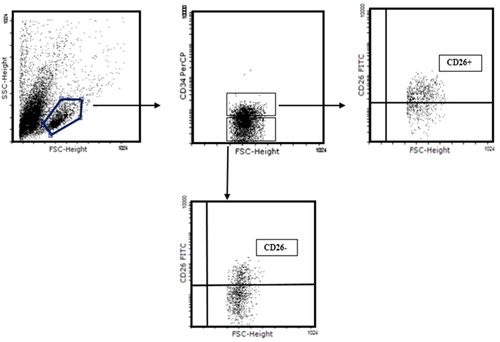
Figure 5. Evaluation of CD34+ CD26+and CD34+CD26− cells derived from cord blood mononuclear cells at different time points. The harvested cells evaluated by FACS and mean (SD) have shown using no cytokines (A), SCF+ FL+ IL2 + IL7+ IL15 (B), SCF+ FL (C), SCF+ FL+ IL2 (D), SCF+ FL+ IL7 (E) and SCF+ FL+ IL15 (F).
Discussion
The present study demonstrated the role of important cytokines (IL2, IL7, and IL15) on the expansion of CD26 cells as a subpopulation of CD34 + cells in mononuclear cord blood cells.
Previous studies have shown that CD26 cells have a negative effect on the engraftment of CD34 + cells, either in bone marrow or in cord blood (CitationChristopherson et al. 2007, 2012, CitationCampbell et al. 2007, CitationPrabhash et al. 2010). The amount of cord blood cells are rare; therefore, to overcome this problem, and to achieve efficient transplantation, more cells from several full-term babies delivered should be collected, or the cells should be expanded by using a combination of cytokines (CitationXu and Reems 2001, CitationHofmeister et al. 2007, CitationMayani et al. 1993, CitationKita et al. 2011, CitationLi et al. 2010, CitationOran and Shpall 2012, CitationRen and Jiang 2013). The results showed a significant effect of IL7 and IL2 on the CD26 fraction, and this effect was different from that seen with IL15.
Several studies have reported that various cytokines, including IL2, IL7, and IL15 have important roles in immune repopulating cells (CitationZhang et al. 2011, CitationSirskyj et al. 2008, CitationNotarangelo et al. 2000). For T cell expansion and in the generation of NK cells, IL2 has crucial role. Moreover, IL2 is mostly being used as an elementary cytokine during transplantation for blood cancers (CitationCavazzana-Calvo et al. 1996, CitationMeazza et al. 2011, CitationZhang et al. 2011, CitationLe-Barillec et al. 2005, CitationMetcalfe et al. 2012). Our data showed that IL2 increased the CD26 + fraction in the initial period of the study and then reduced it after day 14, but the CD34 + CD26− population slightly increased; this is a valuable finding to improve the engraftment and repopulation of recipients.
IL7 is involved in proliferation and survival of T cells and also in B cell development, as well as in IL7-dependent NK cell population (CitationParrish et al. 2009, CitationCavazzana-Calvo et al. 1996, CitationSitnicka et al. 2003, Citation2007, CitationLe-Barillec et al. 2005). Here, we found that IL7 has a significant role in expansion of CD26 + cells in cord blood mononuclear cells in vitro. It affected CD26 + expansion by at least 2-fold more than IL2 and IL15. However, the CD26 + cell expansion reduced when it was used in combination with IL2 and IL15. This reduction can be addressed in sharing IL7 receptors with IL2 and IL15. These receptors are occupied by IL2 and IL15 with high affinity, whereas IL7 is a critical modulator of low-affinity peptide-induced proliferation (CitationFry and Mackall 2002).
IL15 is a key cytokine for NK cell development and has a crucial role in the proliferation and survival of different NK cell subsets (CitationCheng et al. 2009, CitationNozad Charoudeh et al. 2010, CitationVosshenrich et al. 2005, CitationRanson et al. 2003, CitationRanson et al. 2003). NK cells also play an important role in transplantation. Therefore, it is crucial to use IL15 in the expansion and induction of NK cells in cord blood cells. Our data illustrated that IL15 increases the rate of CD26 + cells in cord blood mononuclear cells. IL15 affects both fractions (CD26 + and CD26−) of CD34 + cells. CD26 + and CD26 − cells expanded over time. The cord blood expansion should be carefully performed utilizing IL15, if CD26 + cells are a crucial player. Overall, our findings suggested more CD26 proliferation using IL7 and IL15 separately; however, the influence of IL2 on CD26 reduced with time.
Conclusion
Taken together, our data suggest that using IL7 alone causes more CD26 cell proliferation; however, IL2 influence on CD26 was reduced with time, and it could improve the negative effect of CD26 + cells after transplantation.
Acknowledgments
The authors thank Nazli Saeedi for help with flow cytometry and Fatemeh Ebadi for her editorial help. This work was supported by the Research Council of Medical Science. Hojjatollah Nozad Charoudeh holds the position of Assistant Professor in the Faculty of Medicine.
Authorship
Contribution: A.Z. and H.N.C. designed and conceptualized the research, analyzed the data, and wrote the manuscript. A.Z. purified cell populations, carried out in vitro cultures, and analyzed the data; K.N, B.B, M.S, and H.T.N participated in the study design, and coordinated the sample discussions of data.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alpdogan O, Schmaltz C, Muriglan SJ, Kappel BJ, Perales MA, Rotolo JA, et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265.
- Boelens JJ, Aldenhoven M, Purtill D, Ruggeri A, Defor T, Wynn R, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–3987.
- Bordeaux-Rego P, Luzo A, Costa FF, Olalla Saad ST, Crosara-Alberto DP. Both interleukin-3 and interleukin-6 are necessary for better ex vivo expansion of CD133 + cells from umbilical cord blood. Stem Cells Dev. 2010;19:413–422.
- Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34 + cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–354.
- Carayol G, Robin C, Bourhis JH, Bennaceur‐Griscelli A, Chouaib S, Coulombel L, Caignard A. NK cells differentiated from bone marrow, cord blood and peripheral blood stem cells exhibit similar phenotype and functions. Eur J Immunol. 1998;28:1991–2002.
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, De Coene C, Selz F, Le Deist F, Fischer A. Role of interleukin-2 (IL-2), IL-7, and IL-15 in natural killer cell differentiation from cord blood hematopoietic progenitor cells and from gamma c transduced severe combined immunodeficiency X1 bone marrow cells. Blood. 1996;88:3901–3909.
- Cheng M, Charoudeh HN, Brodin P, Tang Y, Lakshmikanth T, Hoglund P, et al. Distinct and overlapping patterns of cytokine regulation of thymic and bone marrow-derived NK cell development. J Immunol. 2009;182:1460–1468.
- Christopherson KW II, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003.
- Christopherson KW II, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34 + or lineage-human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice. Stem Cells Dev. 2007;16:355–360.
- Christopherson KW, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–4686.
- Christopherson KW, Frank RR, Jagan S, Paganessi LA, Gregory SA, Fung HC. CD26 protease inhibition improves functional response of un-fractionated cord blood, bone marrow, and mobilized peripheral blood cells to CXCL12/SDF-1. Exp Hematol. 2012;40:945–952.
- Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1α-mediated chemotaxis of human cord blood CD34 + progenitor cells. J Immunol. 2002;169:7000–7008.
- Cordero OJ, Salgado FJ, Vinuela JE, Nogueira M. Interleukin-12 enhances CD26 expression and dipeptidyl peptidase IV function on human activated lymphocytes. Immunobiology. 1997;197:522–533.
- Delaney C, Ratajczak MZ, Laughlin MJ. Strategies to enhance umbilical cord blood stem cell engraftment in adult patients. Expert Rev Hematol. 2010;3:273–283.
- Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343.
- Eric-Nikolic A, Matic IZ, Dordevic M, Milovanovic Z, Markovic I, Dzodic R, et al. Serum DPPIV activity and CD26 expression on lymphocytes in patients with benign or malignant breast tumors. Immunobiology. 2011;216:942–946.
- Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–3904.
- Hofmeister C, Zhang J, Knight K, Le P, Stiff P. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transpl. 2007;39:11–23.
- Kahne T, Lendeckel U, Wrenger S, Neubert K, Ansorge S, Reinhold D. Dipeptidyl peptidase IV: a cell surface peptidase involved in regulating T cell growth (review). Int J Mol Med. 1999;4:3–15.
- Kita K, Lee JO, Finnerty CC, Herndon DN. Cord blood-derived hematopoietic stem/progenitor cells: current challenges in engraftment, infection, and ex vivo expansion. Stem Cells Int. 2011;2011:276193.
- Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, Di Santo JP. Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol. 2005;175:1735–1740.
- Li Y, Schmidt-Wolf IG, Wu YF, Huang SL, Wei J, Fang J, et al. Optimized protocols for generation of cord blood-derived cytokine-induced killer/natural killer cells. Anticancer Res. 2010;30:3493–3499.
- Mayani H, Dragowska W, Lansdorp PM. Cytokine-induced selective expansion and maturation of erythroid versus myeloid progenitors from purified cord blood precursor cells. Blood. 1993;81:3252–3258.
- Meazza R, Azzarone B, Orengo AM, Ferrini S. Role of common-gamma chain cytokines in NK cell development and function: perspectives for immunotherapy. J Biomed Biotechnol. 2011;2011:861920.
- Metcalfe C, Cresswell P, Barclay AN. Interleukin-2 signalling is modulated by a labile disulfide bond in the CD132 chain of its receptor. Open Biol. 2012;2:110036.
- Notarangelo LD, Giliani S, Mella P, Schumacher RF, Mazza C, Savoldi G, et al. Combined immunodeficiencies due to defects in signal transduction: defects of the gammac-JAK3 signaling pathway as a model. Immunobiology. 2000;202:106–119.
- Nozad Charoudeh H, Tang Y, Cheng M, Cilio CM, Jacobsen SE, Sitnicka E. Identification of an NK/T cell-restricted progenitor in adult bone marrow contributing to bone marrow- and thymic-dependent NK cells. Blood. 2010;116:183–192.
- Oran B, Shpall E. Umbilical cord blood transplantation: a maturing technology. Hematology Am Soc Hematol Educ Program. 2012;2012:215–222.
- Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW, et al. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182:4255–4266.
- Prabhash K, Khattry N, Bakshi A, Karandikar R, Joshi A, Kannan S, et al. CD26 expression in donor stem cell harvest and its correlation with engraftment in human haematopoietic stem cell transplantation: potential predictor of early engraftment. Ann Oncol. 2010;21:582–588.
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003;100:2663–2668.
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893.
- Rao M, Ahrlund‐Richter L, Kaufman DS. Concise review: cord blood banking, transplantation and induced pluripotent stem cell: success and opportunities. Stem Cells. 2012;30:55–60.
- Ren Z, Jiang Y. Umbilical cord blood hematopoietic stem cell expansion ex vivo. J Blood Disorders Transf 2013;S3:2.
- Salgado FJ, Vela E, Martin M, Franco R, Nogueira M, Cordero OJ. Mechanisms of CD26/dipeptidyl peptidase IV cytokine-dependent regulation on human activated lymphocytes. Cytokine. 2000;12:1136–1141.
- Sirskyj D, Theze J, Kumar A, Kryworuchko M. Disruption of the gamma c cytokine network in T cells during HIV infection. Cytokine. 2008;43:1–14.
- Sitnicka E, Brakebusch C, Martensson IL, Svensson M, Agace WW, Sigvardsson M, et al. Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis. J Exp Med. 2003;198:1495–1506.
- Sitnicka E, Buza-Vidas N, Ahlenius H, Cilio CM, Gekas C, Nygren JM, et al. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964.
- Ueda T, Tsuji K, Yoshino H, Ebihara Y, Yagasaki H, Hisakawa H, et al. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest. 2000;105:1013–1021.
- Vanham G, Kestens L, De Meester I, Vingerhoets J, Penne G, Vanhoof G, et al. Decreased expression of the memory marker CD26 on both CD4 + and CD8 + T lymphocytes of HIV-infected subjects. J Acquir Immune Defic Syndr. 1993;6:749–757.
- Vitale M, Sivori S, Pende D, Augugliaro R, Di Donato C, Amoroso A, et al. Physical and functional independency of p70 and p58 natural killer (NK) cell receptors for HLA class I: their role in the definition of different groups of alloreactive NK cell clones. Proc Natl Acad Sci U S A. 1996;93:1453–1457.
- Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221.
- Vosshenrich CA, Samson-Villeger SI, Di Santo JP. Distinguishing features of developing natural killer cells. Curr Opin Immunol. 2005;17:151–158.
- Wagner J, Steinbuch M, Kernan N, Broxmayer H, Gluckman E. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet. 1995;346:214–219.
- Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618.
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601.
- Xu R, Reems JA. Umbilical cord blood progeny cells that retaina CD34 + phenotype after ex vivo expansion have less engraftment potential than unexpanded CD34 + cells. Transfusion. 2001;41:213–218.
- Yu DM, Wang XM, McCaughan GW, Gorrell MD. Extraenzymatic functions of the dipeptidyl peptidase IV-related proteins DP8 and DP9 in cell adhesion, migration and apoptosis. FEBS J. 2006;273:2447–2460.
- Zhang Q, Wang HY, Liu X, Bhutani G, Kantekure K, Wasik M. IL-2R common gamma-chain is epigenetically silenced by nucleophosphin-anaplastic lymphoma kinase (NPM-ALK) and acts as a tumor suppressor by targeting NPM-ALK. Proc Natl Acad Sci U S A. 2011;108:11977–11982.